Remediation of Lead Contamination by Aspergillus niger and Phosphate Rocks under Different Nitrogen Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. FAp, PG, and Fungal Strains Preparation
2.2. Pb Remediation by A. niger and Phosphate under Different Nitrogen
2.3. TCLP-Pb Extraction from Precipitates
2.4. Instrumentation
2.5. Statistical Analysis
3. Results
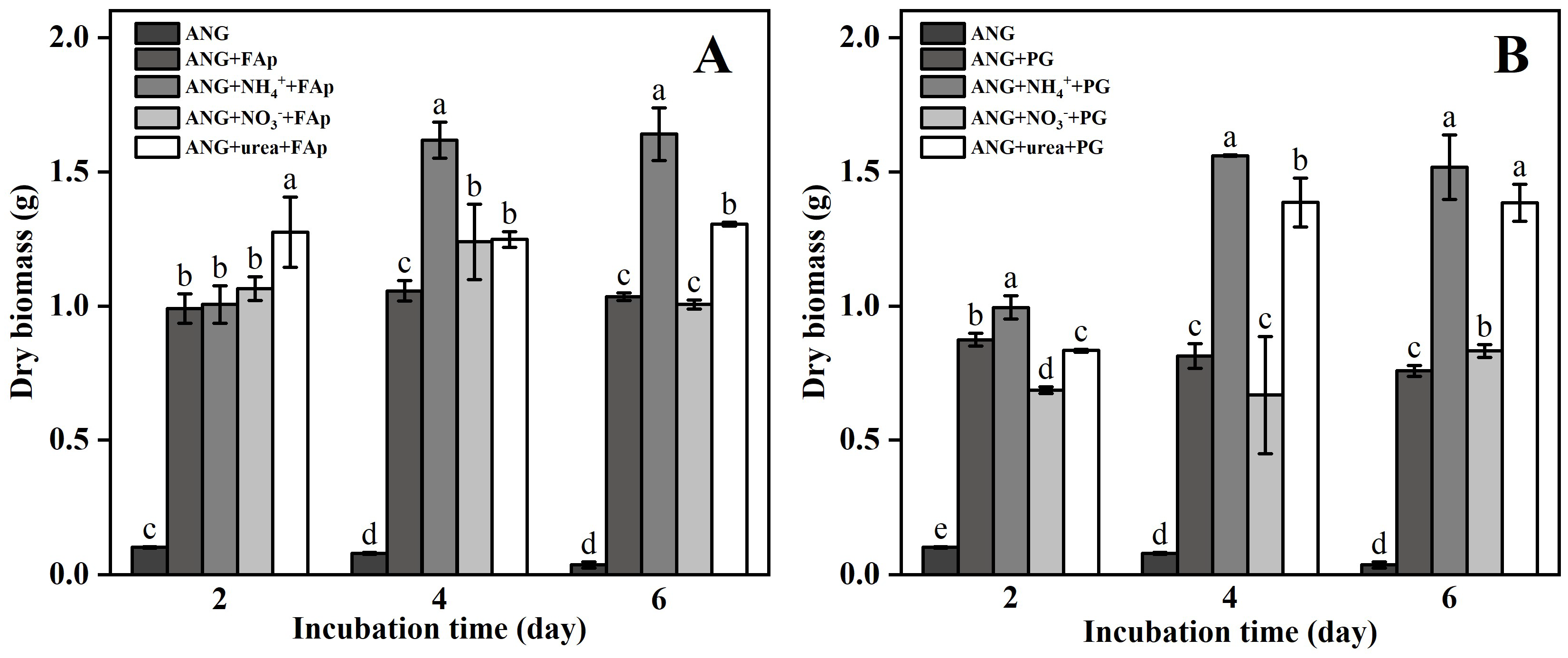
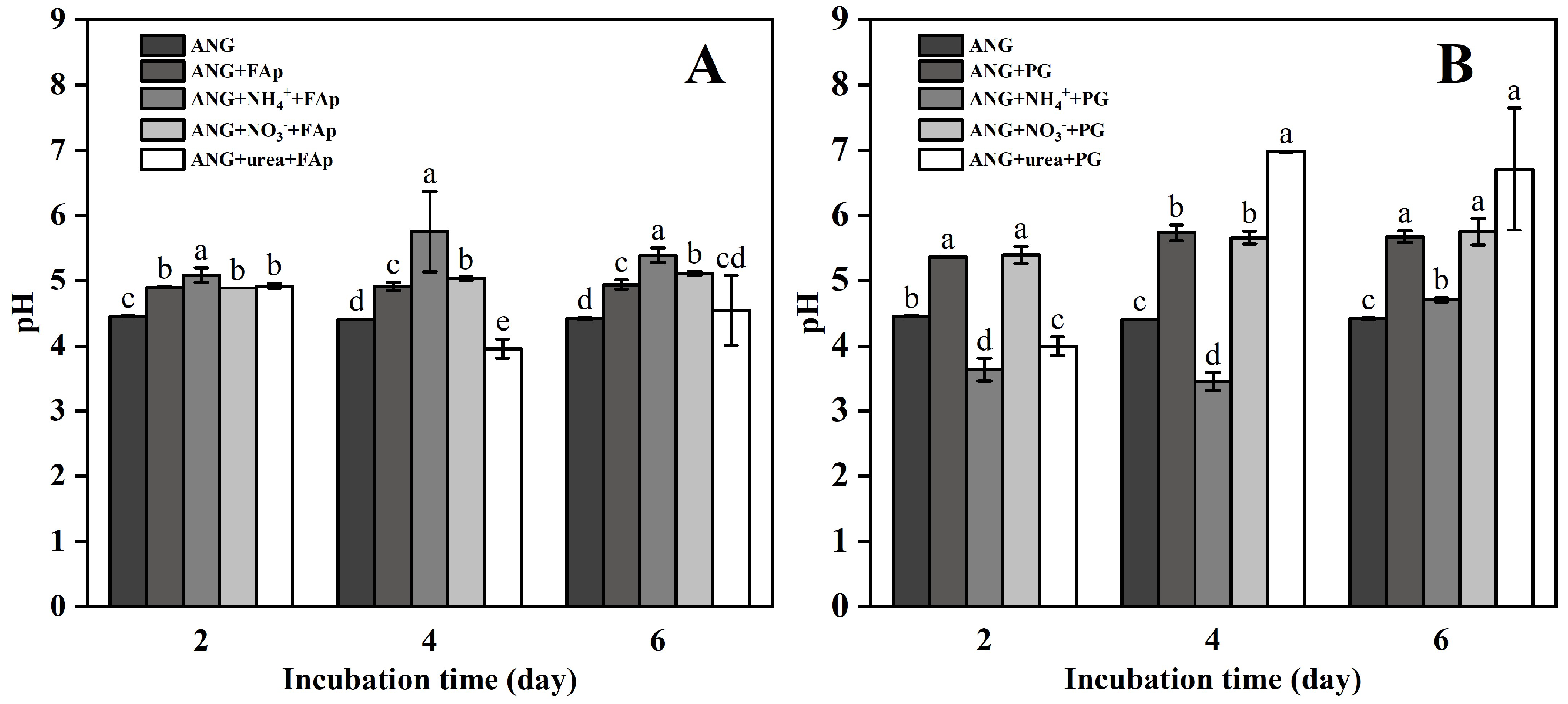
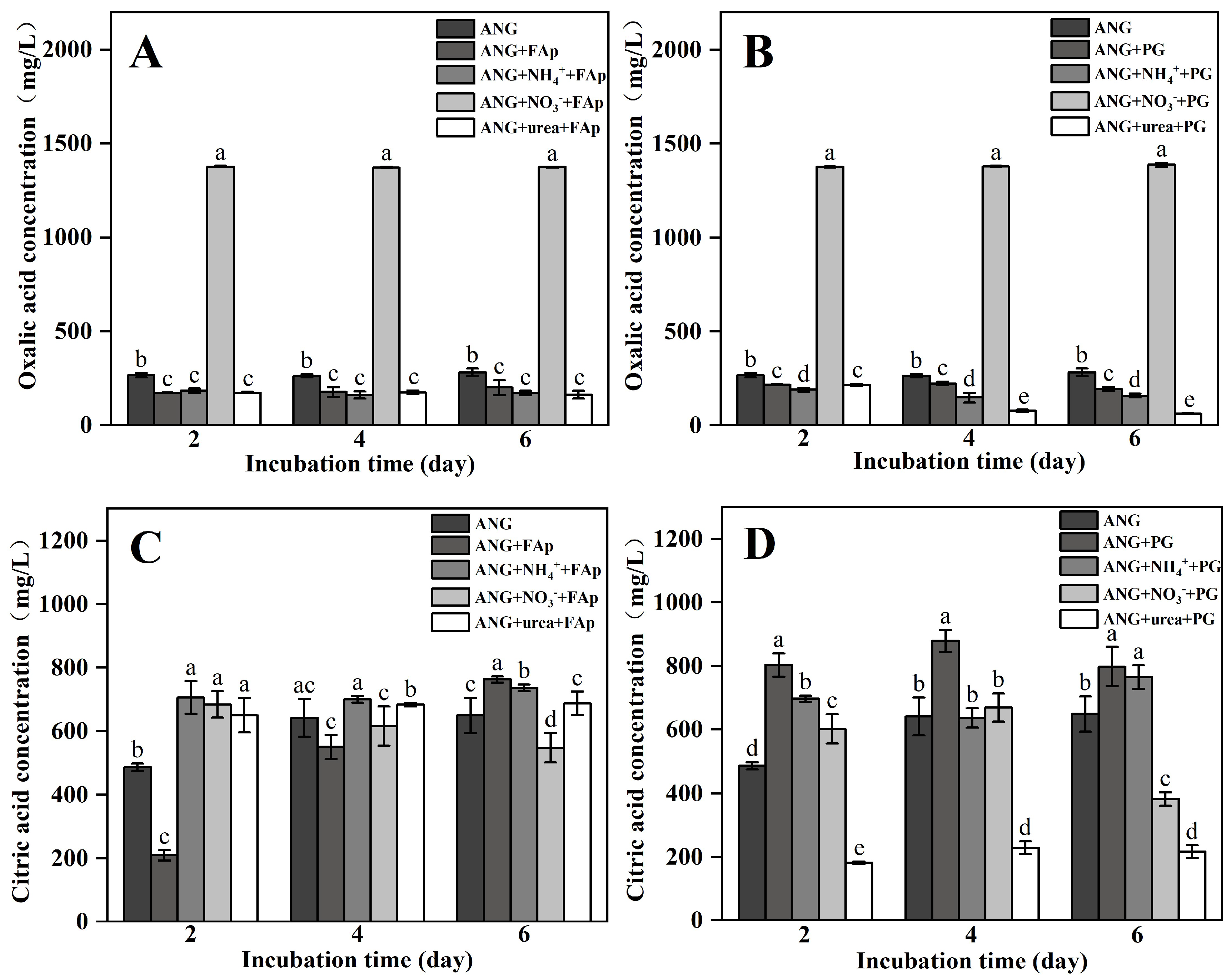
3.1. Fungal Biomass, pH, and Oxalic and Citric Acid Secreted by A. niger
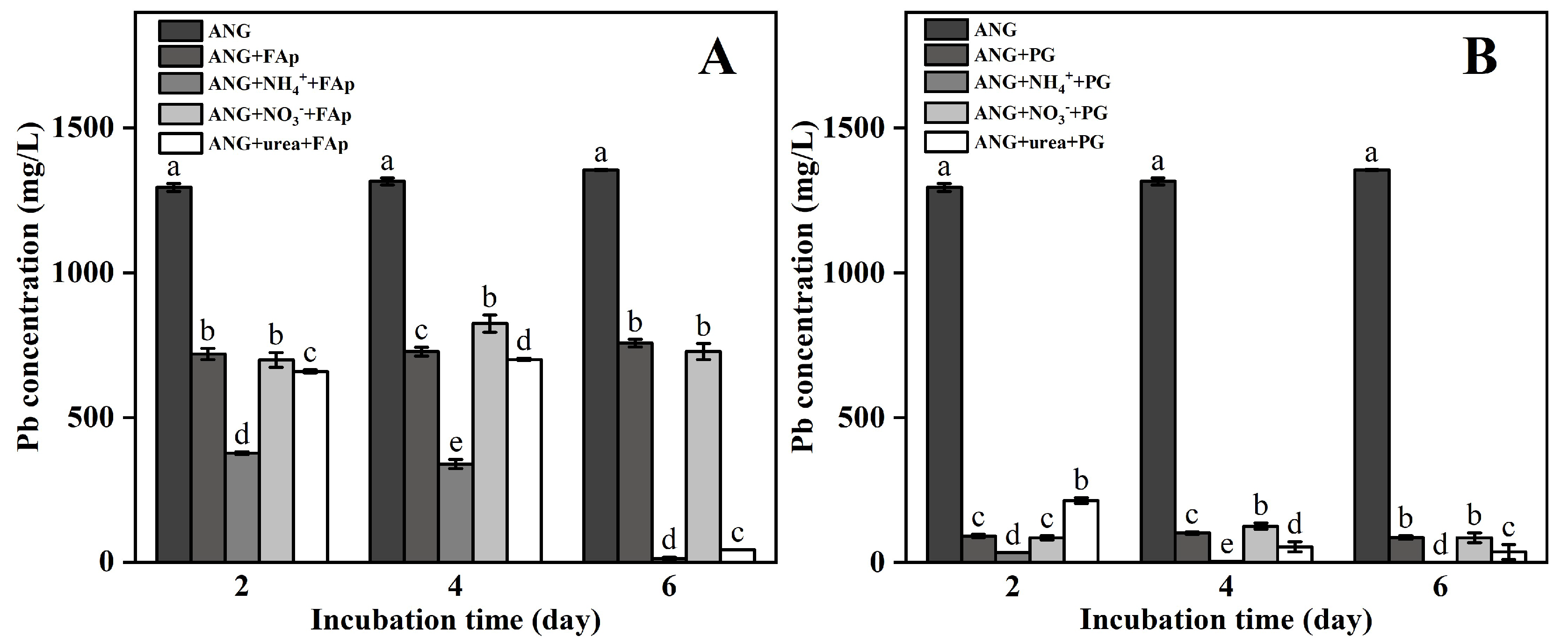
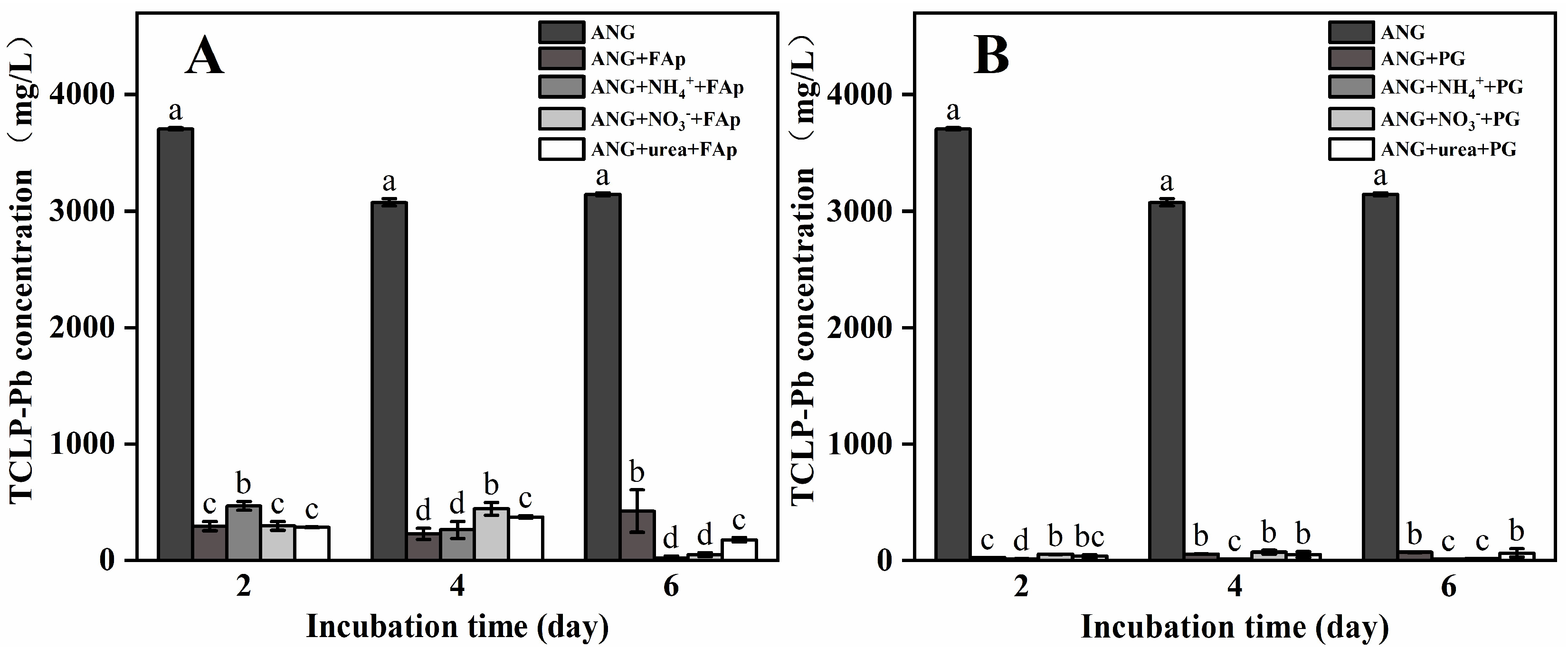
3.2. Pb and TCLP-Pb Concentrations
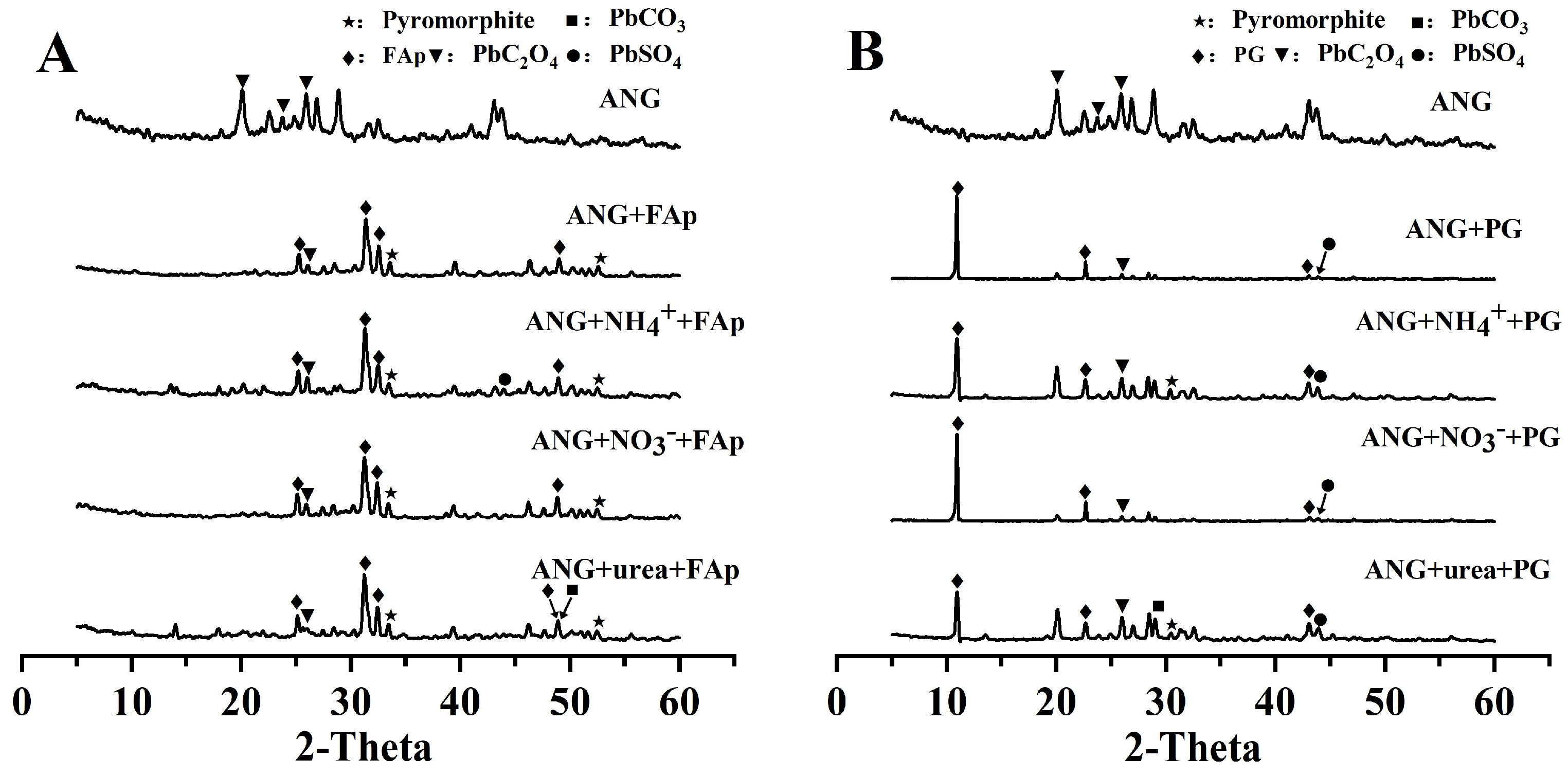
3.3. XRD Analysis
3.4. SEM–EDS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, S.; Tripathi, I.P. Lead pollution—An overview. Int. Res. J. Environ. Sci. 2012, 1, 84–86. [Google Scholar]
- Zeng, G.; Wan, J.; Huang, D.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.; Gong, X.; Wang, R.; Jiang, D. Precipitation, adsorption and rhizosphere effect: The mechanisms for phosphate-induced Pb immobilization in soils—A review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Pattee, O.H.; Pain, D.J. Chapter 15-lead in the environment. Handb. Ecotoxicol. 2003, 2, 373–408. [Google Scholar]
- Kachenko, A.G.; Singh, B. Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 2006, 169, 101–123. [Google Scholar] [CrossRef]
