Coordinating High Yield and Superior Eating Quality of Rice: A Case Study of Hybrid Varieties Derived from Longke638S and Jing4155S
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Crop Management
2.3. Plant Sampling and Measurement
2.4. Determination of Grain Quality
2.4.1. Milling and Appearance Grain Quality
2.4.2. Determination of Amylose Content
2.4.3. Determination of Gel Consistency
2.4.4. Determination of Gelatinization Temperature
2.5. RNA Sequencing
2.6. Statistical Analysis
3. Results
3.1. Grain Yield, Grain Quality and Nitrogen Use Efficiency of Hybrid Varieties
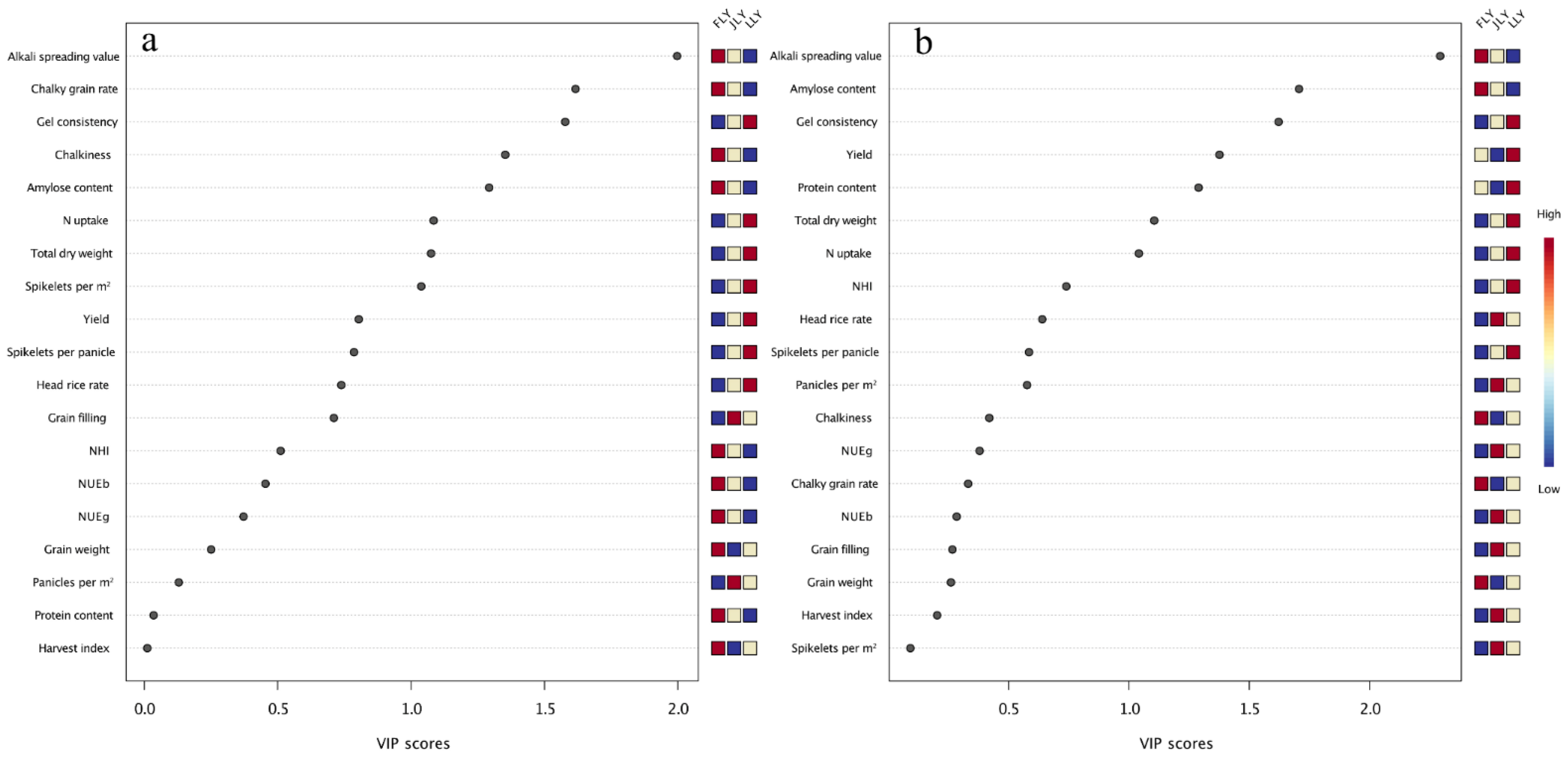
3.2. Correlation between Yield and Quality-Related Traits for Hybrid Varieties
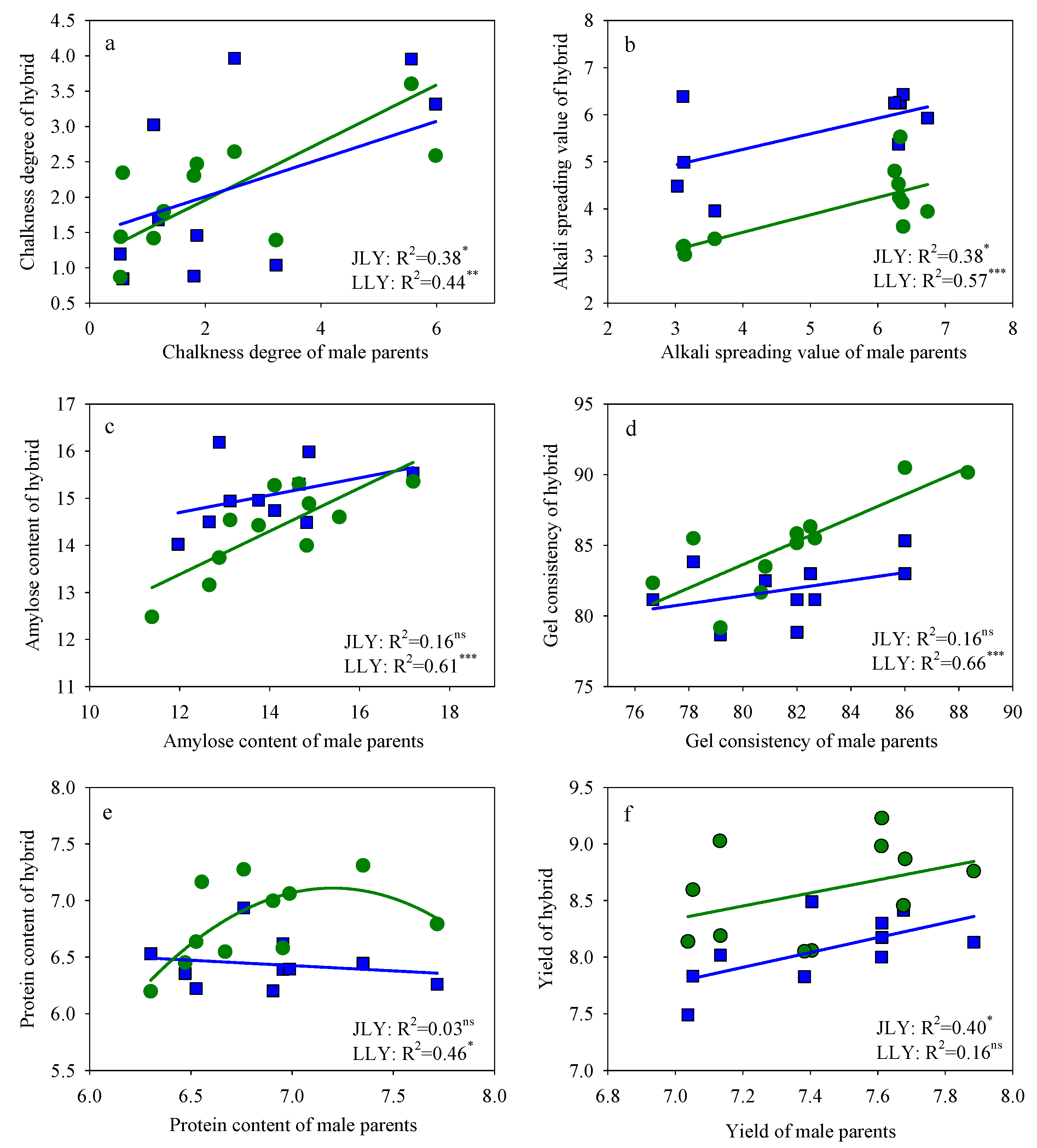
3.3. Grain Yield, Grain Quality, and Nitrogen Use Efficiency of the Male Parents
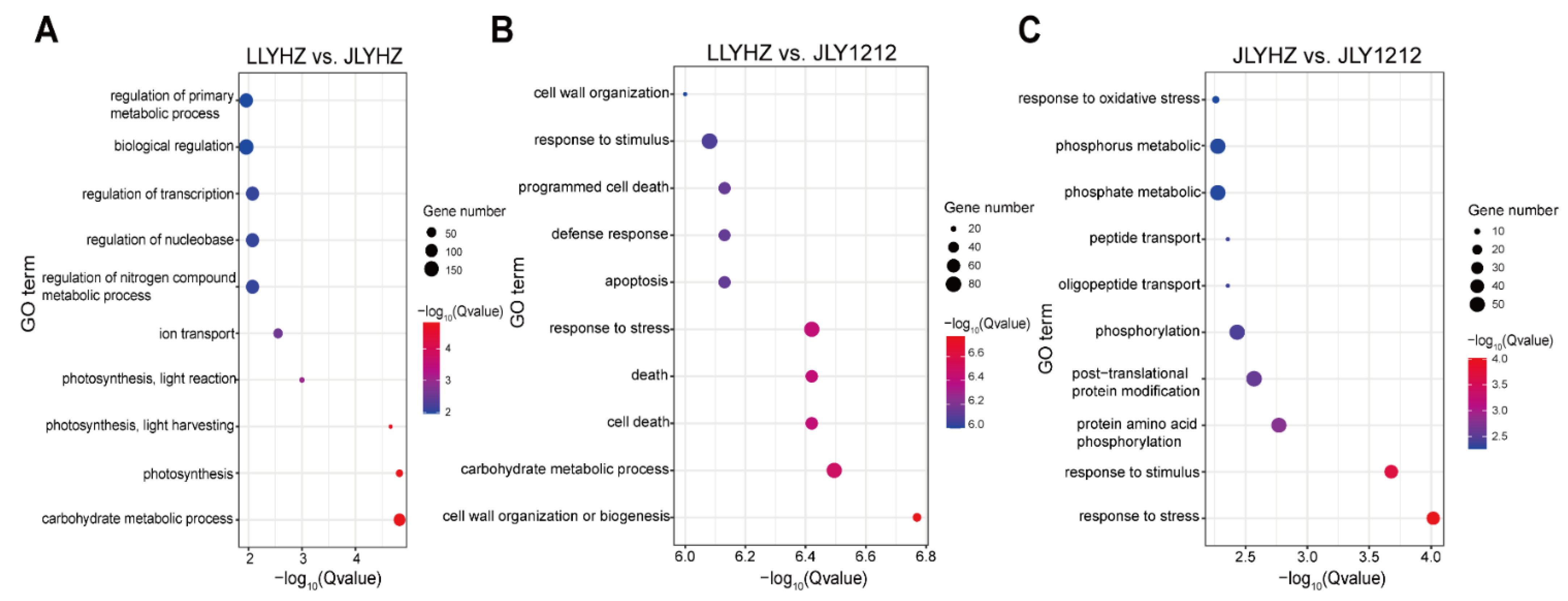
3.4. Transcriptomic Analysis of LLYHZ, JLYHZ and JLY1212
4. Discussion
4.1. Physiological and Molecular Traits Limiting the Grain Yield of Hybrid Varieties Derived from Jing4155S
4.2. Physiological and Molecular Traits for Improving Grain Quality of Hybrid Varieties Derived from Longke638S
4.3. Coordination of High Yield and Superior Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L. Strategy of hybrid rice breeding. Hybrid Rice 1987, 1, 1–3. [Google Scholar]
- Peng, S.B.; Cassman, K.G.; Virmani, S.S.; Sheehy, J.; Khush, G.S. Yield potential trends of tropical rice since the release of IR8 and the challenge of increasing rice yield potential. Crop Sci. 1999, 39, 1552–1559. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Peng, S.B. Yield potential and nitrogen use efficiency of China’s super rice. J. Integr. Agric. 2017, 16, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.B. Dilemma and way-out of hybrid rice during the transition period in China. Acta Agron. Sin. 2016, 42, 313–319. [Google Scholar] [CrossRef]
- Zhang, Q.F. Strategies for developing green super rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G.; et al. Rational design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, D.; He, Y. Rice grain quality-traditional traits for high quality rice and health-plus substances. Mol. Breed. 2019, 40, 1. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.; Wang, Y.; Li, H.; Gao, K. Preliminary study on quality traits of indica hybrid rice. Southest Chnia J. Agric. Sci. 2002, 15, 28–31. [Google Scholar]
