Hydraulic Trait Variation with Tree Height Affects Fruit Quality of Walnut Trees under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Walnut Cultivar and Water Treatment
2.3. Measurement Protocols
2.3.1. Soil and Plant Water Status
2.3.2. Physiological Traits
2.3.3. Morphological Traits
2.3.4. Nonstructural Carbohydrates
2.4. Data Analysis
3. Results
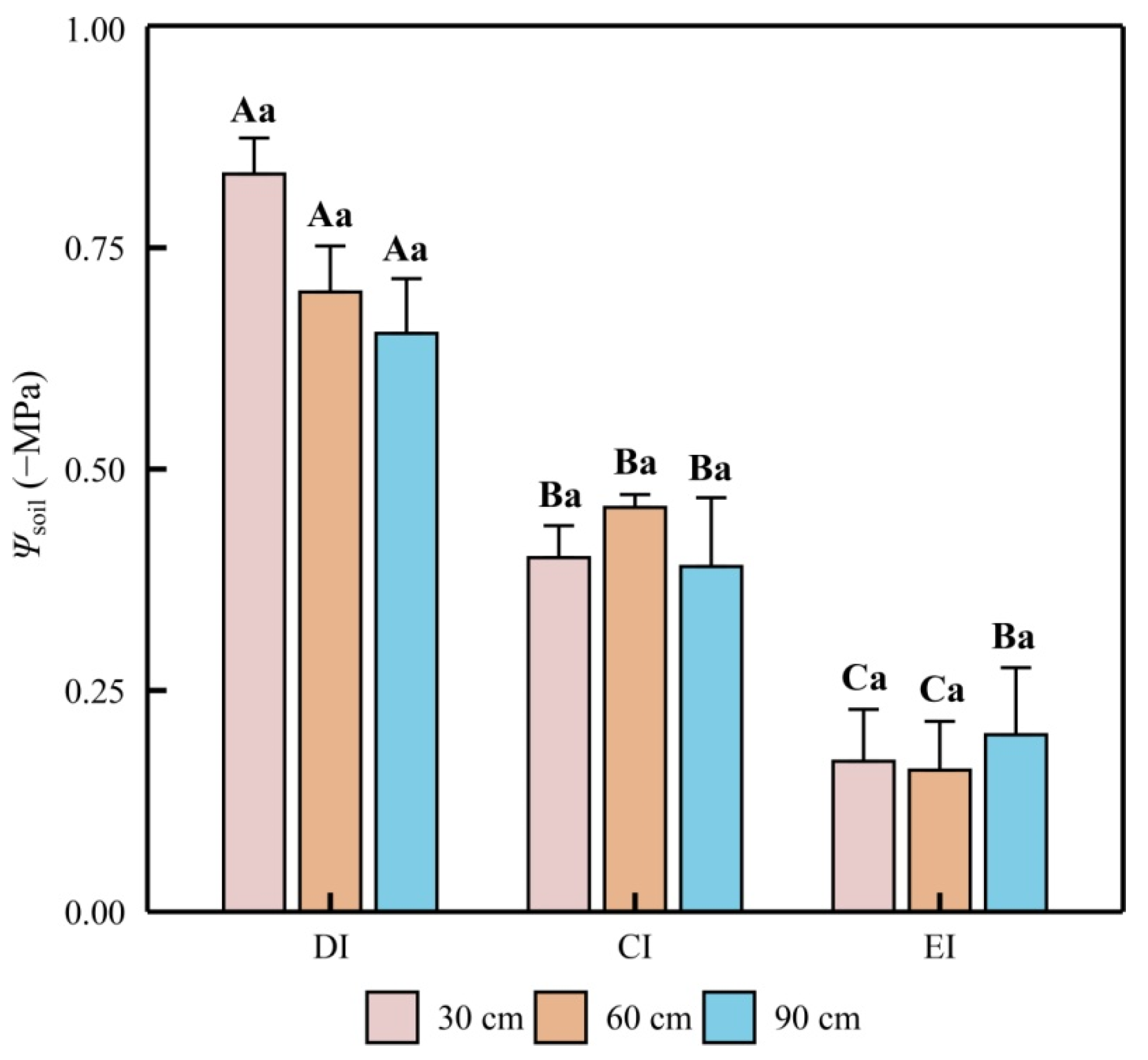
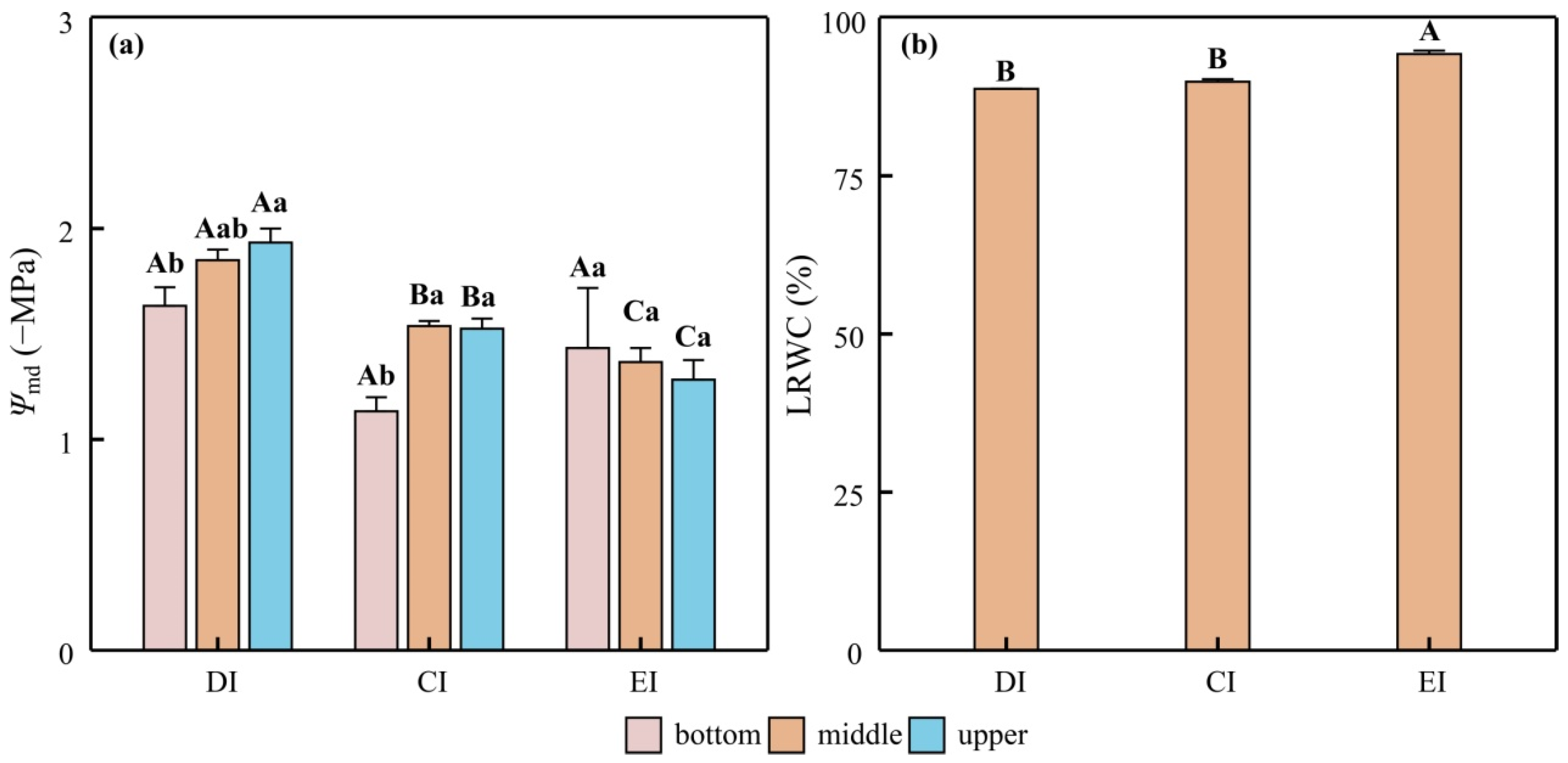
3.1. Soil and Plant Water Potential
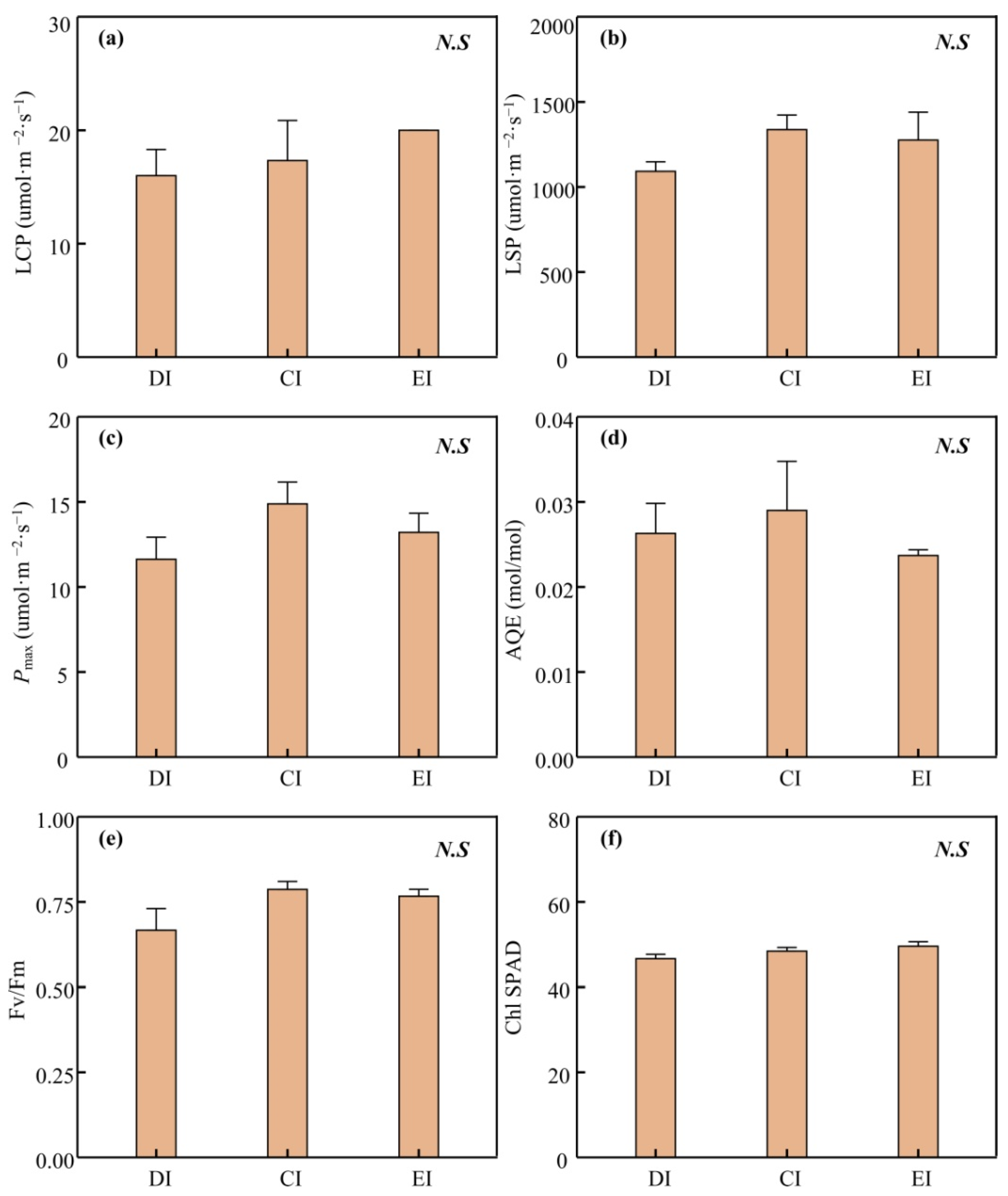
3.2. Physiological Trait Performance and Nonstructural Carbohydrate Variation
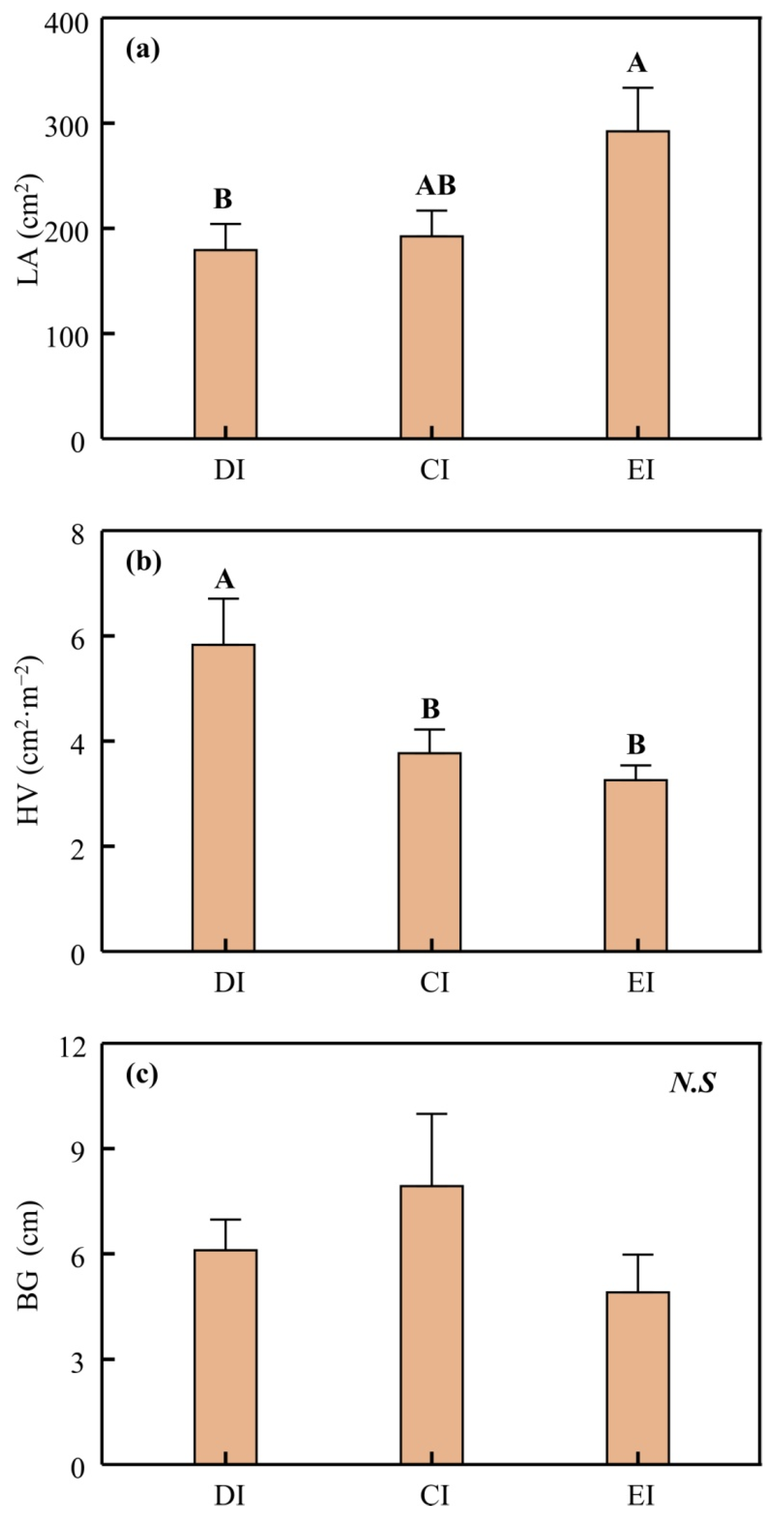
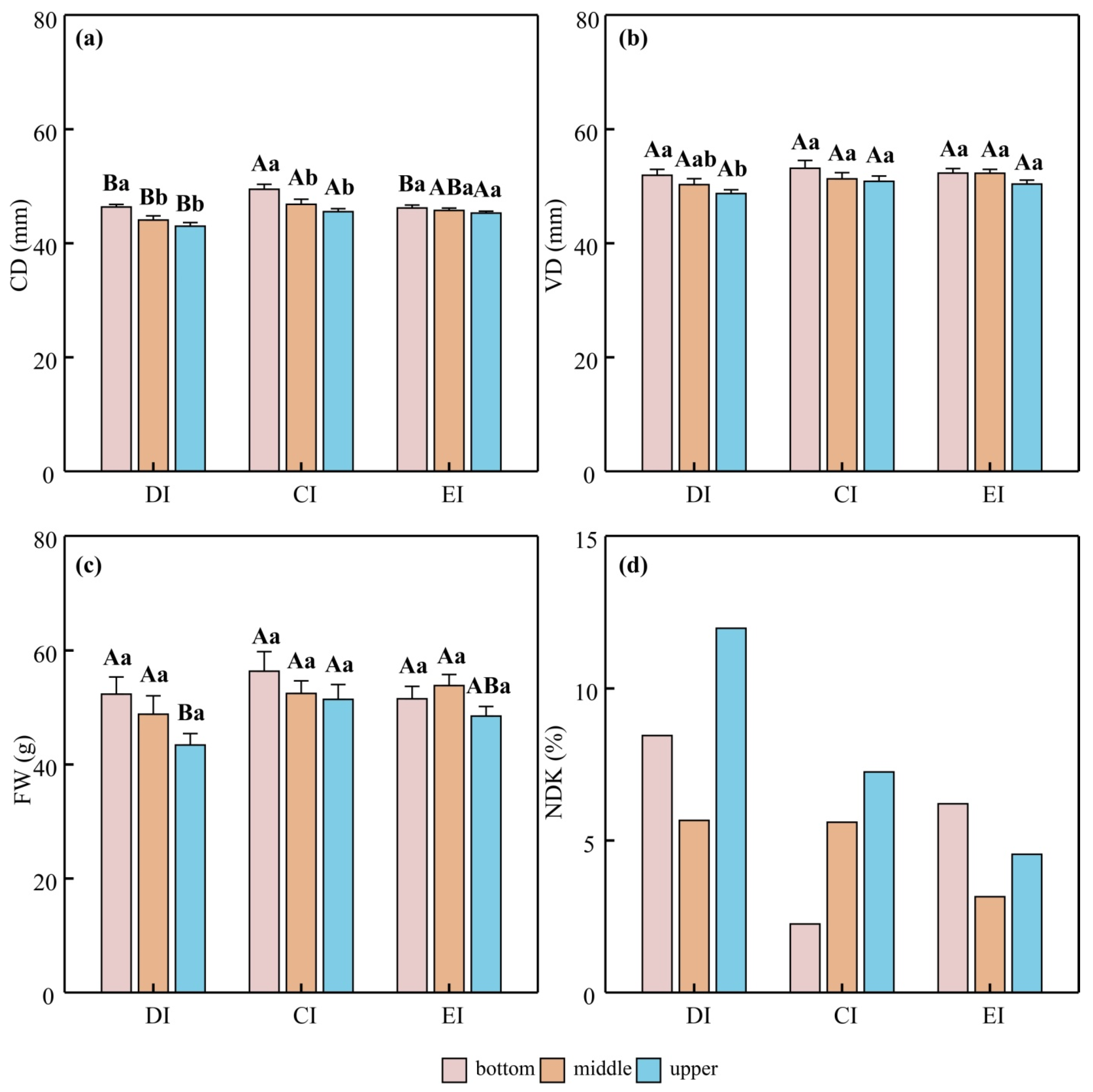
3.3. Morphological Adjustments and Fruit Quality
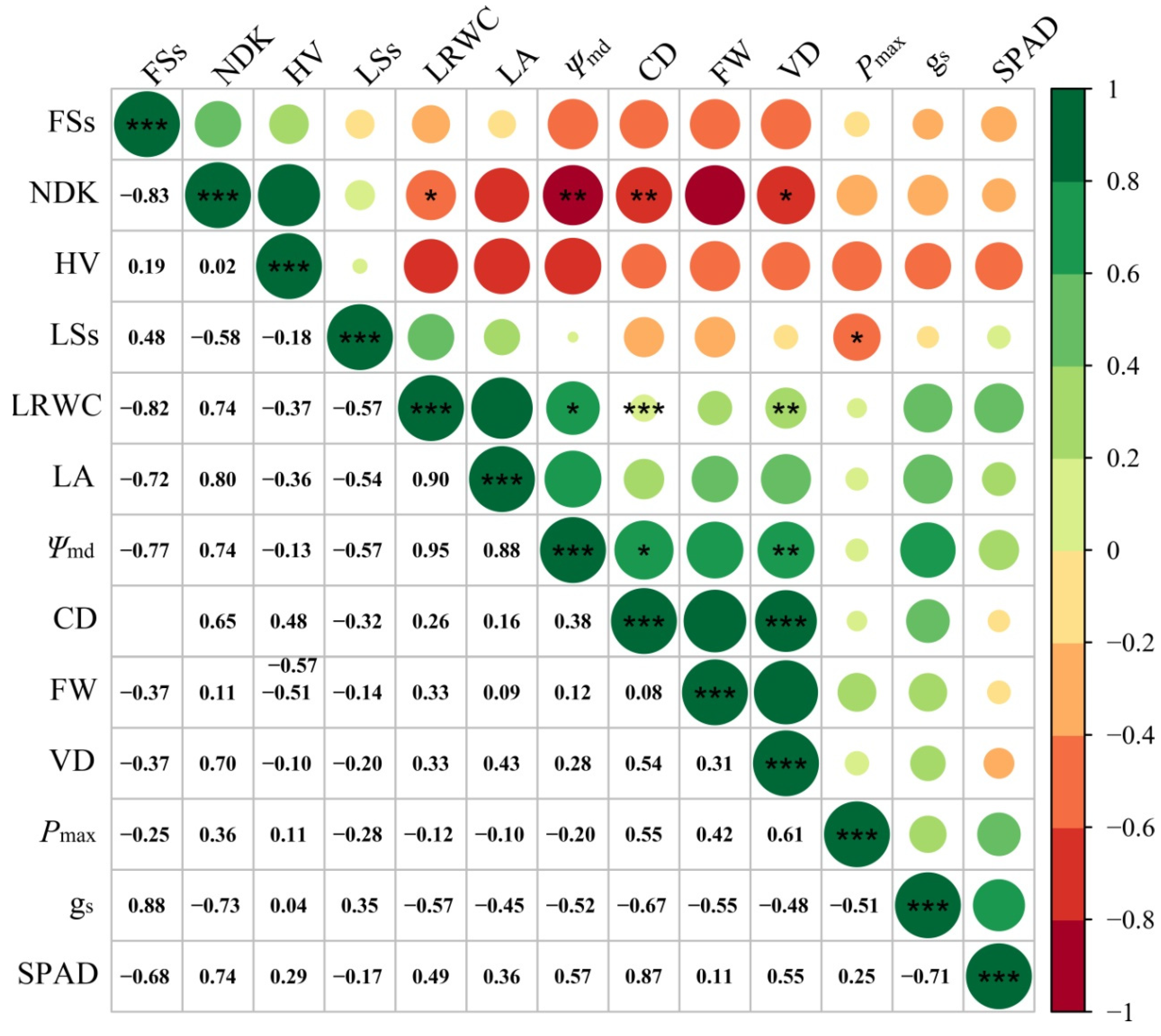
3.4. Correlation among Different Traits
4. Discussion
4.1. Physiological Responses and Morphological Adjustments to Drought
4.2. The Influence of Drought on Nonstructural Carbohydrate Variation and Fruit Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corelligrappadelli, L.; Coston, D.C. Thinning pattern and light environment in peach-tree canopies influence fruit-quality. Hortscience 1991, 26, 1464–1466. [Google Scholar] [CrossRef]
- Girardi, E.A.; Ayres, A.J.; Girotto, L.F.; Pena, L. Tree Growth and Production of Rainfed Valencia Sweet Orange Grafted onto Trifoliate Orange Hybrid Rootstocks under Aw Climate. Agronomy 2021, 11, 2533. [Google Scholar] [CrossRef]
- Genard, M.; Dauzat, J.; Franck, N.; Lescourret, F.; Moitrier, N.; Vaast, P.; Vercambre, G. Carbon allocation in fruit trees: From theory to modelling. Trees-Struct. Funct. 2008, 22, 269–282. [Google Scholar] [CrossRef]
- Vaast, P.; Angrand, J.; Franck, N.; Dauzat, J.; Genard, M. Fruit load and branch ring-barking affect carbon allocation and photosynthesis of leaf and fruit of Coffea arabica in the field. Tree Physiol. 2005, 25, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos de Carvalho, H.W.; de Carvalho, L.M.; Teodoro, A.V.; de Barros, I.; Girardi, E.A.; Soares Filho, W.d.S.; Passos, O.S. Yield, fruit quality, and survival of ‘Pera’ sweet orange on eight rootstocks in tropical cohesive soils. Pesqui. Agropecu. Bras. 2021, 56, E021151. [Google Scholar] [CrossRef]
- Albarracín, V.; Hall, A.J.; Searles, P.S.; Rousseaux, M.C. Responses of vegetative growth and fruit yield to winter and summer mechanical pruning in olive trees. Sci. Hortic. 2017, 225, 185–194. [Google Scholar] [CrossRef]
