Integrated Approaches for Adsorption and Incorporation Testing of Green-Synthesized TiO2NPs Mediated by Seed-Priming Technology in Punica granatum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Preparation of Plant Extracts
2.3. Green Synthesis of TiO2 Nanoparticles
2.4. Characterization of TiO2 Nanoparticles
2.5. Preparations of Nanopriming Solutions and Seed Priming
2.6. Assaying TiO2NPs Adsorption on a Seed Surface by SEM Analysis
2.7. Energy Dispersive X-ray (EDX)
2.8. Assaying TiO2NPs Internalization by Transmission Electron Microscopy (TEM)
2.9. Inductively Coupled Plasma Spectrometer (ICP–OES) Analysis of NPs Uptake
2.10. Energy Dispersive X-ray Fluorescence (EDXRF) Analysis of NPs Uptake
3. Results
3.1. Titanium Dioxide Nanoparticles (TiO2NPs) Characterization
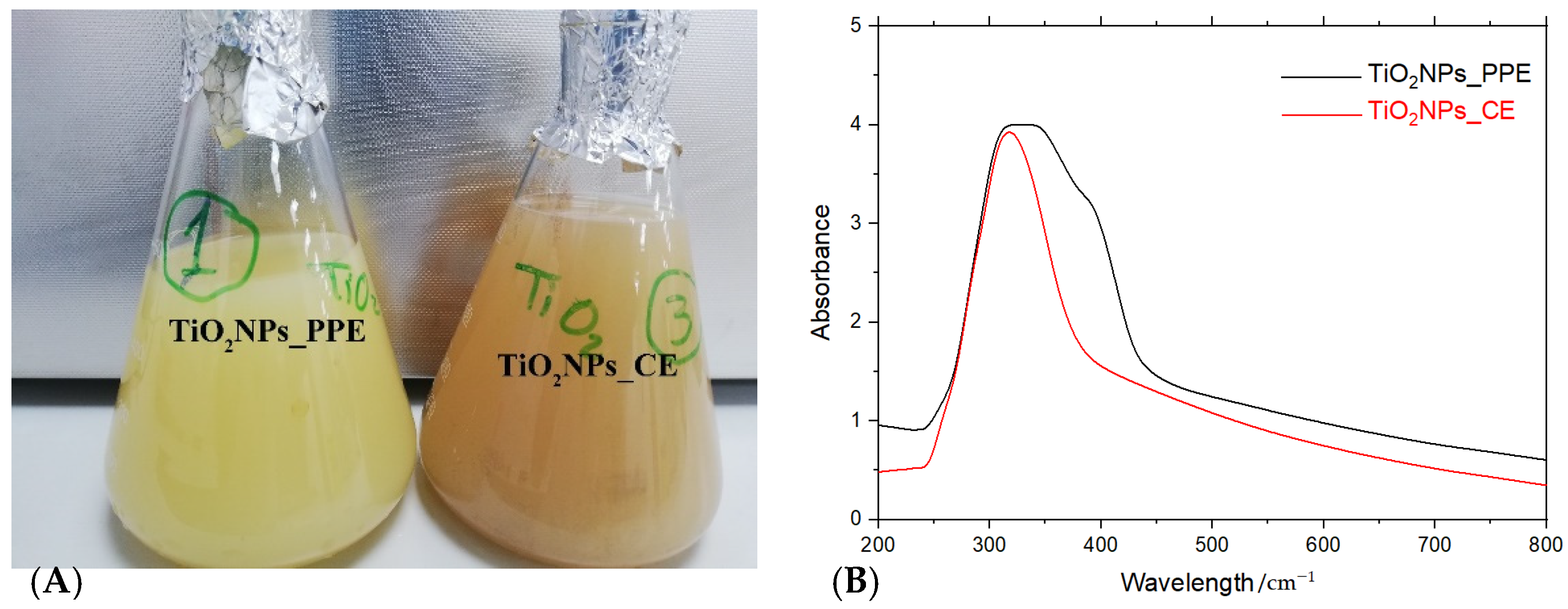
3.1.1. UV–Vis Spectroscopy
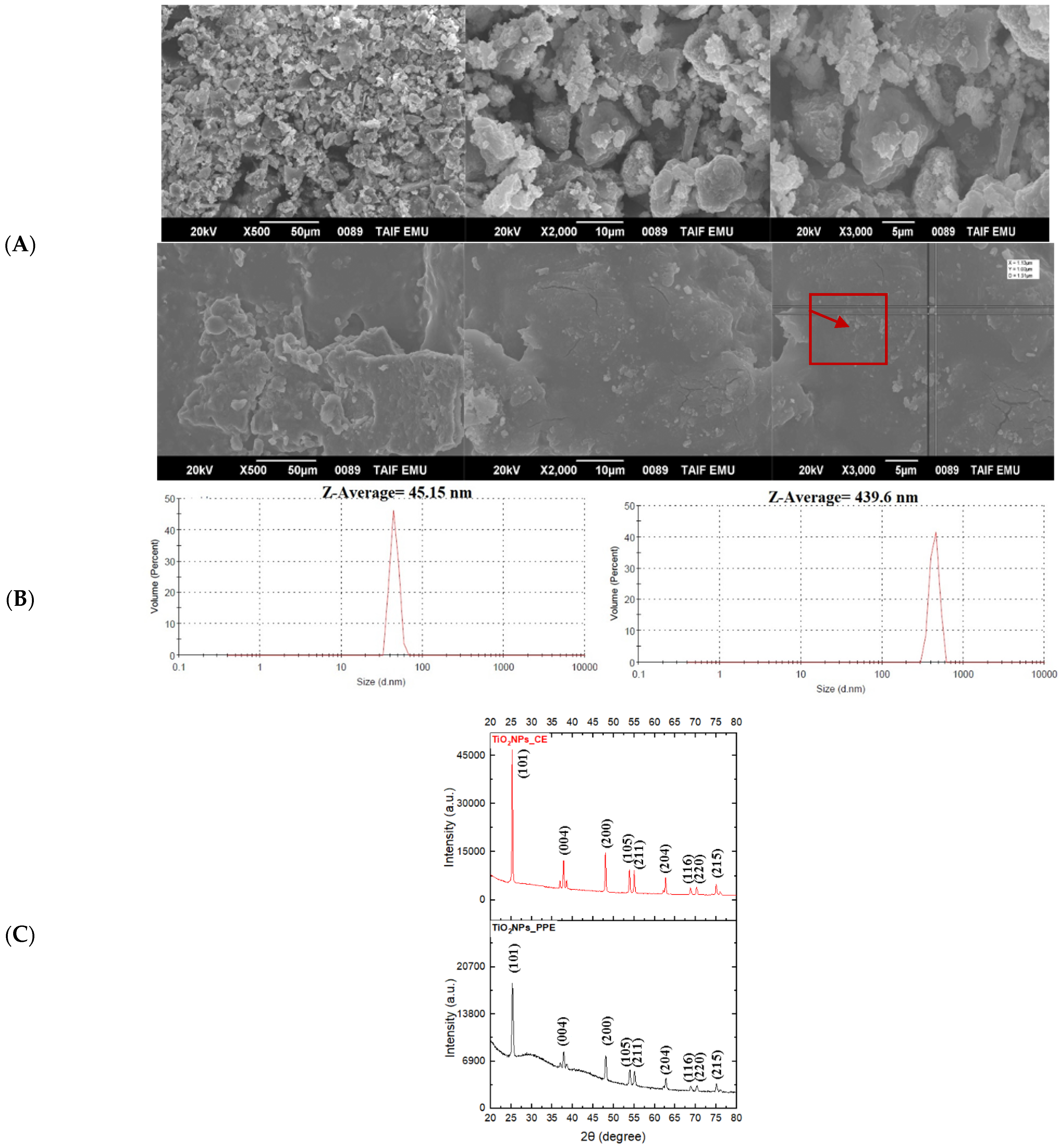
3.1.2. Scanning Electron Microscopy (SEM)
3.1.3. Size Distribution and Zeta Potential
3.1.4. X-ray Diffraction
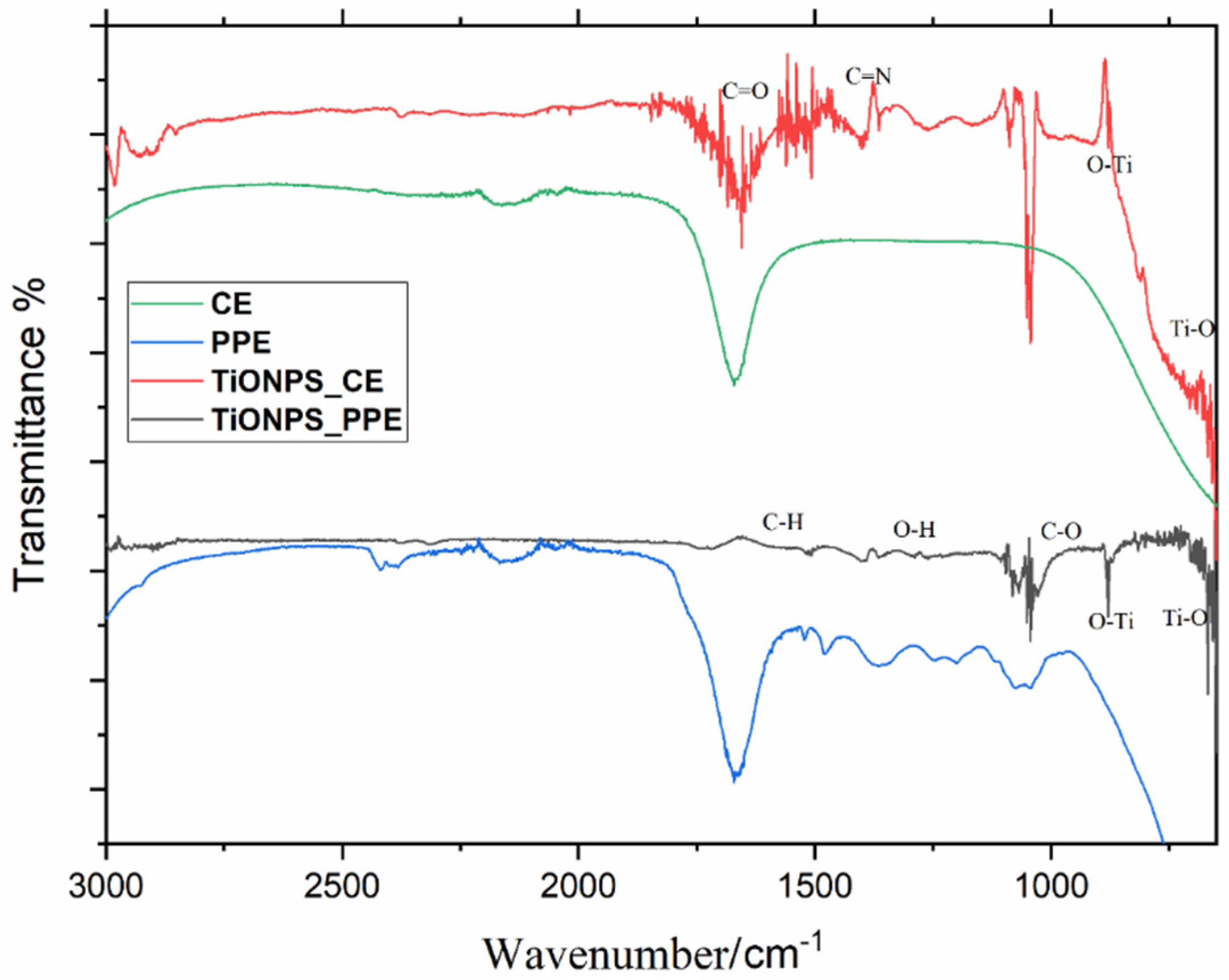
3.1.5. Fourier Transform Infrared Spectroscopy (FTIR)