- Paoliello, M.M.; De Capitani, E.M. Occupational and environmental human lead exposure in Brazil. Environ. Res. 2007, 103, 288–297. [Google Scholar] [CrossRef]
- Meena, V.; Dotaniya, M.L.; Saha, J.K.; Das, H.; Patra, A.K. Impact of Lead Contamination on Agroecosystem and Human Health; Spring: Cham, Switzerland, 2019; pp. 67–82. [Google Scholar]
- Zhang, L.; Li, W.; Cao, H.; Hu, D.; Chen, X.; Guan, Y.; Tang, J.; Gao, H. Ultra-efficient sorption of Cu2+ and Pb2+ ions by light biochar derived from Medulla tetrapanacis. Bioresour. Technol. 2019, 291, 121818. [Google Scholar] [CrossRef]
- Dikilitas, M.; Karakas, S.; Ahmad, P. Effect of Lead on Plant and Human DNA Damages and Its Impact on the Environment; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 41–67. [Google Scholar]
- Xiaoqiong, S.; Ke, F.; Ge, C.; Zhang, J.; Li, S.; Li, C.; Li, J. Core–shell magnetic Fe3O4@ZnS nanoparticles As highly efficient adsorbents for the removal of Pb2+ from water. Russ. J. Phys. Chem. A. 2019, 93, 522–527. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Bai, T.; Tao, J.; Guo, J.; Yang, M.; Wang, S.; Hu, S. Lead immobilization by geological fluorapatite and fungus Aspergillus niger. J. Hazard. Mater. 2016, 320, 386–392. [Google Scholar] [CrossRef]
- Mahmoud, E.; Abd El-Kader, N. Heavy metal immobilization in contaminated soils using phosphogypsum and rice straw compost. Land Degrad. Dev. 2015, 26, 819–824. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Rhue, D.R.; Appel, C.S. Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ. Pollut. 2004, 131, 435–444. [Google Scholar] [CrossRef]