- Fernández, F.J.; Ladux, J.L.; Searles, P.S. Dynamics of shoot and fruit growth following fruit thinning in olive trees: Same season and subsequent season responses. Sci. Hortic. 2015, 192, 320–330. [Google Scholar] [CrossRef]
- Almeida, W.L.; Avila, R.T.; Perez-Molina, J.P.; Barbosa, M.L.; Marcal, D.M.S.; de Souza, R.P.B.; Martino, P.B.; Cardoso, A.A.; Martins, S.C.V.; DaMatta, F.M. The interplay between irrigation and fruiting on branch growth and mortality, gas exchange and water relations of coffee trees. Tree Physiol. 2021, 41, 35–49. [Google Scholar] [CrossRef]
- Rao, N.K.S. Arid Zone Fruit Crops. In Abiotic Stress Physiology of Horticultural Crops; Rao, N.K.S., Shivashankara, K.S., Laxman, R.H., Eds.; Springer India: New Delhi, India, 2016; pp. 223–234. [Google Scholar]
- Ezzat, A.; Salama, A.-M.; Szabo, S.; Yaseen, A.A.; Molnar, B.; Holb, I.J. Deficit Irrigation Strategies on Tree Physiological and Chemical Properties: Treatment Effects, Prediction Based Model Analyses and Inter-Correlations. Agronomy 2021, 11, 1361. [Google Scholar] [CrossRef]
- Nagaz, K.; El Mokh, F.; Ben Hassen, N.; Masmoudi, M.M.; Ben Mechlia, N.; Baba Sy, M.O.; Belkheiri, O.; Ghiglieri, G. Impact of Deficit Irrigation on Yield and Fruit Quality of Orange Trees (Citrus Sinensis, L. Osbeck, CV. Meski Maltaise) in Southern Tunisia. Irrig. Drain. 2020, 69, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Faghih, S.; Zamani, Z.; Fatahi, R.; Liaghat, A. Effects of deficit irrigation and kaolin application on vegetative growth and fruit traits of two early ripening apple cultivars. Biol. Res. 2019, 52, 43. [Google Scholar] [CrossRef] [Green Version]
- Cui, N.; Du, T.; Li, F.; Tong, L.; Kang, S.; Wang, M.; Liu, X.; Li, Z. Response of vegetative growth and fruit development to regulated deficit irrigation at different growth stages of pear-jujube tree. Agric. Water Manag. 2009, 96, 1237–1246. [Google Scholar] [CrossRef]
- Rahmati, M.; Davarynejad, G.H.; Genard, M.; Bannayan, M.; Azizi, M.; Vercambre, G. Peach Water Relations, Gas Exchange, Growth and Shoot Mortality under Water Deficit in Semi-Arid Weather Conditions. PLoS ONE 2015, 10, e0120246. [Google Scholar] [CrossRef] [Green Version]
- Vélez-Sánchez, J.E.; Molina-Ochoa, M.J.; Rodríguez-Hernández, P. Effect of regulated deficit irrigation (RDI) on the vegetative growth of pear variety Triunfo de Viena (Pyrus communis L.). Rev. UDCA Actual. Divulg. Científica 2021, 24, e2007. [Google Scholar] [CrossRef]