- Liao, F.; Zhou, K.; Yang, H.; Xu, Q. Comparison of grain quality between F1 hybrids and their parents in indica hybrid rice. Chin. J. Rice Sci. 2003, 17, 134–140. [Google Scholar]
- Lin, H.; Wang, Z.; Li, H.; Pang, Q. Analysis on characteristics of rice varieties registered in China in 2019. Chnia Rice 2020, 26, 16–22. [Google Scholar]
- Zhu, G.; Peng, S.; Huang, J.; Cui, K.; Nie, L.; Wang, F. Genetic improvements in rice yield and concomitant increases in radiation- and nitrogen-use efficiency in middle reaches of Yangtze River. Sci. Rep. 2016, 6, 21049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Sun, F.; Yuan, S.; Peng, S.; Wang, F. Different mechanisms underlying the yield advantage of ordinary hybrid and super hybrid rice over inbred rice under low and moderate N input conditions. Field Crops Res. 2018, 216, 150–157. [Google Scholar] [CrossRef]
- Wei, H.Y.; Zhang, H.C.; Blumwald, E.; Li, H.L.; Cheng, J.Q.; Dai, Q.G.; Huo, Z.Y.; Xu, M.; Guo, B.W. Different characteristics of high yield formation between inbred japonica super rice and inter-sub-specific hybrid super rice. Field Crops Res. 2016, 198, 179–187. [Google Scholar] [CrossRef]
- Nakamura, Y. Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: Rice endosperm as a model tissue. Plant Cell Physiol. 2002, 43, 718–725. [Google Scholar] [CrossRef]
- Ohdan, T.; Francisco, P.B.; Sawada, T.; Hirose, T.; Terao, T.; Satoh, H.; Nakamura, Y. Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J. Exp. Bot. 2005, 56, 3229–3244. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Qian, Q.; Liu, Q.; Yan, M.; Liu, X.; Yan, C.; Liu, G.; Gao, Z.; Tang, S.; Zeng, D.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasulu, N.; Zhang, C.; Tiozon, R.N.; Liu, Q. Post-genomics revolution in the design of premium quality rice in a high-yielding background to meet consumer demands in the 21st century. Plant Commun. 2022, 3, 100271. [Google Scholar] [CrossRef]
- Kashiwagi, T. Effects of rice grain protein QTL, TGP12, on grain composition, yield components, and eating quality with different nitrogen applications. Field Crops Res. 2021, 263, 108051. [Google Scholar] [CrossRef]
- Fu, C.; Hu, X.; Fu, X.; Qin, P.; Wang, K.; Li, C.; Wu, T.; Liu, S.; Yang, Y. Breeding and application of TGMS line Longke 638S with blast-resistance, good-quality and high combining-ability in middle-season indica rice. Chnia Rice 2021, 27, 61–66. [Google Scholar]