- Manecki, M.; Maurice, P.A.; Traina, S.J. Uptake of aqueous Pb by Cl−, F−, and OH− apatites: Mineralogic evidence for nucleation mechanisms. Am. Mineral. 2000, 85, 932–942. [Google Scholar] [CrossRef]
- Tian, D.; Wang, W.; Su, M.; Zheng, J.; Wu, Y.; Wang, S.; Li, Z.; Hu, S. Remediation of lead-contaminated water by geological fluorapatite and fungus Penicillium oxalicum. Environ. Sci. Pollut. Res. 2018, 25, 21118–21126. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, L.; Zheng, Y.; Tian, D.; Su, M.; Zhang, F.; Ma, S.; Hu, S. Characterizing the mechanisms of lead immobilization via bioapatite and various clay minerals. ACS Earth Space Chem. 2017, 1, 152–157. [Google Scholar] [CrossRef]
- Tian, D.; Cheng, X.; Wang, L.; Hu, J.; Zhou, N.; Xia, J.; Xu, M.; Zhang, L.; Gao, H.; Ye, X.; et al. Remediation of lead-contaminated water by red yeast and different types of phosphate. Front. Bioeng. Biotech. 2022, 10, 775058. [Google Scholar] [CrossRef]
- Tian, D.; Xia, J.; Zhou, N.; Xu, M.; Li, X.; Zhang, L.; Du, S.; Gao, H. The utilization of phosphogypsum as a sustainable phosphate-based fertilizer by Aspergillus niger. Agron 2022, 12, 646. [Google Scholar] [CrossRef]
- Yang, J.; Ma, L.; Liu, H.; Guo, Z.; Bounkhong, K. Chemical behavior of fluorine and phosphorus in chemical looping gasification using phosphogypsum as an oxygen carrier. Chemosphere 2020, 248, 125979. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, L.; Lin, Q.; Cao, J.X. Analysis of phosphogypsum’s physical and chemical properties and making of anhydrite cement from phosphogypsum. Appl. Mech. Mater. 2013, 275–277, 2131–2135. [Google Scholar] [CrossRef]