- Chen, F.; Cui, N.; Jiang, S.; Li, H.; Wang, Y.; Gong, D.; Hu, X.; Zhao, L.; Liu, C.; Qiu, R. Effects of water deficit at different growth stages under drip irrigation on fruit quality of citrus in the humid areas of South China. Agric. Water Manag. 2022, 262, 107407. [Google Scholar] [CrossRef]
- Miras-Avalos, J.M.; Egea, G.; Nicolas, E.; Genard, M.; Vercambre, G.; Moitrier, N.; Valsesia, P.; Gonzalez-Real, M.M.; Bussi, C.; Lescourret, F. QualiTree, a virtual fruit tree to study the management of fruit quality. II. Parameterisation for peach, analysis of growth-related processes and agronomic scenarios. Trees-Struct. Funct. 2011, 25, 785–799. [Google Scholar] [CrossRef]
- Catalan, P.; Delgado, J.A.; Jimenez, M.D.; Balaguer, L. Sink strength manipulation in branches of a Mediterranean woody plant suggests sink-driven allocation of biomass in fruits but not of nutrients in seeds. Acta Physiol. Plant. 2016, 38, 1–6. [Google Scholar] [CrossRef]
- Marsal, J.; Basile, B.; Solari, L.; DeJong, T.M. Influence of branch autonomy on fruit, scaffold, trunk and root growth during Stage III of peach fruit development. Tree Physiol. 2003, 23, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, X. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.S.G. Arid Environments: Their Nature and Extent. In Arid Zone Geomorphology; Wiley: Hoboken, NJ, USA, 2011; pp. 1–16. [Google Scholar]
- Alae-Carew, C.; Nicoleau, S.; Bird, F.A.; Hawkins, P.; Tuomisto, H.L.; Haines, A.; Dangour, A.D.; Scheelbeek, P.F.D. The impact of environmental changes on the yield and nutritional quality of fruits, nuts and seeds: A systematic review. Environ. Res. Lett. 2020, 15, 023002. [Google Scholar] [CrossRef]
- Touil, S.; Richa, A.; Fizir, M.; Argente Garcia, J.E.; Skarmeta Gomez, A.F. A review on smart irrigation management strategies and their effect on water savings and crop yield. Irrig. Drain. 2022, 1–21. [Google Scholar] [CrossRef]
- Conesa, M.R.; de la Rosa, J.M.; Pablo Fernandez-Trujillo, J.; Domingo, R.; Perez-Pastor, A. Deficit irrigation in commercial mandarin trees: Water relations, yield and quality responses at harvest and after cold storage. Span. J. Agric. Res. 2018, 16, e1201. [Google Scholar] [CrossRef] [Green Version]