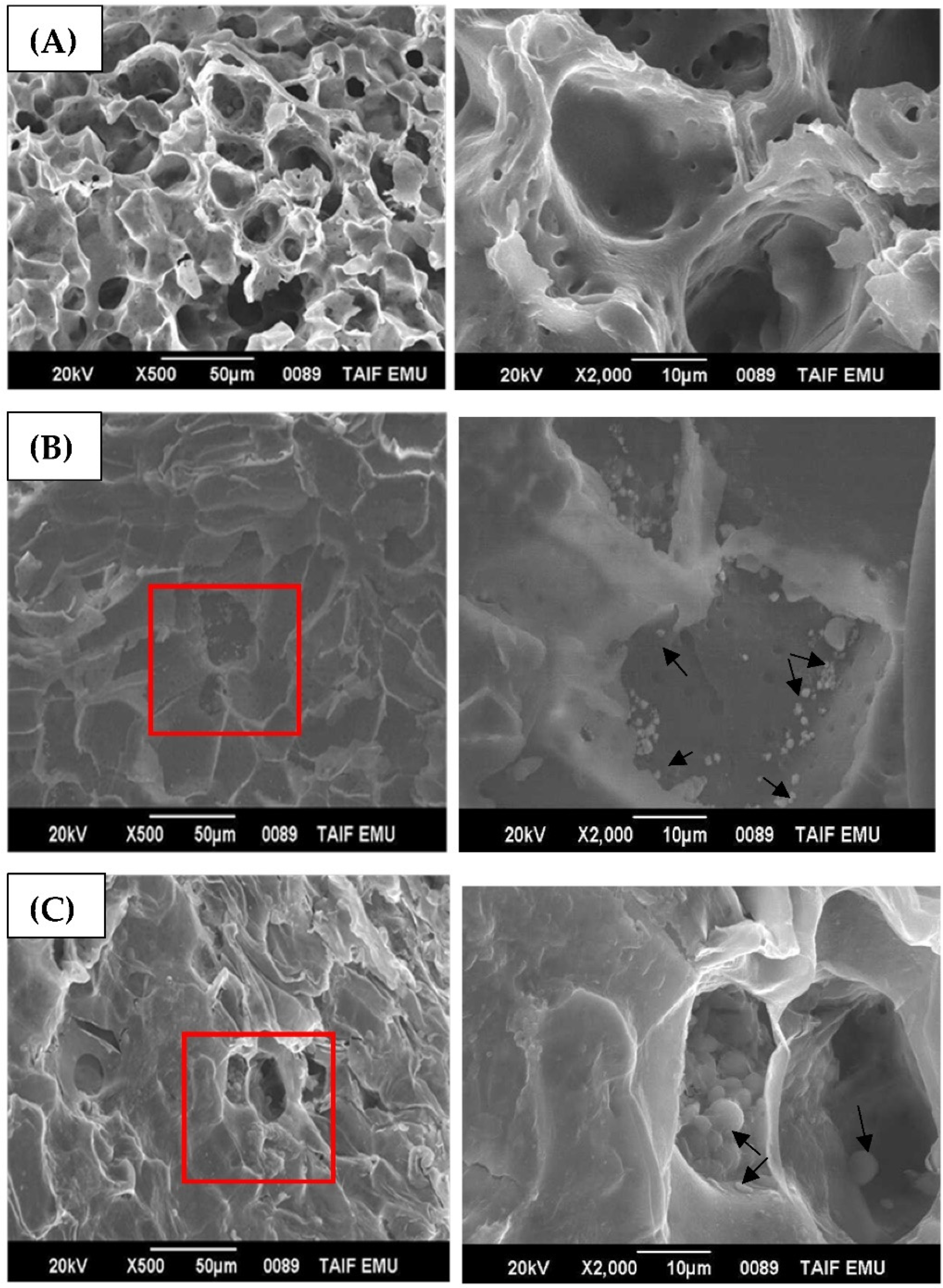
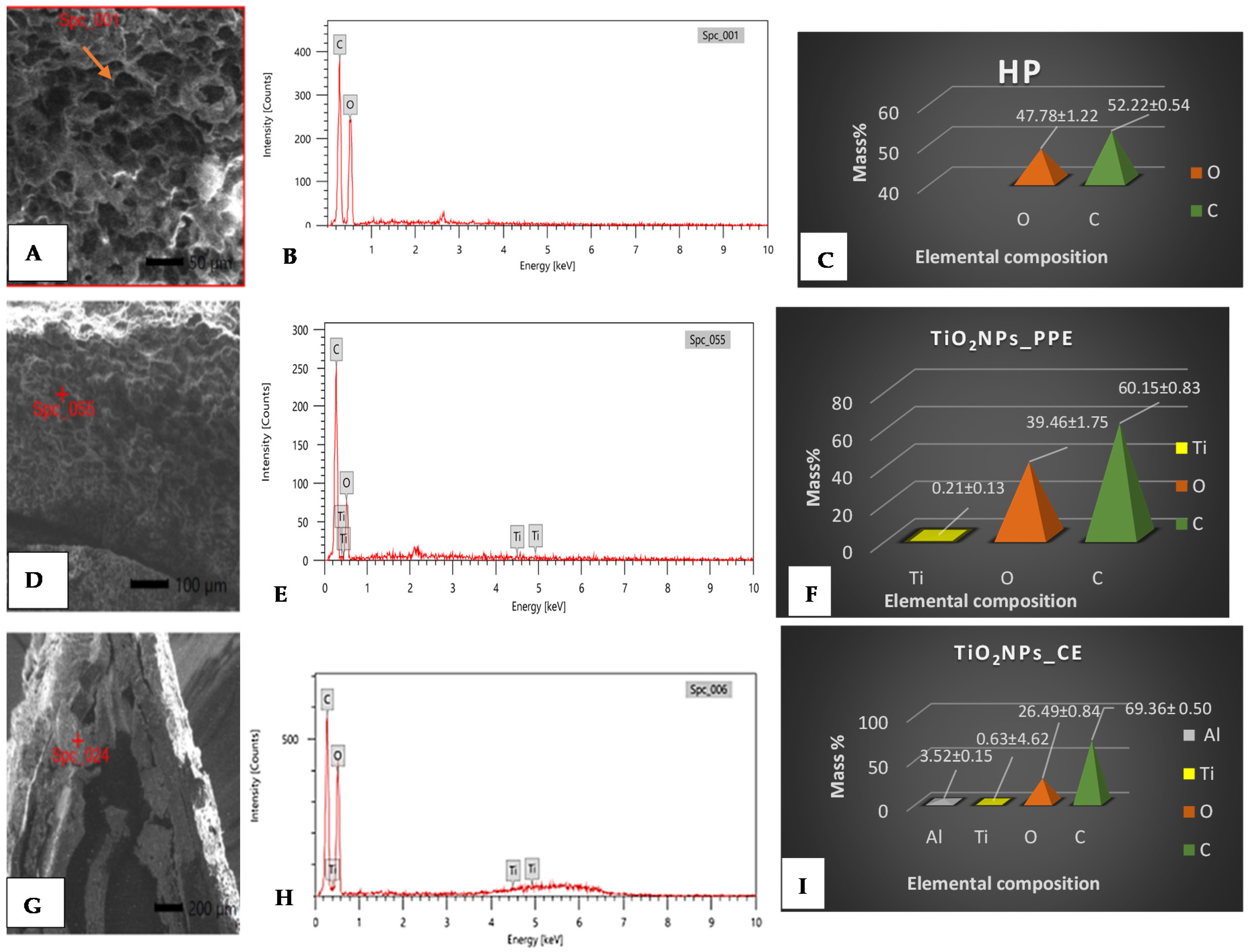
3.2. TiO2NP Adsorption and Localization on Seed Coats
SEM/EDX Analysis
3.3. TiO2NP Uptake in Seeds
3.3.1. TEM Analysis
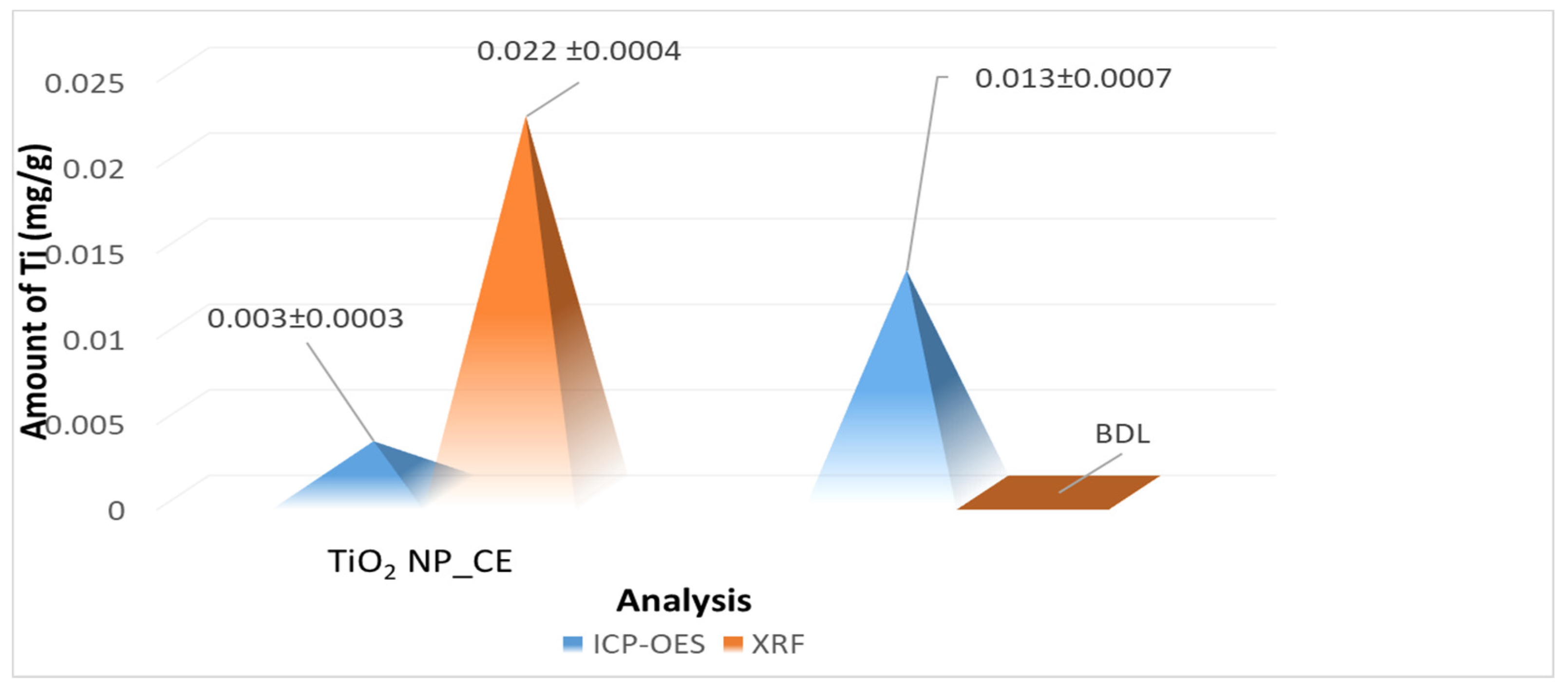
3.3.2. X-ray Fluorescence (XRF) and ICP–OES Analyses
4. Discussion
5. Conclusions
- TiO2 nanoparticles from aqueous extracts of pomegranate fruit peel (PPE) and coffee (CE) were successfully synthesized using the green synthesis method in pure anatase form.
- The evaluation of the uptake and internalization by seeds via different analytical techniques showed that nanopriming with TiO2NPs had positive efficacy on the uptake and incorporation by pomegranate (P. granatum L.) seeds.
