Mitigation of GHG Emissions from Soils Fertilized with Livestock Chain Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Crop and Soil Analyses
2.3. GHG Fluxes
2.4. Statistical Analysis
3. Results
3.1. Crop Yield and Soil Organic C
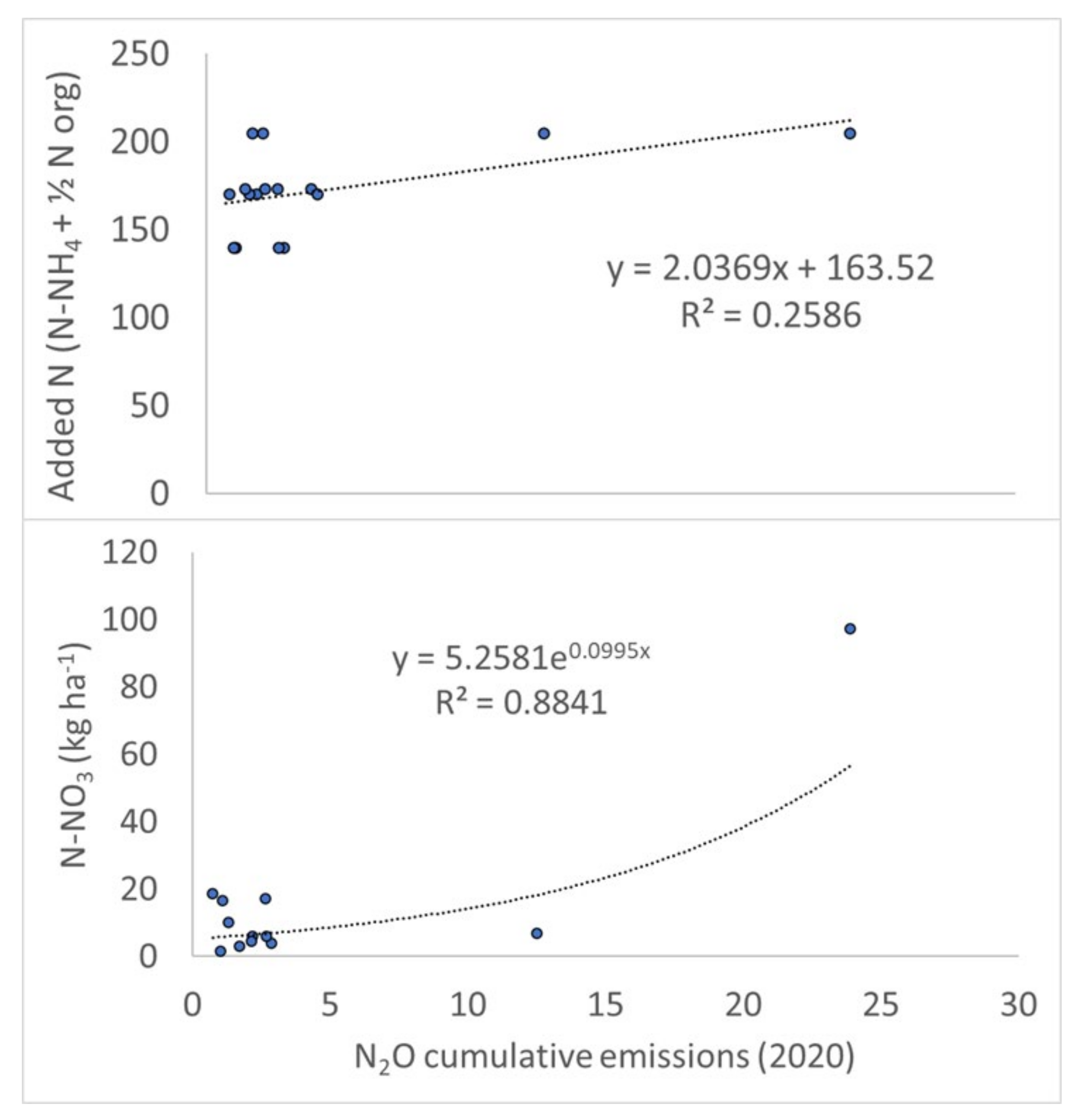
3.2. NH4 and NO3 Dynamics and Accumulation in Soil
3.3. GHGs Fluxes
4. Discussion
4.1. Fertilizer Types
4.2. Biochar Contribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The global methane budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosystems 2006, 74, 207–228. [Google Scholar] [CrossRef]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Change 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Kammann, C.; Ratering, S.; Eckhard, C.; Müller, C. Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J. Environ. Qual. 2012, 41, 1052–1066. [Google Scholar] [CrossRef]
- De Vries, F.T.; Van Groenigen, J.W.; Hoffland, E.; Bloem, J. Nitrogen losses from two grassland soils with different fungal biomass. Soil Biol. Biochem. 2011, 43, 997–1005. [Google Scholar] [CrossRef]
- Lugato, E.; Leip, A.; Jones, A. Mitigation potential of soil carbon management overestimated by neglecting N2O emissions. Nat. Clim. Change 2018, 8, 219–223. [Google Scholar] [CrossRef]
- Mosier, A.R. Exchange of gaseous nitrogen compounds between agricultural systems and the atmosphere. Plant Soil 2001, 228, 17–27. [Google Scholar] [CrossRef]
- Khalil, K.; Mary, B.; Renault, P. Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol. Biochem. 2004, 36, 687–699. [Google Scholar] [CrossRef]
- Liu, X.J.; Mosier, A.R.; Halvorson, A.D.; Zhang, F.S. Tillage and nitrogen application effects on nitrous and nitric oxide emissions from irrigated corn fields. Plant Soil 2005, 276, 235–249. [Google Scholar] [CrossRef]
- Carmo, J.B.; Filoso, S.; Zotelli, L.C.; De Sousa, E.R.; Pitombo, L.M.; Duarte-Neto, P.J.; Vargas, V.P.; Andrade, C.A.; Gava, G.J.; Rossetto, R.; et al. Infield greenhouse gas emissions from sugarcane soils in Brazil: Effects from synthetic and organic fertilizer application and crop trash accumulation. GCB Bioenergy 2012, 5, 267–280. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Hellebrand, H.J.; Scholz, V.; Kern, J. Fertiliser induced nitrous oxide emissions during energy crop cultivation on loamy sand soils. Atmos. Environ. 2008, 42, 8403–8411. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Zheng, X.; Wang, Y. Quantifying direct N2O emissions in paddy fields during rice growing season in mainland China: Dependence on water regime. Atmos. Environ. 2007, 41, 8030–8042. [Google Scholar] [CrossRef]