- Desoky, E.-S.M.; Mansour, E.; Yasin, M.A.T.; El-Sobky, E.-S.E.A.; Rady, M.M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Span. J. Agric. Res. 2020, 18, e0802. [Google Scholar] [CrossRef]
- Xu, H.-J.; Wang, X.-P.; Zhao, C.-Y. Drought sensitivity of vegetation photosynthesis along the aridity gradient in northern China. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102418. [Google Scholar] [CrossRef]
- Guizani, M.; Dabbou, S.; Maatallah, S.; Montevecchi, G.; Hajlaoui, H.; Rezig, M.; Helal, A.N.; Kilani-Jaziri, S. Physiological responses and fruit quality of four peach cultivars under sustained and cyclic deficit irrigation in center-west of Tunisia. Agric. Water Manag. 2019, 217, 81–97. [Google Scholar] [CrossRef]
- Toumi, I.; Ghrab, M.; Nagaz, K. Vegetative growth, yield, and water productivity of an early maturing peach cultivar under deficit irrigation strategies in a warm and arid area MOTS CLES. Irrig. Drain. 2022, 1–10. [Google Scholar] [CrossRef]
- Bandurska, H. Drought Stress Responses: Coping Strategy and Resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 13098–13103. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.J.; Veneklaas, E.J.; Lambers, H.; Burgess, S.S. Leaf water relations during summer water deficit: Differential responses in turgor maintenance and variation in leaf structure among different plant communities in south-western Australia. Plant Cell Environ. 2008, 31, 1791–1802. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Acevedo, E.; Fereres, E.; Henderson, D.W. Water Stress, Growth, and Osmotic Adjustment. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1976, 273, 479–500. [Google Scholar]
- Salmon, Y.; Lintunen, A.; Dayet, A.; Chan, T.; Dewar, R.; Vesala, T.; Holtta, T. Leaf carbon and water status control stomatal and nonstomatal limitations of photosynthesis in trees. New Phytol. 2020, 226, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Kang, S.; Wang, M.; Pang, X.; Wang, J.; Li, Z. Effect of regulated deficit irrigation on water use efficiency and fruit quality of pear-jujube tree in greenhouse. Trans. Chin. Soc. Agric. Eng. 2006, 22, 37–43. [Google Scholar]
- Ben Ahmed, C.; Ben Rouina, B.; Boukhris, M. Effects of water deficit on olive trees cv. Chemlali under field conditions in arid region in Tunisia. Sci. Hortic. 2007, 113, 267–277. [Google Scholar] [CrossRef]