- Shen, Z.; Tian, D.; Zhang, X.; Tang, L.; Su, M.; Zhang, L.; Li, Z.; Hu, S.; Hou, D. Mechanisms of biochar assisted immobilization of Pb2+ by bioapatite in aqueous solution. Chemosphere 2018, 190, 260–266. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, Z.; Jiang, L.; Su, M.; Feng, Z.; Zhang, L.; Wang, S.; Li, Z.; Hu, S. A new insight into lead (II) tolerance of environmental fungi based on a study of Aspergillus niger and Penicillium oxalicum. Environ. Microbiol. 2019, 21, 471–479. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, T.; Xiao, Q.; Wang, P.; Tian, H. Effect of 4-chloro-2-methylphenoxy acetic acid on tomato gene expression and rhizosphere bacterial communities under inoculation with phosphate-solubilizing bacteria. J. Hazard. Mater. 2021, 416, 125767. [Google Scholar] [CrossRef]
- Jones, D.L.; Oburger, E. Solubilization of Phosphorus by Soil Microorganisms; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 169–198. [Google Scholar]
- Tian, D.; Wang, L.; Hu, J.; Zhang, L.; Zhou, N.; Xia, J.; Xu, M.; Yusef, K.K.; Wang, S.; Li, Z.; et al. A study of P release from Fe-P and Ca-P via the organic acids secreted by Aspergillus niger. J. Microbiol. 2021, 59, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Cameselle, C.; Rodríguez-Couto, S.; Sanromán, Á. Influence of milk whey, nitrogen and phosphorus concentration on oxalic acid production by Aspergillus niger. Bioprocess Eng. 1999, 20, 1–5. [Google Scholar] [CrossRef]