- McSwiney, C.P.; Robertson, G.P. Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Glob. Change Biol. 2005, 11, 1712–1719. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Brüggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Glob. Change Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Change Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Gao, X.; Guan, X.; Zhang, W.; Zhang, Y.; Shi, X.; Chen, X. Nitrous oxide emissions in Chinese vegetable systems: A meta-analysis. Environ. Pollut. 2018, 239, 375–383. [Google Scholar] [CrossRef]
- Xia, L.; Lam, S.K.; Wolf, B.; Kiese, R.; Chen, D.; Butterbach-Bahl, K. Trade-offs between soil carbon sequestration and reactive nitrogen losses under straw return in global agroecosystems. Glob. Change Biol. 2018, 24, 5919–5932. [Google Scholar] [CrossRef] [PubMed]
- Velthof, G.L.; Kuikman, P.J.; Oenema, O. Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biol. Fertil. Soils 2003, 37, 221–230. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Johansen, A.; Carter, M.S.; Jensen, E.S.; Hauggard-Nielsen, H.; Ambus, P. Effects of digestate from anaerobically digested cattle slurry and plant materials on soil microbial community and emission of CO2 and N2O. Appl. Soil Ecol. 2013, 63, 36–44. [Google Scholar] [CrossRef]
- Dietrich, M.; Fongen, M.; Foereid, B. Greenhouse gas emissions from digestate in soil. Int. J. Recycl. Org. Waste Agric. 2020, 9, 1–19. [Google Scholar]
- Fiedler, S.R.; Augustin, J.; Wrage-Mönnig, N.; Jurasinski, G.; Gusovius, B.; Glatzel, S. Potential short-term losses of N2O and N2 from high concentrations of biogas digestate in arable soils. Soil 2017, 3, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.L.; Clarke, M.L.; Othman, M.; Ramsden, S.J.; West, H.M. Biochar-mediated reductions in greenhouse gas emissions from soil amended with anaerobic digestates. Biomass Bioenergy 2015, 79, 39–49. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; David, W.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.V.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Siguah, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef] [Green Version]
- Case, S.D.; McNamara, N.P.; Reay, D.S.; Stott, A.W.; Grant, H.K.; Whitaker, J. Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biol. Biochem. 2015, 81, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Case, S.D.; McNamara, N.P.; Reay, D.S.; Whitaker, J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil–the role of soil aeration. Soil Biol. Biochem. 2012, 51, 125–134. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Singh, B.; Joseph, S.; Kimber, S.; Cowie, A.; Chan, K.Y. Biochar and emissions of non-CO2 greenhouse gases from soil. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2009; pp. 259–282. [Google Scholar]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an electron shuttle between bacteria and Fe(III) minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344. [Google Scholar] [CrossRef]