- Mullet, J.E.; Whitsitt, M.S. Plant cellular responses to water deficit. Plant Growth Regul. 1996, 20, 119–124. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Meinzer, F.C.; Hao, G.-Y.; Scholz, F.G.; Bucci, S.J.; Takahashi, F.S.C.; Villalobos-Vega, R.; Giraldo, J.P.; Cao, K.-F.; Hoffmann, W.A.; et al. Size-dependent mortality in a Neotropical savanna tree: The role of height-related adjustments in hydraulic architecture and carbon allocation. Plant Cell Environ. 2009, 32, 1456–1466. [Google Scholar] [CrossRef]
- Ryan, M.G.; Phillips, N.; Bond, B.J. The hydraulic limitation hypothesis revisited. Plant Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The limits to tree height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef]
- McGregor, I.R.; Helcoski, R.; Kunert, N.; Tepley, A.J.; Gonzalez-Akre, E.B.; Herrmann, V.; Zailaa, J.; Stovall, A.E.L.; Bourg, N.A.; McShea, W.J.; et al. Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytol. 2021, 231, 601–616. [Google Scholar] [CrossRef]
- Fang, L.-D.; Ning, Q.-R.; Guo, J.-J.; Gong, X.-W.; Zhu, J.-J.; Hao, G.-Y. Hydraulic limitation underlies the dieback of Populus pseudo-simonii trees in water-limited areas of northern China. For. Ecol. Manag. 2020, 483, 118764. [Google Scholar] [CrossRef]
- Ambrose, A.R.; Sillett, S.C.; Dawson, T.E. Effects of tree height on branch hydraulics, leaf structure and gas exchange in California redwoods. Plant Cell Environ. 2009, 32, 743–757. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Pittermann, J.; Dawson, T.E. Hydraulic efficiency and safety of branch xylem increases with height in Sequoia sempervirens (D. Don) crowns. Plant Cell Environ. 2006, 29, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGranahan, G.; Leslie, C. Walnut. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer US: Boston, MA, USA, 2012; pp. 827–846. [Google Scholar]
- Liu, B.; Liang, J.; Zhao, D.; Wang, K.; Jia, M.; Wang, J. Morphological and Compositional Analysis of Two Walnut (Juglans regia L.) Cultivars Growing in China. Plant Foods Hum. Nutr. 2020, 75, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, P.; Woeste, K.E.; Chiocchini, F.; Olimpieri, I.; Tortolano, V.; Clark, J.; Hemery, G.E.; Mapelli, S.; Malvolti, M.E. Landscape genetics of Persian walnut (Juglans regia L.) across its Asian range. Tree Genet. Genomes 2014, 10, 1027–1043. [Google Scholar] [CrossRef]