- de Oliveira Mendes, G.; Murta, H.M.; Valadares, R.V.; da Silveira, W.B.; da Silva, I.R.; Costa, M.D. Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner. Eng. 2020, 155, 106458. [Google Scholar] [CrossRef]
- Guo, C.; Tian, W.; Wang, Z.; Han, F.; Su, M.; Wu, Y.; Li, Z.; Hu, S. Reduction of Pb availability during surficial leaching in different types of soils with addition of apatite and oxalic acid. J. Soils Sediments 2018, 19, 741–749. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Yang, L.; Liu, L.; Qian, L.W.; Tian, K.L.; Zhang, Q.; Cao, D.J. Combined forms of Pb and its detoxification and absorption in Cladophora rupestris subcells. Spectrochim. Acta. A 2021, 248, 119190. [Google Scholar] [CrossRef]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.; Junier, P. Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar]
- Cerezine, P.C.; Nahas, E.; Banzatto, D.A. Soluble phosphate accumulation by Aspergillus niger from fluorapatite. Appl. Microbiol. Biotechnol. 1988, 29, 501–505. [Google Scholar] [CrossRef]
- Shen, Z.; Zhen, L.; Alessi, D.S. Stabilization-based soil remediation should consider long-term challenges. Front. Environ. Sci. Eng. 2018, 12, 16. [Google Scholar] [CrossRef]