- Brevik, E.C. Soils and climate change: Gas fluxes and soil processes. Soil Horiz. 2012, 53, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Guenet, B.; Gabrielle, B.; Chenu, C.; Arrouays, D.; Balesdent, J.; Bernoux, M.; Bruni, E.; Caliman, J.P.; Cardinael, R.; Chen, S.; et al. Can N2O emissions offset the benefits from soil organic carbon storage? Glob. Change Biol. 2021, 27, 237–256. [Google Scholar] [CrossRef]
- Adviento-Borbe, M.A.; Pittelkow, C.M.; Anders, M.; van Kessel, C.; Hill, J.E.; McClung, A.M.; Linquist, B.A. Optimal fertilizer nitrogen rates and yield-scaled global warming potential in drill seeded rice. J. Environ. Qual. 2013, 42, 1623–1634. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in temperate mixed hardwood forest. Glob. Change Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Adviento-Borbe, M.A.; Kaye, J.P.; Bruns, M.A.; McDaniel, M.D.; McCoy, M.; Harkcom, S. Soil greenhouse gas and ammonia emissions in long-term maize-based cropping systems. Soil Sci. Soc. Am. J. 2010, 74, 1623–1634. [Google Scholar] [CrossRef]
- Arthurson, V. Closing the global energy and nutrient cycles through application of biogas residues to agricultural land-potential benefits and drawbacks. Energies 2009, 2, 226–242. [Google Scholar] [CrossRef] [Green Version]
- Riva, C.; Orzi, V.; Carozzi, M.; Acutis, M.; Boccasile, G.; Lonati, S.; Tambone, F.; D’Imporzano, G.; Adani, F. Short-term experiments in using digestate products as substitutes for mineral (N) fertilizer: Agronomic performance, odours, and ammonia emission impacts. Sci. Total Environ. 2016, 547, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.P.; Pekrun, C.; Möller, K. Organic matter composition of digestates has a stronger influence on N2O emissions than the supply of ammoniacal Nitrogen. Agronomy 2021, 11, 2215. [Google Scholar] [CrossRef]
- Chu, H.; Fujii, T.; Morimoto, S.; Lin, X.; Yagi, K.; Hu, J.; Zhang, J. Community structure of ammonia-oxidizing bacteria under long-term application of mineral fertilizer and organic manure in a sandy loam. Appl. Environ. Microbiol. 2007, 73, 458–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Nielsen, M.P.; Scheutz, C.; Jensen, L.S.; Christensen, T.H.; Nielsen, S.; Bruun, S. Effects of sewage sludge stabilization on fertilizer value and greenhouse gas emissions after soil application. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 506–516. [Google Scholar]
- Rodhe, L.K.K.; Ascue, J.; Willén, A.; Persson, B.V.; Nordberg, Å. Greenhouse gas emissions from storage and field application of anaerobically digested and non-digested cattle slurry. Agric. Ecosyst. Environ. 2015, 199, 358–368. [Google Scholar] [CrossRef]