- Fu, C.; Hu, X.; Qin, P.; Fu, X.; Sun, Z.; Yang, G.; Wang, K.; Yang, Y. Breeding and application of medium indica TGMS line Jing 4155S with blast resistance, good grain quality and high combining ability in rice. Chnia Rice 2021, 36, 18–24. [Google Scholar]
- Yu, S.B.; Ali, J.; Zhou, S.C.; Ren, G.J.; Xie, H.A.; Xu, J.L.; Yu, X.Q.; Zhou, F.S.; Peng, S.B.; Ma, L.Y.; et al. From Green Super Rice to green agriculture: Reaping the promise of functional genomics research. Mol. Plant 2022, 15, 9–26. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.; Wang, S.; Wang, Y.; Chen, X.; Zhang, Y.; Gao, C.; et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 2015, 47, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; He, Y.; Yin, X.; Zhong, X.; Yan, B.; Wu, Y.; Chen, J.; Li, X.; Zhai, K.; Huang, Y.; et al. Ca(2+) sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 2021, 184, 5391–5404. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Pan, C.; Li, Y.; Wu, Y.; Cai, Y.; Lu, Y.; Wang, R.; Yu, L.; Shi, W.; Kang, H.; et al. Genomic insight into balancing high yield, good quality, and blast resistance of japonica rice. Genome Biol. 2021, 22, 283. [Google Scholar] [CrossRef]
- Wu, L.L.; Yuan, S.; Huang, L.Y.; Sun, F.; Zhu, G.L.; Li, G.H.; Fahad, S.; Peng, S.B.; Wang, F. Physiological mechanisms underlying the high-grain yield and high-nitrogen use efficiency of elite rice varieties under a low rate of nitrogen application in China. Front. Plant Sci. 2016, 7, 1024. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Dai, W.; Yang, J.; Tang, S.; Huang, Y.; Ma, J.; Wang, Z.; He, C.; Zhang, L. Popularization and application innovation of new rice varieties taking Jingliangyou 1212 as a case. China Rice 2020, 26, 85–87. [Google Scholar]
- Li, X.X.; Huang, L.Y.; Peng, S.B.; Wang, F. Inter-annual climate variability constrains rice genetic improvement in China. Food Energy Secur. 2021, 11, e299. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.Z.; Zhou, S.G.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mevik, B.H.; Wehrens, R. The pls package: Principal component and partial least squares regression in R. J. Stat. Softw. 2007, 18, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M. Contributions from Jed Wing and Steve Weston and Andre Williams. Caret Classif. Regres. Train. 2008, 3, 40–45. [Google Scholar]