- Beer, R.; Kaiser, F.; Schmidt, K.; Ammann, B.; Carraro, G.; Grisa, E.; Tinner, W. Vegetation history of the walnut forests in Kyrgyzstan (Central Asia): Natural or anthropogenic origin? Quat. Sci. Rev. 2008, 27, 621–632. [Google Scholar] [CrossRef]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Q.; Wang, G.; Ning, W. Effect of Soil Texture and Age on Soil Exchangeable Calcium and Magnesium in the Early Bearing Walnut Orchard. J. Agric. Sci. Technol. 2017, 19, 132–137. [Google Scholar]
- Shen, H.; Xu, S.-J.; Hong, L.; Wang, Z.-M.; Ye, W.-H. Growth but Not Photosynthesis Response of a Host Plant to Infection by a Holoparasitic Plant Depends on Nitrogen Supply. PLoS ONE 2013, 8, e75555. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.L.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Li, M.; Chen, Y.; Zhou, Q.; Liu, W.; Liang, G.; Jia, Z. Important Physiological Changes Due to Drought Stress on Oat. Front. Ecol. Evol. 2021, 9, 644726. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Water Relations. In Plant Physiological Ecology; Lambers, H., Chapin, F.S., Pons, T.L., Eds.; Springer New York: New York, NY, USA, 2008; pp. 163–223. [Google Scholar]
- Ackerly, D. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr. 2004, 74, 25–44. [Google Scholar] [CrossRef]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, L.; Tyree, M.T. 5-Leaf Hydraulics and Its Implications in Plant Structure and Function. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Academic Press: Burlington, NJ, USA, 2005; pp. 93–114. [Google Scholar]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Ishida, A.; Yamamura, Y.; Hori, Y. Roles of leaf water potential and soil-to-leaf hydraulic conductance in water use by understorey woody plants. Ecol. Res. 1992, 7, 213–223. [Google Scholar] [CrossRef]
- Lin, T.; Zheng, X.; Zheng, H. Seasonal variations in leaf and branch trace elements and the influence of a 3-yr 100% rainfall exclusion on Pinus massoniana Lamb. Peerj 2020, 8, e9935. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, R.; Ackerly, D.D. Ecological relevance of minimum seasonal water potentials. Physiol. Plant. 2006, 127, 353–359. [Google Scholar] [CrossRef]