- Köster, J.R.; Cardenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Senbayram, M.; Well, R.; Giesemann, A.; Dittert, K. Anaerobic digestates lower N2O emissions compared to cattle slurry by affecting rate and product stoichiometry of denitrification—An N2O isotopomer case study. Soil Biol. Biochem. 2015, 84, 65–74. [Google Scholar] [CrossRef]
- Amon, B.; Kryvoruchko, V.; Amon, T.; Zechmeister-Boltenstern, S. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agric. Ecosyst. Environ. 2006, 112, 153–162. [Google Scholar] [CrossRef]
- Clemens, J.; Trimborn, M.; Weiland, P.; Amon, B. Mitigation of greenhouse gas emissions by anaerobic digestion of cattle slurry. Agric. Ecosyst. Environ. 2006, 112, 171–177. [Google Scholar] [CrossRef]
- Pilegaard, K.; Skiba, U.; Ambus, P.; Beier, C.; Brüggemann, N.; Butterbach-Bahl, K.; Dick, J.; Dorsey, J.; Duyzer, J.; Gallagher, M.; et al. Factors controlling regional differences in forest soil emission of nitrogen oxides (NO and N2O). Biogeosciences 2006, 3, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Baral, K.R.; Labouriau, R.; Olesen, J.E.; Petersen, S.O. Nitrous oxide emissions and nitrogen use efficiency of manure and digestates applied to spring barley. Agric. Ecosyst. Environ. 2017, 239, 188–198. [Google Scholar] [CrossRef]
- Senbayram, M.; Chen, R.; Budai, A.; Bakken, L.; Dittert, K. N2O emission and the N2O/(N2O + N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric. Ecosyst. Environ. 2012, 147, 4–12. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A. Emissions of carbon dioxide and methane from fields fertilized with digestate from an agricultural biogas plant. Int. Agrophysics 2018, 32, 29. [Google Scholar] [CrossRef] [Green Version]
- Eickenscheidt, T.; Freibauer, A.; Heinichen, J.; Augustin, J.; Drösler, M. Short-term effects of biogas digestate and cattle slurry application on greenhouse gas emissions affected by N availability from grasslands on drained fen peatlands and associated organic soils. Biogeosciences 2014, 11, 6187–6207. [Google Scholar] [CrossRef] [Green Version]
- Dutaur, L.; Verchot, L.V. A global inventory of the CH4 sink. Global Biogeochem. Cycles 2007, 21, GB4013. [Google Scholar] [CrossRef]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813. [Google Scholar] [CrossRef]
- Biernat, L.; Taube, F.; Loges, R.; Kluss, C.; Reinsch, T. Nitrous oxide emissions and methane uptake from organic and conventionally managed arable crop rotations on farms in Northwest Germany. Sustainability 2020, 12, 3240. [Google Scholar] [CrossRef] [Green Version]