- Huang, L.; Yang, D.; Li, X.; Peng, S.; Wang, F. Coordination of high grain yield and high nitrogen use efficiency through large sink size and high post-heading source capacity in rice. Field Crops Res. 2019, 233, 49–58. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xue, H.W. OsLEC1/OsHAP3E participates in the determination of meristem identity in both vegetative and reproductive developments of rice. J. Integr. Plant Biol. 2013, 55, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Lee, B.; Kim, C.; Kim, S.J.; Yim, J.; Han, J.J.; Lee, S.; Kim, S.R.; An, G. The OsFOR1 gene encodes a polygalacturonase-inhibiting protein (PGIP) that regulates floral organ number in rice. Plant Mol. Biol. 2003, 53, 357–369. [Google Scholar] [CrossRef]
- Tanaka, W.; Toriba, T.; Ohmori, Y.; Yoshida, A.; Kawai, A.; Mayama-Tsuchida, T.; Ichikawa, H.; Mitsuda, N.; Ohme-Takagi, M.; Hirano, H.Y. The YABBY gene TONGARI-BOUSHI1 is involved in lateral organ development and maintenance of meristem organization in the rice spikelet. Plant Cell 2012, 24, 80–95. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Wang, X.; Zhang, L.; Yang, Z.; Xin, X.; Wu, S.; Sun, C.; Liu, J.; Yang, J.; Luo, X. Identification and characterization of OsEBS, a gene involved in enhanced plant biomass and spikelet number in rice. Plant Biotechnol. J. 2013, 11, 1044–1057. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, Y.; Charnikhova, T.; Mulder, P.P.; Heijmans, J.; Hoogenboom, A.; Agalou, A.; Michel, C.; Morel, J.B.; Dreni, L.; et al. OsJAR1 is required for JA-regulated floret opening and anther dehiscence in rice. Plant Mol. Biol. 2014, 86, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, R.M.; Dong, S.; Tang, H.; Ahmad, F.; Zhang, H. Functional analysis of OsHSBP1 and OsHSBP2 revealed their involvement in the heat shock response in rice (Oryza sativa L.). J. Exp. Bot. 2012, 63, 6003–6016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Zeng, D.; Cheng, F.; Tian, Z.; Guo, L.; Su, Y.; Yan, M.; Jiang, H.; Dong, G.; Huang, Y.; et al. ALK, the key gene for gelatinization temperature, is a modifier gene for gel consistency in rice. J. Integr. Plant Biol. 2011, 53, 756–765. [Google Scholar]
- Zhang, L.; Zhao, L.; Lin, L.; Zhao, L.; Liu, Q.; Wei, C. A Novel Mutation of OsPPDKB, Encoding Pyruvate Orthophosphate Dikinase, Affects Metabolism and Structure of Starch in the Rice Endosperm. Int. J. Mol. Sci. 2018, 19, 2268. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Ono, M.; Utsumi, C.; Steup, M. Functional interaction between plastidial starch phosphorylase and starch branching enzymes from rice during the synthesis of branched maltodextrins. Plant Cell Physiol. 2012, 53, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Gu, Z.; Chen, Z.; Yu, J.; Chu, R.; Tan, H.; Zhao, D.; Fan, X.; Zhang, C.; Li, Q.; et al. Improving rice eating and cooking quality by coordinated expression of the major starch synthesis-related genes, SSII and Wx, in endosperm. Plant Mol. Biol. 2021, 106, 419–432. [Google Scholar] [CrossRef]