- The EM analysis offered definitive determination of the NPs in the plant tissues and information about the distribution of the NPs within the tissues. The total elemental analysis using EDX, XRF, and ICP–OES also provided complementary information to the SEM/TEM, reflecting the significance of an integrative approach for verifying the incorporation of nanoparticles inside seed tissues.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multilocations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef] [Green Version]
- Sarikhani, H.; Valipour, M.; Chehregani, A. Fruit growth and patterns of lignification in the seeds of four Iranian pomegranate (Punica granatum L.) cultivars. J. Hortic. Sci. Biotechnol. 2014, 89, 268–272. [Google Scholar] [CrossRef]
- Phat, P.; Sheikh, S.; Lim, J.H.; Kim, T.B.; Seong, M.H.; Chon, H.G.; Shin, Y.K.; Song, Y.J.; Noh, J. Enhancement of Seed Germination and Uniformity in Triploid Watermelon (Citrullus lanatus (Thunb.) Matsum. and Nakai). Korean J. Hortic. Sci. Technol. 2015, 33, 932–940. [Google Scholar] [CrossRef] [Green Version]
- El-Sanatawy, A.M.; Ash-Shormillesy, S.M.A.I.; Qabil, N.; Awad, M.F.; Mansour, E. Seed Halo-Priming Improves Seedling Vigor, Grain Yield, and Water Use Efficiency of Maize under Varying Irrigation Regimes. Water 2021, 13, 2115. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Khan, H.; Munsif, F.; Nie, L. Ascorbic Acid Priming Enhances Seed Germination and Seedling Growth of Winter Wheat under Low Temperature Due to Late Sowing in Pakistan. Agronomy 2019, 9, 757. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; He, X. How to improve seed germination with green nanopriming. Seed Sci. Technol. 2021, 49, 81–92. [Google Scholar] [CrossRef]
- Khalaki, M.A.; Moameri, M.; Lajayer, B.A.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021, 93, 13–28. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [Green Version]
- Spielman-Sun, E.; Avellan, A.; Bland, G.D.; Tappero, R.V.; Acerbo, A.S.; Unrine, J.M.; Giraldo, J.P.; Lowry, G.V. Nanoparticle surface charge influences translocation and leaf distribution in vascular plants with contrasting anatomy. Environ. Sci. Nano 2019, 6, 2508–2519. [Google Scholar] [CrossRef]
- An, J.; Hu, P.; Li, F.; Wu, H.; Shen, Y.; White, J.C.; Tian, X.; Li, Z.; Giraldo, J.P. Emerging investigator series: Molecular mechanisms of plant sa-linity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 2020, 7, 2214–2228. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S.I.; Kalaichelvan, P.T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crop. Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Khan, S.T.; Al-Warthan, A.; Siddiqui, M.R.H.; Tahir, M.N.; Tremel, W. Plant extracts as green reductants for the synthesis of silver nanoparticles: Lessons from chemical synthesis. Dalton Trans. 2018, 47, 11988–12010. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Al Sufyani, N.M. Comparative Analysis of Nanosilver Particles Synthesized by Different Approaches and Their Antimicrobial Efficacy. J. Nanomater. 2021, 2021, 2204776. [Google Scholar] [CrossRef]
- Deng, Y.; Petersen, E.J.; Challis, K.E.; Rabb, S.A.; Holbrook, R.D.; Ranville, J.F.; Nelson, B.C.; Xing, B. Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants. Environ. Sci. Technol. 2017, 51, 10615–10623. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Ernest, V.; Mukherjee, A.; Chandrasekaran, N. In Vivo Nanotoxicity Assays in Plant Models. Methods Mol. Biol. 2012, 926, 399–410. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Nicolás-Álvarez, D.E.; Andraca-Adame, J.A.; Chanona-Pérez, J.J.; Méndez-Méndez, J.V.; Borja-Urby, R.; Cayetano-Castro, N.; Martínez-Gutiérrez, H.; López-Salazar, P. Effects of TiO2 Nanoparticles Incorporation into Cells of Tomato Roots. Nanomaterials 2021, 11, 1127. [Google Scholar] [CrossRef]
- Swathi, N.; Sandhiya, D.; Rajeshkumar, S.; Lakshmi, T. Green synthesis of titanium dioxide nanoparticles using Cassia fistula and its antibacterial activity. Int. J. Res. Pharm. Sci. 2019, 10, 856–860. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano Metal Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Tezotto, T.; Favarin, J.L.; Neto, A.P.; Gratão, P.L.; Azevedo, R.A.; Mazzafera, P. Simple procedure for nutrient analysis of coffee plant with energy dispersive X-ray fluorescence spectrometry (EDXRF). Sci. Agric. 2013, 70, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Baghaienezhad, M.; Boroghani, M.; Anabestani, R. Silver nanoparticles Synthesis by coffee residues extract and their antibacterial activity. Nanomed. Res. J. 2020, 5, 29–34. [Google Scholar]