- Dalal, R.C.; Allen, D.E.; Livesley, S.J.; Richards, G. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: A review. Plant Soil 2008, 309, 43–76. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Webster, C.P.; Powlson, D.S. Long-term effects of nitrogen fertilization on methane oxidation in soil of the broadbalk wheat experiment. Soil Biol. Biochem. 1993, 25, 1307–1315. [Google Scholar] [CrossRef]
- Ullah, S.; Frasier, R.; King, L.; Picotte-Anderson, N.; Moore, T.R. Potential fluxes of N2O and CH4 from soils of three forest types in Eastern Canada. Soil Biol. Biochem. 2008, 40, 986–994. [Google Scholar] [CrossRef]
- Möller, K. Effects of biogas digestion on soil organic matter and nitrogen inputs, flows and budgets in organic cropping systems. Nutr. Cycl. Agroecosyst. 2009, 84, 179–202. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Effect of digestate on soil organic carbon and plant-available nutrient content compared to cattle slurry and mineral fertilization. Agronomy 2020, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers—Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J. Plant Nutr. Soil Sci. 2014, 177, 651–670. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Bai, S.H.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. GCB Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global change, nitrification, and denitrification: A review. Glob. Biogeochem. Cycles 2005, 19, GB1007. [Google Scholar] [CrossRef]
- Cooper, R.J.; Wexler, S.K.; Adams, C.A.; Hiscock, K.M. Hydrogeological controls on regional-scale indirect nitrous oxide emission factors for rivers. Environ. Sci. Technol. 2017, 51, 10440–10448. [Google Scholar] [CrossRef]
- Tian, L.; Zhu, B.; Akiyama, H. Seasonal variations in indirect N2O emissions from an agricultural headwater ditch. Biol. Fertil. Soils 2017, 53, 651–662. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.-P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Methane emissions and associated microbial activities from paddy salt-affected soil as influenced by biochar and cow manure addition. Appl. Soil Ecol. 2020, 152, 103531. [Google Scholar]
- Chowdhury, M.A.; de Neergaard, A.; Jensen, L.S. Composting of solids separated from anaerobically digested animal manure: Effect of different bulking agents and mixing ratios on emissions of greenhouse gases and ammonia. Biosyst. Eng. 2014, 124, 63–77. [Google Scholar] [CrossRef]
- Vu, Q.D.; de Neergaard, A.; Tran, T.D.; Hoang, H.T.T.; Vu, V.T.K.; Jensen, L.S. Greenhouse gas emissions from passive composting of manure and digestate with crop residues and biochar on small-scale livestock farms in Vietnam. Environ. Technol. 2015, 36, 2924–2935. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Nguyen, H.D.T.; Hegarty, R.S. Defaunation and its impacts on ruminal fermentation, enteric methane production and animal productivity. Livest. Res. Rural. Dev. 2020, 32, 4. [Google Scholar]