- Zhao, K.; Tung, C.W.; Eizenga, G.C.; Wright, M.H.; Ali, M.L.; Price, A.H.; Norton, G.J.; Islam, M.R.; Reynolds, A.; Mezey, J.; et al. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat. Commun. 2011, 2, 467. [Google Scholar] [CrossRef]

| Variety | Yield in 2019 (t ha−1) | Yield in 2020 (t ha−1) | Average Yield (t ha−1) | Coefficient of Variation (%) |
|---|---|---|---|---|
| LLYHZ | 9.12 | 9.23 | 9.18 | 0.85 |
| LLY534 | 9.23 | 8.87 | 9.05 | 2.81 |
| LLY1206 | 9.23 | 8.76 | 8.99 | 3.69 |
| LLY8612 | 8.89 | 8.98 | 8.94 | 0.71 |
| LLY149 | 8.44 | 9.03 | 8.73 | 4.78 |
| LLY1377 | 8.78 | 8.60 | 8.69 | 1.46 |
| JLYHZ | 8.93 | 8.17 | 8.55 | 6.29 |
| JLY1206 | 8.73 | 8.13 | 8.43 | 5.03 |
| JLY1988 | 8.43 | 8.41 | 8.42 | 0.17 |
| LLY1988 | 8.35 | 8.46 | 8.40 | 0.93 |
| LLYHLZ | 8.71 | 8.05 | 8.38 | 5.57 |
| LLY3189 | 8.44 | 8.19 | 8.32 | 2.13 |
| JLYHLZ | 8.65 | 7.83 | 8.24 | 7.04 |
| JLY8612 | 8.18 | 8.00 | 8.09 | 1.57 |
| JLY1377 | 8.18 | 7.83 | 8.00 | 3.09 |
| JLY3189 | 7.90 | 8.02 | 7.96 | 1.07 |
| LLY1125 | 7.62 | 8.14 | 7.88 | 4.67 |
| JLY1212 | 7.19 | 8.49 | 7.84 | 11.72 |
| JLY1125 | 8.00 | 7.49 | 7.74 | 4.66 |
| JLY1199 | 7.15 | 8.30 | 7.72 | 10.53 |
| LLY1212 | 7.31 | 8.06 | 7.68 | 6.90 |
| FLY4 | 7.99 | 8.30 | 8.14 | 2.69 |
| LSD (0.05) | 0.84 | 0.60 | — | — |
| Variety | HR (%) | CGR (%) | CD (%) | ASV | GC (mm) | AC (%) | PC (%) | HR (%) | CGR (%) | CD (%) | ASV | GC (mm) | AC (%) | PC (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | |||||||||||||
| LLYHZ | 63.2 | 5.7 | 1.3 | 3.3 | 83.7 | 9.4 | 7.5 | 66.8 | 7.7 | 1.4 | 3.2 | 85.8 | 13.7 | 7.3 |
| LLY534 | 66.1 | 6.3 | 1.2 | 4.0 | 83.3 | 11.5 | 7.2 | 67.0 | 9.9 | 1.8 | 4.1 | 81.7 | 14.6 | 6.5 |
| LLY1206 | 59.6 | 5.7 | 1.0 | 5.0 | 80.0 | 9.5 | 7.0 | 65.3 | 5.4 | 0.9 | 4.2 | 86.3 | 13.2 | 6.8 |
| LLY8612 | 57.4 | 7.3 | 2.1 | 3.0 | 86.0 | 10.0 | 6.7 | 61.8 | 14.7 | 2.6 | 3.4 | 85.5 | 14.0 | 6.2 |
| LLY149 | 41.4 | 5.7 | 1.2 | 3.0 | 86.0 | 8.8 | 7.1 | 58.4 | 7.4 | 1.4 | 3.0 | 90.2 | 12.5 | 7.2 |
| LLY1377 | 58.9 | 9.7 | 2.0 | 5.0 | 83.3 | 11.4 | 6.9 | 64.6 | 12.2 | 2.3 | 4.8 | 79.2 | 15.3 | 7.1 |