- Halim, C. Evaluating the applicability of a modified toxicity characteristic leaching procedure (TCLP) for the classification of cementitious wastes containing lead and cadmium. J. Hazard. Mater. 2003, 103, 125–140. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, C.; Zeng, G.; Huang, D.; Hu, L.; Huang, C.; Wu, H.; Wang, L. Synthesis and evaluation of a new class of stabilized nano-chlorapatite for Pb immobilization in sediment. J. Hazard. Mater. 2016, 320, 278–288. [Google Scholar] [CrossRef]
- Dursun, A.Y.; Uslu, G.; Cuci, Y.; Aksu, Z. Bioaccumulation of copper(II), lead(II) and chromium(VI) by growing Aspergillus niger. Process Biochem. 2003, 38, 1647–1651. [Google Scholar] [CrossRef]
- Ousmanova, D.; Parker, W. Fungal generation of organic acids for removal of lead from contaminated soil. Water Air Soil Pollut. 2006, 179, 365–380. [Google Scholar] [CrossRef]
- Xenidis, A.; Stouraiti, C.; Papassiopi, N. Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron. J. Hazard. Mater. 2010, 177, 929–937. [Google Scholar] [CrossRef]
- Tian, D.; Su, M.; Zou, X.; Zhang, L.; Tang, L.; Geng, Y.; Qiu, J.; Wang, S.; Gao, H.; Li, Z. Influences of phosphate addition on fungal weathering of carbonate in the red soil from karst region. Sci. Total. Environ. 2021, 755, 142570. [Google Scholar] [CrossRef]
- Meng, L.; Pan, S.; Zhou, L.; Santasup, C.; Su, M.; Tian, D.; Li, Z. Evaluating the survival of Aspergillus niger in a highly polluted red soil with addition of Phosphogypsum and bioorganic fertilizer. Environ. Sci. Pollut. Res. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.M.; Chen, J.; Naidu, R. Not all phosphate fertilizers immobilize lead in soils. Water Air Soil Pollut. 2013, 224, 1–7. [Google Scholar] [CrossRef]