- Kammann, C.; Ippolito, J.; Hagemann, N.; Borchard, N.; Cayuela, M.L.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Jeffery, S.; Kern, J.; Novak, J.; et al. Biochar as a tool to reduce the agricultural greenhouse-gas burden–knowns, unknowns and future research needs. J. Environ. Eng. Landsc. Manag. 2017, 25, 114–139. [Google Scholar] [CrossRef]
- Wang, J.; Pan, X.; Liu, Y.; Zhang, X.; Xiong, Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 2012, 360, 287–298. [Google Scholar] [CrossRef]
- Abagandura, G.O.; Chintala, R.; Sandhu, S.S.; Kumar, S.; Schumacher, T.E. Effects of biochar and manure applications on soil carbon dioxide, methane, and nitrous oxide fluxes from two different soils. J. Environ. Qual. 2019, 6, 1664–1674. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
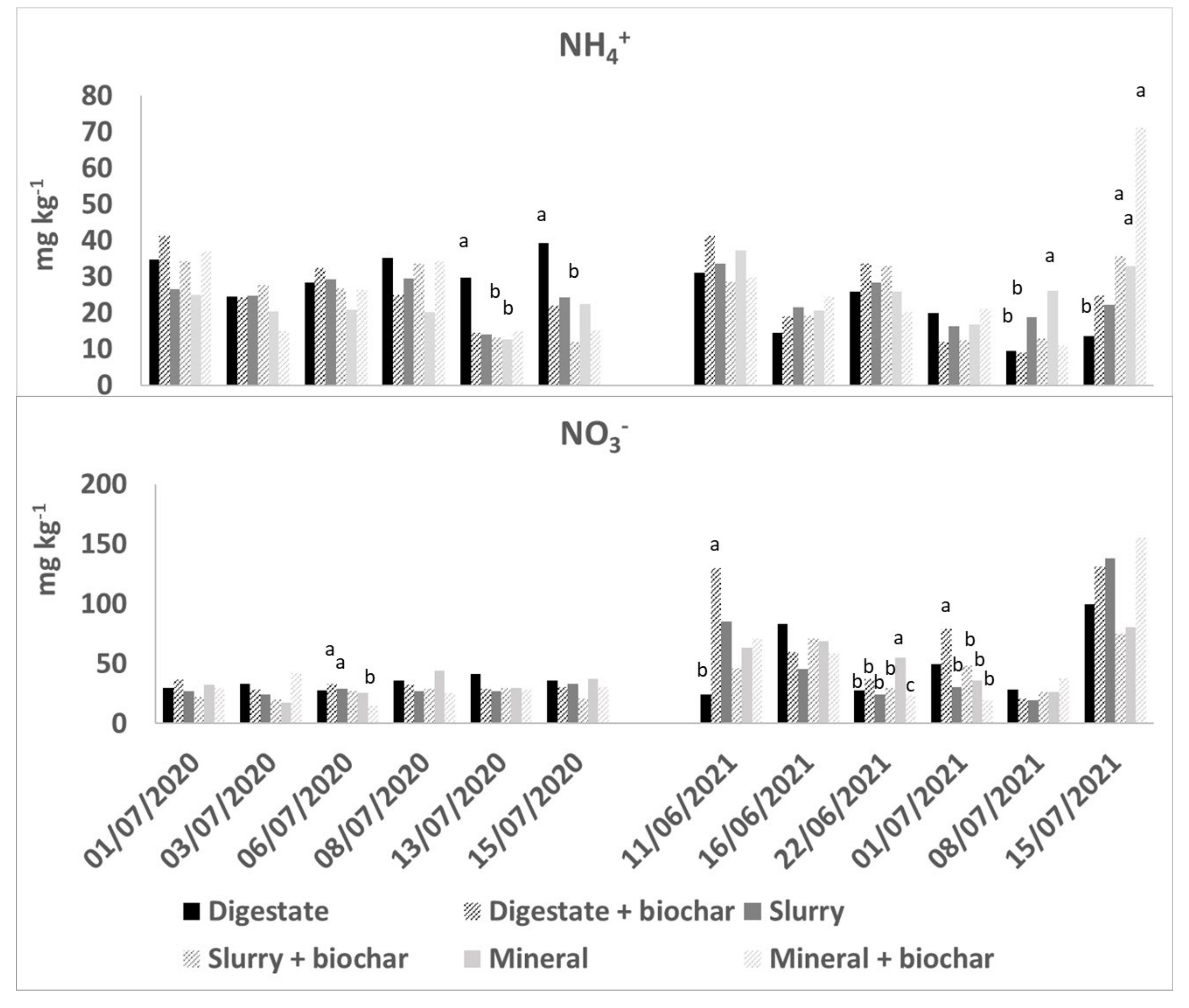
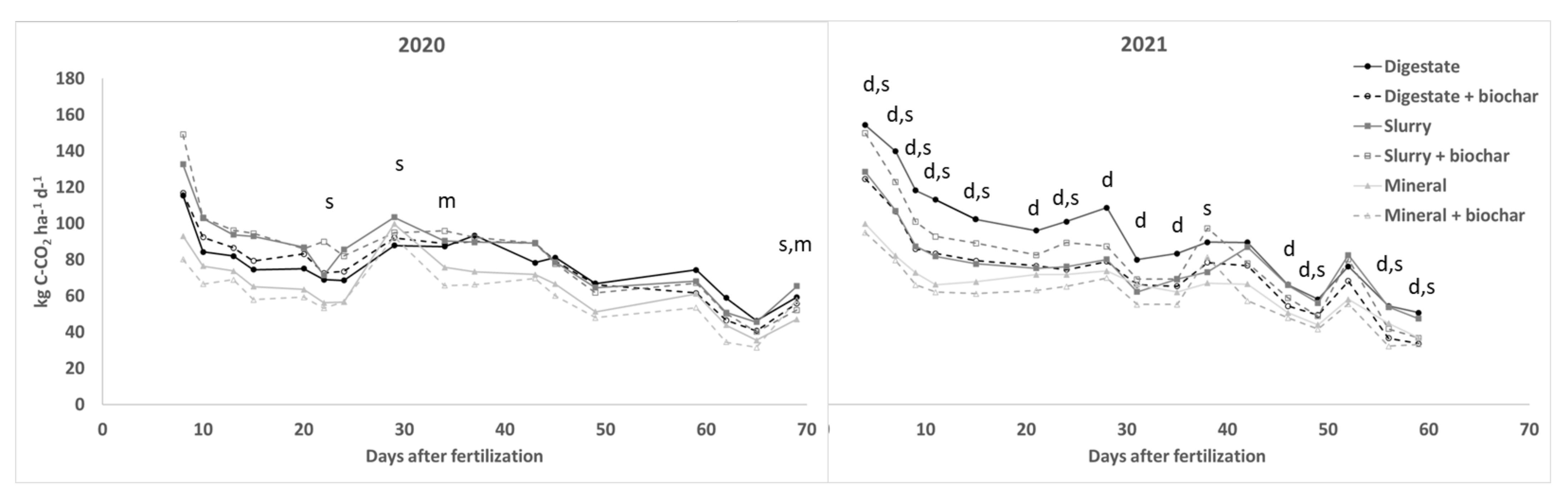

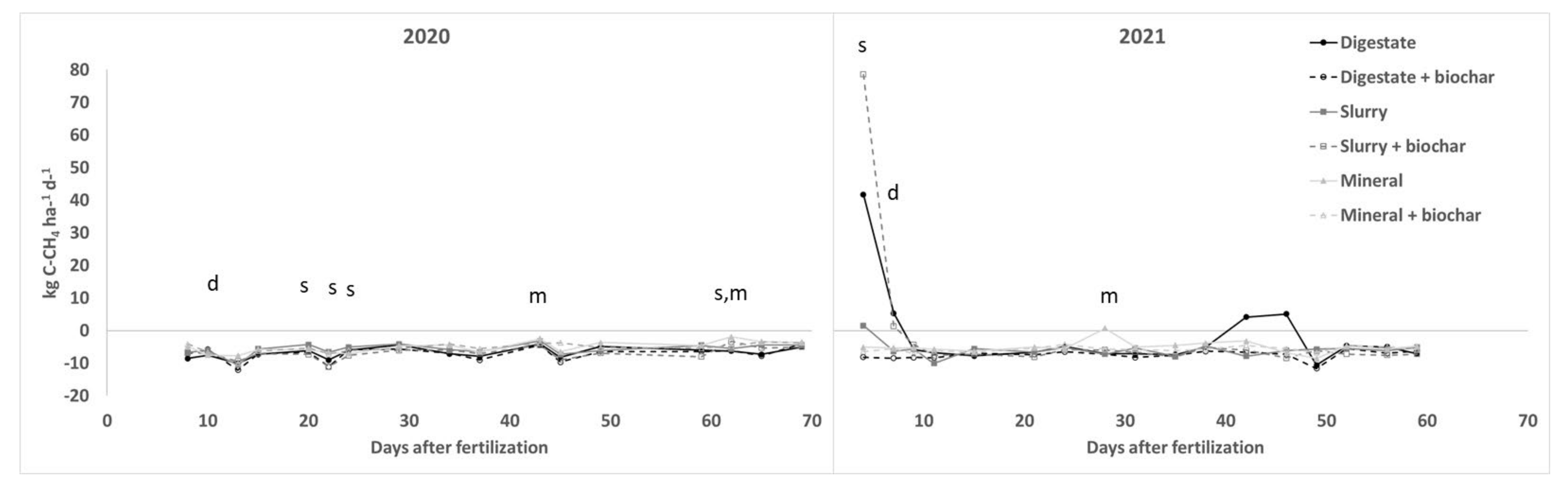
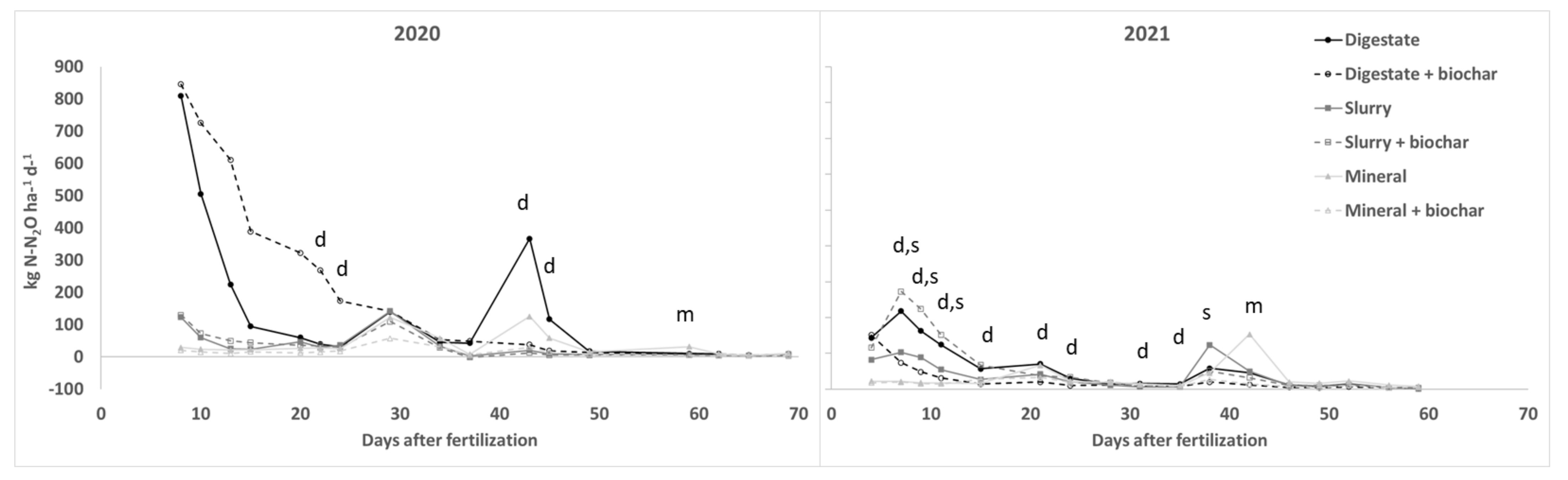
| 2020 | 2021 | |
|---|---|---|
| Fertilization (33% urea; 100% organic) and ploughing | June 23 | June 7 |
| Harrowing and sowing | June 24 | June 8 |
| Pre-emergency weeding | June 25 | June 9 |
| Static chambers installation | June 29 | June 10 |
| Gas monitoring | 17 events | 17 events |
| N mineral sampling | 6 events | 6 events |
| Irrigation | 3 events | 6 events |
| Post emergency weeding | July 17 | July 6 |
| In-season (top-dress) urea fertilization (67%) | July 15 | July 7 |
| End of gas sampling | August 31 | August 5 |
| Digestate | Slurry | |||
|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | |
| Moisture content (%) | 92.1 | 93.1 | 94.6 | 94.7 |
| Norg content (g kg−1) | 2.43 | 1.61 | 4.32 | 1.64 |
| N-NH4 content (g kg−1) | 2.57 | 1.48 | 0.98 | 0.46 |
| Effective N (%) | 0.38 | 0.23 | 0.31 | 0.13 |
| Added dose (Mg ha−1) | 54 | 74 | 45 | 133 |
| N added (Kg ha−1) | 270 | 229 | 239 | 279 |
| Effective N added (Kg ha−1) | 205 | 170 | 140 | 173 |
| Yield | TOC | N-NH4 | N-NO3 | ||
|---|---|---|---|---|---|
| Gg ha−1 | Gg ha−1 | Kg ha−1 | Kg ha−1 | ||
| 2020 | Digestate | 16.4 (1) a,b,c | 44 (0.4) d,e,f | 6.7 (2.3) b | 5.7 (1.0) a |
| Digestate + biochar | 16.9 (1) a,b,c | 49 (2) c,g | 7.1 (0.1) b | 50.1 (47) a | |
| Slurry | 14.9 (0.2) a,b,c | 39 (0.8) f | 7.3 (0.7) b | 10.2 (6.3) a | |
| Slurry + biochar | 14.9 (1) a,b,c | 49 (0.6) c,d,g | 7.7 (1.1) b | 3.7 (2.2) a | |
| Mineral | 13.6 (1) c | 39 (1) f | 6.2 (1.8) b | 11.6 (5.7) a | |
| Mineral + biochar | 14.2 (0.8) b,c | 56 (3) a,b | 13.0 (2.0) a | 14.4 (4.2) a | |
| 2021 | Digestate | 16.6 (0.5) a,b,c | 46 (3) d,e,g | 8.6 (0.5) b | 2.9 (1.1) a |
| Digestate + biochar | 18.6 (3) a,b | 50 (2) c,g | 10.2 (0.2) a,b | 29.5 (3.6) a | |