| JLYHZ | 63.1 | 9.0 | 2.3 | 5.0 | 82.3 | 10.4 | 6.7 | 66.0 | 15.6 | 3.0 | 6.4 | 81.2 | 16.2 | 6.9 |
| JLY1206 | 64.2 | 10.3 | 1.6 | 6.0 | 79.0 | 11.2 | 7.0 | 66.3 | 6.3 | 1.2 | 6.3 | 83.0 | 14.5 | 6.3 |
| JLY1988 | 60.0 | 17.0 | 4.4 | 4.7 | 83.0 | 10.4 | 7.5 | 64.4 | 15.7 | 4.0 | 5.0 | 83.0 | 14.9 | 6.2 |
| LLY1988 | 58.0 | 10.0 | 2.3 | 3.0 | 87.7 | 9.4 | 7.8 | 63.7 | 11.9 | 2.6 | 3.2 | 90.5 | 14.5 | 6.6 |
| LLYHLZ | 64.4 | 11.7 | 2.9 | 4.3 | 85.7 | 11.2 | 7.2 | 65.1 | 12.2 | 2.5 | 4.5 | 82.3 | 15.3 | 6.5 |
| LLY3189 | 58.4 | 16.3 | 4.4 | 4.3 | 84.3 | 11.4 | 7.3 | 63.3 | 16.7 | 3.6 | 3.9 | 85.2 | 15.4 | 7.0 |
| JLYHLZ | 45.7 | 8.7 | 1.9 | 6.0 | 80.7 | 11.6 | 7.1 | 66.9 | 7.3 | 1.5 | 5.4 | 81.2 | 14.7 | 6.4 |
| JLY8612 | 52.1 | 19.0 | 5.8 | 5.0 | 84.0 | 10.9 | 6.7 | 63.3 | 14.9 | 3.3 | 4.0 | 83.8 | 14.5 | 6.5 |
| JLY1377 | 58.3 | 10.0 | 2.0 | 6.0 | 79.3 | 11.5 | 7.1 | 66.9 | 5.6 | 0.9 | 6.3 | 78.7 | 15.3 | 6.4 |
| JLY3189 | 61.4 | 17.3 | 3.9 | 6.0 | 80.3 | 11.5 | 7.3 | 64.5 | 18.9 | 4.0 | 5.9 | 78.8 | 15.5 | 6.2 |
| LLY1125 | 43.0 | 12.0 | 2.5 | 5.0 | 81.3 | 10.4 | 7.2 | 65.4 | 7.7 | 1.4 | 5.5 | 85.5 | 14.4 | 6.6 |
| JLY1212 | 45.8 | 11.0 | 2.4 | 6.0 | 80.3 | 11.3 | 7.6 | 66.7 | 4.9 | 0.8 | 6.4 | 82.5 | 16.0 | 6.4 |
| JLY1125 | 59.8 | 13.3 | 2.5 | 6.0 | 80.7 | 11.2 | 7.5 | 66.1 | 7.1 | 1.0 | 6.3 | 81.2 | 15.0 | 6.4 |
| JLY1199 | 54.2 | 8.7 | 2.0 | 5.3 | 81.7 | 9.6 | 7.3 | 63.4 | 8.0 | 1.7 | 4.5 | 85.3 | 14.0 | 6.6 |
| LLY1212 | 59.3 | 3.3 | 0.8 | 4.3 | 82.7 | 11.6 | 7.5 | 64.0 | 12.0 | 2.3 | 3.6 | 83.5 | 14.9 | 7.3 |
| FLY4 | 42.2 | 24.3 | 5.2 | 6.0 | 80.3 | 12.5 | 7.3 | 63.7 | 18.5 | 4.1 | 6.3 | 79.0 | 16.8 | 6.5 |
| LSD (0.05) | 3.3 | 3.7 | 1.1 | 0.6 | 2.5 | 0.4 | 0.7 | 2.0 | 4.9 | 1.3 | 0.8 | 4.0 | 0.7 | 0.5 |
| Variety | N Uptake (kg ha−1) | NHI (%) | NUEg (kg kg−1) | NUEb (kg kg−1) | N Uptake (kg ha−1) | NHI (%) | NUEg (kg kg−1) | NUEb (kg kg−1) |
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | |||||||
| LLYHZ | 150.1 | 68.5 | 54.3 | 110.8 | 142.6 | 68.1 | 56.0 | 108.3 |
| LLY534 | 142.0 | 65.8 | 54.8 | 115.1 | 130.5 | 70.1 | 63.8 | 120.5 |
| LLY1206 | 156.3 | 62.8 | 53.7 | 108.4 | 136.0 | 70.0 | 61.4 | 116.8 |
| LLY8612 | 133.8 | 65.9 | 58.7 | 123.1 | 134.5 | 67.9 | 65.2 | 121.7 |
| LLY149 | 136.8 | 65.2 | 54.7 | 113.7 | 145.5 | 74.0 | 61.8 | 115.3 |
| LLY1377 | 133.9 | 66.3 | 57.5 | 120.9 | 148.5 | 63.5 | 53.5 | 116.3 |
| JLYHZ | 135.2 | 64.6 | 57.2 | 117.8 | 138.4 | 65.8 | 56.6 | 111.1 |
| JLY1206 | 131.5 | 66.0 | 56.4 | 120.8 | 118.2 | 71.0 | 67.5 | 124.6 |