- Mathew, S.S.; Sunny, N.E.; Shanmugam, V. Green synthesis of anatase titanium dioxide nanoparticles using Cuminum cyminum seed extract; effect on Mung bean (Vigna radiata) seed germination. Inorg. Chem. Commun. 2021, 126, 108485. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Bekele, E.T.; Gonfa, B.A.; Sabir, F.K. Use of Different Natural Products to Control Growth of Titanium Oxide Nanoparticles in Green Solvent Emulsion, Characterization, and Their Photocatalytic Application. Bioinorg. Chem. Appl. 2021, 2021, 6626313. [Google Scholar] [CrossRef]
- Waleczek, M.; Dendooven, J.; Dyachenko, P.; Petrov, A.Y.; Eich, M.; Blick, R.H.; Detavernier, C.; Nielsch, K.; Furlan, K.P.; Zierold, R. Influence of Alumina Addition on the Optical Properties and the Thermal Stability of Titania Thin Films and Inverse Opals Produced by Atomic Layer Deposition. Nanomaterials 2021, 11, 1053. [Google Scholar] [CrossRef]
- Abu-Dalo, M.; Jaradat, A.; Albiss, B.A.; Al-Rawashdeh, N.A.F. Green synthesis of TiO2 NPs/pristine pomegranate peel extract nanocomposite and its antimicrobial activity for water disinfection. J. Environ. Chem. Eng. 2019, 7, 103370. [Google Scholar] [CrossRef]
- Al Qarni, F.; Alomair, N.A.; Mohamed, H.H. Environment-Friendly Nanoporous Titanium Dioxide with Enhanced Photocatalytic Activity. Catalysts 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, C.A.; Silambarasan, D.; Sarika, R.; Selvam, V. Synthesis and characterization of TiO2. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Sutradhar, P.; Saha, M.; Maiti, D. Microwave synthesis of copper oxide nanoparticles using tea leaf and coffee powder extracts and its antibacterial activity. J. Nanostruct. Chem. 2014, 4, 86. [Google Scholar] [CrossRef] [Green Version]
- Aisida, S.O.; Madubuonu, N.; Alnasir, M.H.; Ahmad, I.; Botha, S.; Maaza, M.; Ezema, F.I. Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. Appl. Nanosci. 2020, 10, 305–315. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Bagheri, S.; Shameli, K.; Abd Hamid, S.B. Synthesis and Characterization of Anatase Titanium Dioxide Nanoparticles Using Egg White Solution via Sol-Gel Method. J. Chem. 2013, 2013, 848205. [Google Scholar] [CrossRef]
- Mayedwa, N.; Mongwaketsi, N.; Khamlich, S.; Kaviyarasu, K.; Matinise, N.; Maaza, M. Green synthesis of nickel oxide, palladium and palladium oxide synthesized via Aspalathus linearis natural extracts: Physical properties & mechanism of formation. Appl. Surf. Sci. 2018, 446, 266–272. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Ambika, S.; Sangili, A.; Nithya, P.; Sumathi, R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol. 2017, 171, 117–124. [Google Scholar]
- Srivastava, S.K.; Singh, V.B. Ab initio and DFT studies of the structure and vibrational spectra of anhydrous caffeine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 45–50. [Google Scholar] [CrossRef]
- Bello, O.S.; Adegoke, K.A.; Akinyunni, O.O. Preparation and characterization of a novel adsorbent from Moringa oleifera leaf. Appl. Water Sci. 2017, 7, 1295–1305. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.J.; Wu, S.G.; Huang, L.; Head, J.; Chen, D.R.; Kong, I.C. Phytotoxicity of Metal Oxide Nanoparticles is Related to Both Dissolved Metals Ions and Adsorption of Particles on Seed Surfaces. J. Pet. Environ. Biotechnol. 2012, 3, 126. [Google Scholar] [CrossRef] [Green Version]
- Kurczyńska, E.; Godel-Jędrychowska, K.; Sala, K.; Milewska-Hendel, A. Nanoparticles—Plant Interaction: What We Know, Where We Are? Appl. Sci. 2021, 11, 5473. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Z.; Sun, D.W. Bioinspired Nanomodification Strategies: Moving from Chemical-Based Agrosystems to Sustainable Agriculture. ACS Nano 2021, 15, 12655–12686. [Google Scholar] [CrossRef]
- Xiong, C.; Zheng, Y.; Feng, Y.; Yao, C.; Ma, C.; Zheng, X.; Jiangd, J. Preparation of a novel chloromethylated polystyrene-2-amino- 1,3,4-thiadiazole chelating resin and its adsorption properties and mechanism for separation and recovery of Pt (IV) from aqueous solutions. J. Mater. Chem. A 2014, 2, 5379–5386. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. Int. 2019, 26, 19859–19870. [Google Scholar] [CrossRef]