- Yun, L.; Bi, H.; Ma, W.; Tian, X.; Cui, Z. Spatial distribution characteristics of root system of walnut trees in the walnut-peanut intercropping system in the loess region of western shanxi. J. Northeast For. Univ. 2010, 38, 67–70. [Google Scholar]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants- ngegative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.A. Adaptation of photosynthetic processes to stress. Science 1975, 188, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.A.; Schwartz, J.M.; Johnson, G.N. From empirical to theoretical models of light response curves-linking photosynthetic and metabolic acclimation. Photosynth. Res. 2020, 145, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Photosynthesis. In Plant Physiological Ecology; Lambers, H., Chapin, F.S., Pons, T.L., Eds.; Springer: New York, NY, USA, 2008; pp. 11–99. [Google Scholar]
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and future of genetic improvement. Tree Genet. Genomes 2018, 14, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos Guimaraes, Z.T.; Hardt Ferreira dos Santos, V.A.; Ferreira, M.J. Chlorophyll a fluorescence parameters are related to the leaf economics spectrum of tropical tree species in a mixed plantation. Trees-Struct. Funct. 2022, 36, 763–775. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Xu, G.-Q.; Li, Y.; Wu, X. Deepening Rooting Depths Improve Plant Water and Carbon Status of a Xeric Tree during Summer Drought. Forests 2019, 10, 592. [Google Scholar] [CrossRef] [Green Version]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Carter, J.L.; White, D.A. Plasticity in the Huber value contributes to homeostasis in leaf water relations of a mallee Eucalypt with variation to groundwater depth. Tree Physiol. 2009, 29, 1407–1418. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.-Q.; Mi, X.-J.; Ma, J.; Ma, J.; Tang, L.-S. Impact of groundwater depth on hydraulic performance and growth of Haloxylon ammodendron in a desert region of central Asia. Ecohydrology 2021, e2394. [Google Scholar] [CrossRef]
- Tsuji, C.; Dannoura, M.; Desalme, D.; Angeli, N.; Takanashi, S.; Kominami, Y.; Epron, D. Drought affects the fate of non-structural carbohydrates in hynoki cypress. Tree Physiol. 2022, 42, 784–796. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Feliz, L.; Baca-Bocanegra, B.; Hernández-Hierro, J.M.; Heredia, F.J.; Barroso, J.M.; Rato, A.E. Comparative study on the use of three different near infrared spectroscopy recording methodologies for varietal discrimination of walnuts. Talanta 2020, 206, 120189. [Google Scholar] [CrossRef]