| Slurry | 16.9 (0.2) a,b,c | 46 (0.8) d,e,g | |||
| Slurry + biochar | 15.6 (3) a,b,c | 59 (3) a | |||
| Mineral | 19.2 (1) a | 42 (1) e,f | 10.3 (0.7) a,b | 27.6 (19.4) a | |
| Mineral + biochar | 14.0 (1) c | 54 (1) b,c | 11.2 (1.2) a,b | 21.5 (12.6) a |
| 2020 | 2021 | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAF | 8 | 10 | 13 | 15 | 20 | 22 | 24 | 29 | 34 | 37 | 43 | 45 | 49 | 59 | 62 | 65 | 69 | 4 | 7 | 9 | 11 | 15 | 21 | 24 | 28 | 31 | 35 | 38 | 42 | 46 | 49 | 52 | 56 | 59 |
| DAY | 1/7 | 3/7 | 6/7 | 8/7 | 13/7 | 15/7 | 17/7 | 22/7 | 27/7 | 30/7 | 5/8 | 7/8 | 11/8 | 21/8 | 24/8 | 27/8 | 31/8 | 11/6 | 14/6 | 16/6 | 18/6 | 22/6 | 28/6 | 1/7 | 5/7 | 8/7 | 12/7 | 15/7 | 19/7 | 23/7 | 26/7 | 29/7 | 2/8 | 5/8 |
| CO2 | ||||||||||||||||||||||||||||||||||
| Dig. vs Min. | 24 | 10 | 11 | 14 | 18 | 23 | 21 | 12 | 15 * | 27 * | 9 | 22 | 31 * | 22 | 34 | 31 | 26 * | 55 * | 70 * | 62 * | 71 * | 51 * | 34 * | 41 * | 48 * | 21 * | 34 * | 34 | 35 * | 31 * | 32 * | 31 * | 21 | 38 * |
| Slu. vs Min. | 42 | 35 | 27 * | 43 * | 37 * | 27 | 51 * | 4 | 19 * | 22 | 24 * | 18 | 26 | 11 | 16 | 28 | 39 * | 29 * | 30 * | 20 * | 23 * | 15 | 5 | 6 | 9 | −6 | 11 | 9 | 31 | 30 * | 27 * | 42 * | 20 | 29 * |
| Bioch-Dig | 1 | 9 | 6 | 6 | 11 | 5 | 7 | 5 | 2 | −4 | 14 | −3 | −1 | −17 | −21 | −12 | −6 | −19 * | −24 * | −27 * | −26 * | −22 * | −20 * | −26 * | −27 * | −17 * | −22 * | −13 | −14 | −18 * | −15 * | −11 | −33 * | −34 * |
| Bioch-Slu | 12 | 0 | 3 | 2 | −1 | 26 * | −4 | −8 * | 6 | 3 | 0 | −1 | −4 | −1 | −1 | −12 | −20 * | 17 * | 15 * | 15 * | 13 * | 15 * | 10 | 17 * | 9 | 11 | 0 | 33 * | −10 | −11 | −13 * | −3 | −22 * | −23 * |
| Bioch-Min | −14 | −13 | −7 | −11 | −7 | −5 | 0 | −7 | −13 * | −10 | −3 | −10 | −6 | −13 | −21 | −11 | 23 * | −5 | −3 | −9 | −6 | −10 | −12 | −9 | −5 | −16 | −11 | 21 | −14 | −6 | −6 | −4 | −28 | −10 |
| CH4 | ||||||||||||||||||||||||||||||||||
| Dig. vs Min. | 75 | 0 | 31 | 25 | 6 | 34 * | −15 | −6 | 59 * | 21 | 67 * | 27 | 34 | 28 | 235 * | 115 * | 39 | −920 | −202 | 9 | 19 | 23 | 29 | −12 | −1124 | 40 | 62 | 35 | −230 | −176 | 23 | 1 | −13 | 48 |
| Slu. vs Min. | 35 | −19 | 37 | −3 | −26 | −2 | −28 * | −13 | 31 | 0 | 31 | 12 | 68 * | −2 | 197 * | 30 | 25 | −130 | 20 | 6 | 77 * | −12 | 30 | −6 | −1146 * | 5 | 72 * | 25 | 146 | −10 | −33 | 19 | 7 | 9 |
| Bioch-Dig | 4 | 44 | 171 | 309 | 434 | 573 * | 468 * | 1 | 18 | 15 | −90 * | −84 * | −22 | −13 | −36 | −1 | −13 | 5 | −66 * | −70 * | −75 * | −75 * | −71 * | −66 * | −21 | −52 * | −52 * | −65 | −74 | −57 | −39 | −58 | −16 | −29 |
| Bioch-Slu | 5 | 22 | 98 | 87 | −21 | 18 | −19 | −23 | −13 | −151 | −38 | −42 | −22 | −19 | 31 | −12 | −48 | 41 | 164 * | 151 * | 177 * | 150 | −11 | 69 | 59 | 42 | 11 | −59 * | −35 | −12 | 77 | −16 | 4 | −63 |