- Segatto, C.; Ternus, R.; Junges, M.; de Mello, J.M.M.; da Luz, G.L.; Riella, H.G.; Silva, L.L.; Lajús, C.R.; Fiori, M.A. Adsorption and incorporation of the zinc oxide nanoparticles in seeds of corn: Germination performance and antimicrobial protection. Int. J. Adv. Eng. Res. Sci. 2018, 5, 2456–6495. [Google Scholar] [CrossRef] [Green Version]
- Kong, I.C.; Ko, K.-S.; Koh, D.-C. Comparisons of the Effect of Different Metal Oxide Nanoparticles on the Root and Shoot Growth under Shaking and Non-Shaking Incubation, Different Plants, and Binary Mixture Conditions. Nanomaterials 2021, 11, 1653. [Google Scholar] [CrossRef]
- Zhao, L.J.; Peralta-Videa, J.R.; Ren, M.; Varela-Ramirez, A.; Li, C.; Hernandez-Viezcas, J.A.; Aguilera, R.J.; Gardea-Torresdey, J.L. Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: Electron microprobe and confocal microscopy studies. Chem. Eng. J. 2012, 184, 1–8. [Google Scholar] [CrossRef]
- Deng, Y.Q.; White, J.C.; Xing, B.S. Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. Sci. 2014, 15, 552–572. [Google Scholar] [CrossRef] [Green Version]
- Corral-Bobadilla, M.; Lostado-Lorza, R.; Somovilla-Gómez, F.; Escribano-García, R. Effective use of activated carbon from olive stone waste in the biosorption removal of Fe(III) ions from aqueous solutions. Clean. Prod. 2021, 294, 126332. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Morsy, H. Biochar of Spent Coffee Grounds as Per Se and Impregnated with TiO2: Promising Waste-Derived Adsorbents for Balofloxacin. Molecules 2021, 26, 2295. [Google Scholar] [CrossRef] [PubMed]
- Rosson, E.; Garbo, F.; Marangoni, G.; Bertani, R.; Lavagnolo, M.C.; Moretti, E.; Talon, A.; Mozzon, M.; Sgarbossa, P. Activated Carbon from Spent Coffee Grounds: A Good Competitor of Commercial Carbons for Water Decontamination. Appl. Sci. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Arenas, L.; Ortega, M.; García-Martínez, M.J.; Querol, E.; Llamas, J.F. Geochemical characterization of the mining district of Linares (Jaen, Spain) by means of XRF and ICP-AES. J. Geochem. Explor. 2011, 108, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, K.; Samoraj, M.; Tuhy, Ł.; Michalak, I.; Mironiuk, M.; Mikulewicz, M. Using XRF and ICP-OES in Biosorption Studies. Molecules 2018, 23, 2076. [Google Scholar] [CrossRef] [Green Version]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Alsahli, A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Korkmaz, A.D.; Baykal, A.; Almessiere, M.; Ercan, I. Impact of superparamagnetic iron oxide nanoparticles (SPIONs) and ionic iron on physiology of summer squash (Cucurbita pepo): A comparative study. Plant Physiol. Biochem. 2019, 139, 56–65. [Google Scholar] [CrossRef]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Wirick, S.; Bertsch, P.M. Bioavailability of Gold Nanomaterials to Plants: Importance of Particle Size and Surface Coating. Environ. Sci. Technol. 2012, 46, 8467–8474. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Ali, G.A.M.; Hassanein, A.; Attia, A.M.; Marzouk, E.R. Toxicity and Uptake of CuO Nanoparticles: Evaluation of an Emerging Nanofertilizer on Wheat (Triticum aestivum L.) Plant. Sustainability 2022, 14, 4914. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmigid, H.M.; Alyamani, A.A.; Hussien, N.A.; Morsi, M.M.; Alhumaidi, A. Integrated Approaches for Adsorption and Incorporation Testing of Green-Synthesized TiO2NPs Mediated by Seed-Priming Technology in Punica granatum L. Agronomy 2022, 12, 1601. https://doi.org/10.3390/agronomy12071601
Abdelmigid HM, Alyamani AA, Hussien NA, Morsi MM, Alhumaidi A. Integrated Approaches for Adsorption and Incorporation Testing of Green-Synthesized TiO2NPs Mediated by Seed-Priming Technology in Punica granatum L. Agronomy. 2022; 12(7):1601. https://doi.org/10.3390/agronomy12071601
Chicago/Turabian StyleAbdelmigid, Hala M., Amal Ahmed Alyamani, Nahed Ahmed Hussien, Maissa M. Morsi, and Afnan Alhumaidi. 2022. "Integrated Approaches for Adsorption and Incorporation Testing of Green-Synthesized TiO2NPs Mediated by Seed-Priming Technology in Punica granatum L." Agronomy 12, no. 7: 1601. https://doi.org/10.3390/agronomy12071601
APA StyleAbdelmigid, H. M., Alyamani, A. A., Hussien, N. A., Morsi, M. M., & Alhumaidi, A. (2022). Integrated Approaches for Adsorption and Incorporation Testing of Green-Synthesized TiO2NPs Mediated by Seed-Priming Technology in Punica granatum L. Agronomy, 12(7), 1601. https://doi.org/10.3390/agronomy12071601