- Tian, D.; Li, Z.; O’Connor, D.; Shen, Z. The need to prioritize sustainable phosphate-based fertilizers. Soil Use Manag. 2020, 36, 351–354. [Google Scholar] [CrossRef]
- Bandara, A.; Senanayake, G. Leachability of rare-earth, calcium and minor metal ions from natural Fluorapatite in perchloric, hydrochloric, nitric and phosphoric acid solutions: Effect of proton activity and anion participation. Hydrometall 2015, 153, 179–189. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; Lopez, F.A.; Alguacil, F.J.; Lopez-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Silva, U.d.C.; Mendes, G.D.O.; Silva, N.M.R.M.; Duarte, J.L.; Silva, I.R.; Tótola, M.R.; Costa, M.D. Fluoride-tolerant mutants of Aspergillus niger show enhanced phosphate solubilization capacity. PLoS ONE 2014, 9, e110246. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, D. Synthesis and characterization of a new class of stabilized apatite nanoparticles and applying the particles to in situ Pb immobilization in a fire-range soil. Chemosphere 2013, 91, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M.; Bahri-Esfahani, J.; Li, Q.; Rhee, Y.J.; Wei, Z.; Fomina, M.; Liang, X. Oxalate production by fungi: Significance in geomycology, biodeterioration and bioremediation. Fungal Biol. Rev. 2014, 28, 36–55. [Google Scholar] [CrossRef]
- Gharieb, M.M. Nutritional effects on oxalic acid production and solubilization of gypsum by Aspergillus niger. Mycol. Res. 2000, 104, 550–556. [Google Scholar] [CrossRef]