| JLY1988 | 137.4 | 71.7 | 56.6 | 117.5 | 135.8 | 60.7 | 58.2 | 117.0 |
| LLY1988 | 156.3 | 63.2 | 48.4 | 107.3 | 124.5 | 63.8 | 57.5 | 119.7 |
| LLYHLZ | 159.3 | 65.1 | 53.8 | 117.3 | 129.5 | 60.0 | 55.3 | 123.2 |
| LLY3189 | 150.7 | 64.6 | 52.5 | 112.3 | 130.6 | 69.5 | 59.3 | 115.3 |
| JLYHLZ | 126.9 | 66.4 | 55.8 | 115.2 | 133.0 | 68.9 | 64.5 | 120.8 |
| JLY8612 | 118.6 | 64.6 | 56.9 | 128.3 | 127.4 | 62.5 | 56.9 | 114.5 |
| JLY1377 | 135.3 | 66.9 | 56.5 | 117.7 | 126.3 | 67.5 | 62.8 | 122.2 |
| JLY3189 | 140.5 | 67.4 | 54.9 | 114.7 | 127.9 | 65.2 | 62.5 | 119.6 |
| LLY1125 | 129.5 | 62.8 | 52.3 | 109.4 | 139.2 | 66.9 | 60.7 | 114.6 |
| JLY1212 | 131.6 | 66.2 | 52.3 | 110.4 | 125.3 | 69.1 | 63.8 | 119.7 |
| JLY1125 | 149.0 | 59.8 | 47.8 | 104.1 | 120.9 | 66.9 | 62.3 | 123.9 |
| JLY1199 | 137.5 | 58.8 | 47.9 | 116.8 | 138.6 | 62.6 | 56.4 | 113.1 |
| LLY1212 | 148.7 | 62.2 | 49.1 | 113.5 | 138.2 | 65.9 | 53.7 | 115.8 |
| FLY4 | 127.3 | 69.8 | 56.9 | 117.1 | 126.7 | 62.4 | 56.7 | 116.8 |
| LSD (0.05) | 25.1 | 6.1 | 6.8 | 12.1 | 16.0 | 5.0 | 6.15 | 10.0 |
| Variety | Yield (t ha−1) | Biomass (g m−2) | HI (%) | N Uptake (kg ha−1) | NUEg (kg kg−1) | HR (%) | CGR (%) | CD (%) | ASV | GC (mm) | AC (%) | PC (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1125 | 7.04 | 1420.0 | 48.5 | 129.2 | 53.1 | 60.0 | 13.8 | 3.2 | 13.8 | 82.7 | 6.3 | 7.0 |
| R1199 | 7.61 | 1485.7 | 48.1 | 131.0 | 54.7 | 55.1 | 7.0 | 1.2 | 12.0 | 86.0 | 3.0 | 7.0 |
| R1206 | 7.88 | 1511.8 | 46.3 | 139.0 | 50.6 | 60.1 | 4.5 | 0.5 | 12.7 | 82.5 | 6.3 | 7.7 |
| R1212 | 7.40 | 1398.1 | 47.9 | 127.8 | 52.5 | 65.3 | 4.1 | 0.6 | 14.9 | 80.8 | 6.4 | 7.4 |
| R1377 | 7.05 | 1394.8 | 45.3 | 117.5 | 53.9 | 63.9 | 12.5 | 1.8 | 14.7 | 79.2 | 6.3 | 7.0 |
| R1988 | 7.68 | 1398.6 | 45.2 | 118.5 | 53.5 | 60.3 | 12.5 | 2.5 | 13.1 | 86.0 | 3.1 | 6.5 |
| R3189 | 7.13 | 1489.2 | 43.9 | 126.5 | 52.2 | 53.7 | 24.7 | 5.6 | 17.2 | 82.0 | 6.7 | 6.9 |
| R534 | 7.68 | 1526.3 | 48.8 | 129.9 | 57.4 | 66.3 | 7.1 | 1.3 | 15.5 | 80.7 | 6.4 | 6.7 |
| R8612 | 7.61 | 1371.0 | 46.0 | 114.6 | 55.2 | 43.4 | 27.8 | 6.0 | 14.8 | 78.2 | 3.6 | 6.3 |
| R149 | 7.13 | 1356.4 | 47.8 | 112.2 | 57.8 | 57.8 | 3.1 | 0.5 | 11.4 | 88.3 | 3.1 | 6.6 |
| HZ | 7.61 | 1386.1 | 46.9 | 112.5 | 57.8 | 61.3 | 6.8 | 1.1 | 12.9 | 82.0 | 3.1 | 6.8 |
| HSLZ | 7.38 | 1333.2 | 49.3 | 115.0 | 57.1 | 64.3 | 9.8 | 1.9 | 14.1 | 76.7 | 6.3 | 6.5 |
| HHZ | 7.76 | 1477.9 | 49.2 | 125.4 | 58.1 | 64.9 | 5.1 | 0.7 | 16.7 | 78.3 | 6.6 | 6.6 |