- Estiarte, M.; Reixach, J.P.i. Excess carbon: The relationship with phenotypical plasticity in storage and defense functions of plants. Orsis Org. I Sist. 1999, 14, 159–203. [Google Scholar]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.K.; Pavao, G.C.; dos Santos Dias, C.T.; Quaggio, J.A.; de Matos Pires, R.C. Deficit irrigation effect on fruit yield, quality and water use efficiency: A long-term study on Pera-IAC sweet orange. Agric. Water Manag. 2020, 231, 106019. [Google Scholar] [CrossRef]
- Wagner, Y.; Pozner, E.; Bar-On, P.; Ramon, U.; Raveh, E.; Neuhaus, E.; Cohen, S.; Grünzweig, J.; Klein, T. Rapid stomatal response in lemon saves trees and their fruit yields under summer desiccation, but fails under recurring droughts. Agric. For. Meteorol. 2021, 307, 108487. [Google Scholar] [CrossRef]

| Indexes | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Correlation Degree | Correlation Order |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LSs | 0.82 | 0.98 | 0.53 | 0.87 | 0.58 | 0.76 | 0.58 | 0.70 | 0.54 | 0.71 | 4 |
| FSs | 0.57 | 0.76 | 0.90 | 0.60 | 0.84 | 0.81 | 0.70 | 0.60 | 0.88 | 0.74 | 2 |
| Ψmd | 0.86 | 0.59 | 0.84 | 0.93 | 0.90 | 0.86 | 0.85 | 0.84 | 0.85 | 0.84 | 1 |
| FW | 0.84 | 0.85 | 0.34 | 0.43 | 0.78 | 0.85 | 0.95 | 0.54 | 0.96 | 0.73 | 3 |
| FCD | 0.81 | 0.73 | 0.37 | 0.38 | 0.74 | 0.77 | 0.94 | 0.68 | 0.90 | 0.70 | 6 |
| FVD | 0.89 | 0.81 | 0.35 | 0.43 | 0.90 | 0.70 | 0.73 | 0.54 | 0.98 | 0.70 | 5 |
| LWC | 0.94 | 0.52 | 0.39 | 0.76 | 0.81 | 0.85 | 0.50 | 0.73 | 0.56 | 0.67 | 7 |
| gs | 0.57 | 0.38 | 0.53 | 0.49 | 0.79 | 0.78 | 0.80 | 0.63 | 0.57 | 0.61 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Xu, G.; Li, J.; Hu, H. Hydraulic Trait Variation with Tree Height Affects Fruit Quality of Walnut Trees under Drought Stress. Agronomy 2022, 12, 1647. https://doi.org/10.3390/agronomy12071647
Chen T, Xu G, Li J, Hu H. Hydraulic Trait Variation with Tree Height Affects Fruit Quality of Walnut Trees under Drought Stress. Agronomy. 2022; 12(7):1647. https://doi.org/10.3390/agronomy12071647
Chicago/Turabian StyleChen, Tuqiang, Guiqing Xu, Jinyao Li, and Haifang Hu. 2022. "Hydraulic Trait Variation with Tree Height Affects Fruit Quality of Walnut Trees under Drought Stress" Agronomy 12, no. 7: 1647. https://doi.org/10.3390/agronomy12071647
APA StyleChen, T., Xu, G., Li, J., & Hu, H. (2022). Hydraulic Trait Variation with Tree Height Affects Fruit Quality of Walnut Trees under Drought Stress. Agronomy, 12(7), 1647. https://doi.org/10.3390/agronomy12071647