| Bioch-Min | −29 | −38 | −53 | −29 | −53 | −44 | −28 | −53 | −44 | −33 | −76 | −73 | −51 | −84 * | −65 * | 21 | −51 | −20 | −7 | −14 | −15 | 77 | −50 | −16 | 10 | −12 | −22 | −41 | −91 * | −64 | −70 | −53 | −63 | −39 |
| N2O | ||||||||||||||||||||||||||||||||||
| Dig. vs Min. | 2701 * | 2004 | 954 | 322 | 123 | 44 | 20 | 15 | −22 | 451 | 193 | 99 | 12 | −68 * | 2 | 15 | −16 | 546 * | 914 * | 796 * | 597 * | 259 | 5 | 33 | −24 | 10 | 37 * | 28 | −70 * | −38 | −54 * | −33 | −54 * | −52 |
| Slu. vs Min. | 326 | 148 | 18 | 6 | 71 | 6 | 41 | 16 | −43 | −71 | −84 | −84 | −56 | −79 * | −56 * | −11 | −27 | 271 | 380 | 391 | 208 | 70 | −36 | −12 | −39 | −53 * | −40 * | 170 * | −67 * | −45 | −63 * | −44 | −59 * | −46 |
| Bioch-Dig | 4 | 44 | 171 | 309 | 434 | 573 * | 468 * | 1 | 18 | 15 | −90 * | −84 * | −22 | −13 | −36 | −1 | −13 | 5 | −66 * | −70 * | −75 * | −75 * | −71 * | −66 * | −21 | −52 * | −52 * | −65 | −74 | −57 | −39 | −58 | −16 | −29 |
| Bioch-Slu | 5 | 22 | 98 | 87 | −21 | 18 | −19 | −23 | −13 | −151 | −38 | −42 | −22 | −19 | 31 | −12 | −48 | 41 | 164 * | 151 * | 177 * | 150 | −11 | 69 | 59 | 42 | 11 | −59 * | −35 | −12 | 77 | −16 | 4 | −63 |
| Bioch-Min | −29 | −38 | −53 | −29 | −53 | −44 | −28 | −53 | −44 | −33 | −76 | −73 | −51 | −84 * | −65 * | 21 | −51 | −20 | −7 | −14 | −15 | 77 | −50 | −16 | 10 | −12 | −22 | −41 | −91 * | −64 | −70 | −53 | −63 | −39 |
| 2020 | 2021 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organic vs. Mineral Fertilizer (%) | Biochar Effect (%) | Organic vs. Mineral Fertilizer (%) | Biochar Effect (%) | |||||||||
| CO2 | CH4 | N2O | CO2 | CH4 | N2O | CO2 | CH4 | N2O | CO2 | CH4 | N2O | |
| Digestate | 17 # | +31 * | +202 # | 0 | +8 | +24 | +42 *** | −8 | +63 # | −21 *** | +58 * | −59 * |
| Slurry | 25 * | +11 | −18 | 0 | +24 ** | −6 | +18 * | +16 # | +11 | +6 ** | −15 | +49 |
| Mineral | − | − | − | −8 * | +7 | −57 ** | - | − | − | −8 # | +10 | −50 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagomarsino, A.; Valagussa, M.; Scotti, C.; Borrelli, L.; Becagli, C.; Tosca, A. Mitigation of GHG Emissions from Soils Fertilized with Livestock Chain Residues. Agronomy 2022, 12, 1593. https://doi.org/10.3390/agronomy12071593
Lagomarsino A, Valagussa M, Scotti C, Borrelli L, Becagli C, Tosca A. Mitigation of GHG Emissions from Soils Fertilized with Livestock Chain Residues. Agronomy. 2022; 12(7):1593. https://doi.org/10.3390/agronomy12071593
Chicago/Turabian StyleLagomarsino, Alessandra, Massimo Valagussa, Carla Scotti, Lamberto Borrelli, Claudia Becagli, and Alberto Tosca. 2022. "Mitigation of GHG Emissions from Soils Fertilized with Livestock Chain Residues" Agronomy 12, no. 7: 1593. https://doi.org/10.3390/agronomy12071593