- Barroso, C.B.; Pereira, G.T.; Nahas, E. Solubilization of CaHPO4 and AlPO4 by Aspergillus niger in culture media with different carbon and nitrogen sources. Braz. J. Microbiol. 2006, 37, 434–438. [Google Scholar] [CrossRef][Green Version]
- Su, M.; Meng, L.; Zhao, L.; Tang, Y.; Qiu, J.; Tian, D.; Li, Z. Phosphorus deficiency in soils with red color: Insights from the interactions between minerals and microorganisms. Geoderma 2021, 404, 115311. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Zhang, L.; Li, X.; Wang, L.; Yusef, K.K.; Gao, H.; Tian, D. Remediation of Lead Contamination by Aspergillus niger and Phosphate Rocks under Different Nitrogen Sources. Agronomy 2022, 12, 1639. https://doi.org/10.3390/agronomy12071639
Feng Y, Zhang L, Li X, Wang L, Yusef KK, Gao H, Tian D. Remediation of Lead Contamination by Aspergillus niger and Phosphate Rocks under Different Nitrogen Sources. Agronomy. 2022; 12(7):1639. https://doi.org/10.3390/agronomy12071639
Chicago/Turabian StyleFeng, Yi, Liangliang Zhang, Xiang Li, Liyan Wang, Kianpoor Kalkhajeh Yusef, Hongjian Gao, and Da Tian. 2022. "Remediation of Lead Contamination by Aspergillus niger and Phosphate Rocks under Different Nitrogen Sources" Agronomy 12, no. 7: 1639. https://doi.org/10.3390/agronomy12071639
APA StyleFeng, Y., Zhang, L., Li, X., Wang, L., Yusef, K. K., Gao, H., & Tian, D. (2022). Remediation of Lead Contamination by Aspergillus niger and Phosphate Rocks under Different Nitrogen Sources. Agronomy, 12(7), 1639. https://doi.org/10.3390/agronomy12071639