| LSD (0.05) | 0.60 | 185.8 | 3.3 | 20.4 | 5.1 | 2.6 | 3.6 | 1.1 | 0.6 | 4.0 | 0.4 | 0.6 |
| Locus | Group | Locus Name | Gene Function |
|---|---|---|---|
| LOC_Os06g04200 | down | Wx | Eating quality; seed amylose content. |
| LOC_Os01g44220 | down | OsAPL2|osagpl2-3|OsAGPL2|GIF2 | Eating quality; starch biosynthesis |
| LOC_Os05g33570 | down | FLO4|OsPPDKB|OsC4PPDK | Eating quality; seed protein and lipid content |
| LOC_Os08g09230 | down | OsSSIIIa|Flo5 | Eating quality; seed starch content |
| LOC_Os08g40930 | down | OsISA1|OsPHS8 | Eating quality; seed starch content |
| LOC_Os06g12450 | down | ALK|SSIIa | Eating quality; gelatinization temperature; gel consistency |
| LOC_Os03g55090 | down | Pho1 | Seed; seed starch content; grain maturation |
| LOC_Os03g61120 | down | OASA1 | Sterility; trp level in grain; fertility; germination ability |
| LOC_Os02g49370 | down | OsHAP3E | Panicle flower; dwarfism; leaf angle; floral organ formation |
| LOC_Os07g38130 | up | OsFOR1|PGIP | Panicle flower; regulation of floral organ number |
| LOC_Os04g45330 | up | OsYABBY5|OsYAB3|TOB1 | Panicle flower; spikelets morphology |
| LOC_Os05g39990 | up | NA | Flowering; dwarfism; cell wall extensibility; flowering time |
| LOC_Os05g51360 | up | OsEBS | Panicle flower; plant height; leaf size; grain number |
| LOC_Os05g50890 | up | OsJar1|OsGH3.5|OsGH3-5 | Sterility; seed development |
| LOC_Os05g03040 | up | RSR1 | Seed; seed amylose content; grain size |
| LOC_Os06g12210 | up | BU1|OsbHLH174 | Seed; leaf angle; grain size; brassinosteroid sensitivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Wang, K.; Xia, S.; Du, T.; Fu, Y.; Yang, Y.; Wang, F. Coordinating High Yield and Superior Eating Quality of Rice: A Case Study of Hybrid Varieties Derived from Longke638S and Jing4155S. Agronomy 2022, 12, 1628. https://doi.org/10.3390/agronomy12071628
Yang W, Wang K, Xia S, Du T, Fu Y, Yang Y, Wang F. Coordinating High Yield and Superior Eating Quality of Rice: A Case Study of Hybrid Varieties Derived from Longke638S and Jing4155S. Agronomy. 2022; 12(7):1628. https://doi.org/10.3390/agronomy12071628
Chicago/Turabian StyleYang, Wenlong, Kai Wang, Shengming Xia, Ting Du, Yifan Fu, Yuanzhu Yang, and Fei Wang. 2022. "Coordinating High Yield and Superior Eating Quality of Rice: A Case Study of Hybrid Varieties Derived from Longke638S and Jing4155S" Agronomy 12, no. 7: 1628. https://doi.org/10.3390/agronomy12071628






