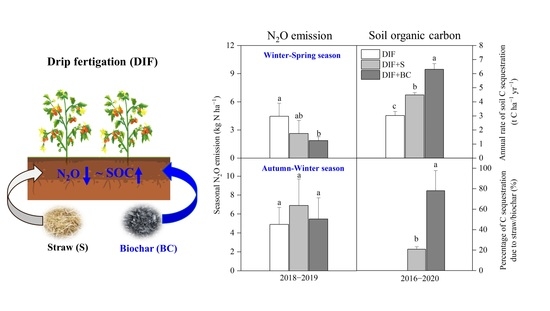
Increasing the Environmental Sustainability of Greenhouse Vegetable Production by Combining Biochar Application and Drip Fertigation—Effects on Soil N2O Emissions and Carbon Sequestrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site and Experiment Design
- (a)
- DIF: drip irrigation with reduced fertilization;
- (b)
- DIF+S: drip irrigation with reduced fertilization, plus the incorporation of maize straw;
- (c)
- DIF+BC: drip irrigation with reduced fertilization, plus the incorporation of biochar.
2.2. Measurement of Soil N2O Emission
2.3. Measurements of SOC and δ13C
2.4. Auxiliary Measurements
2.5. Calculation of Crop Performance and Environmental Parameters
2.6. Statistical Analyses
3. Results
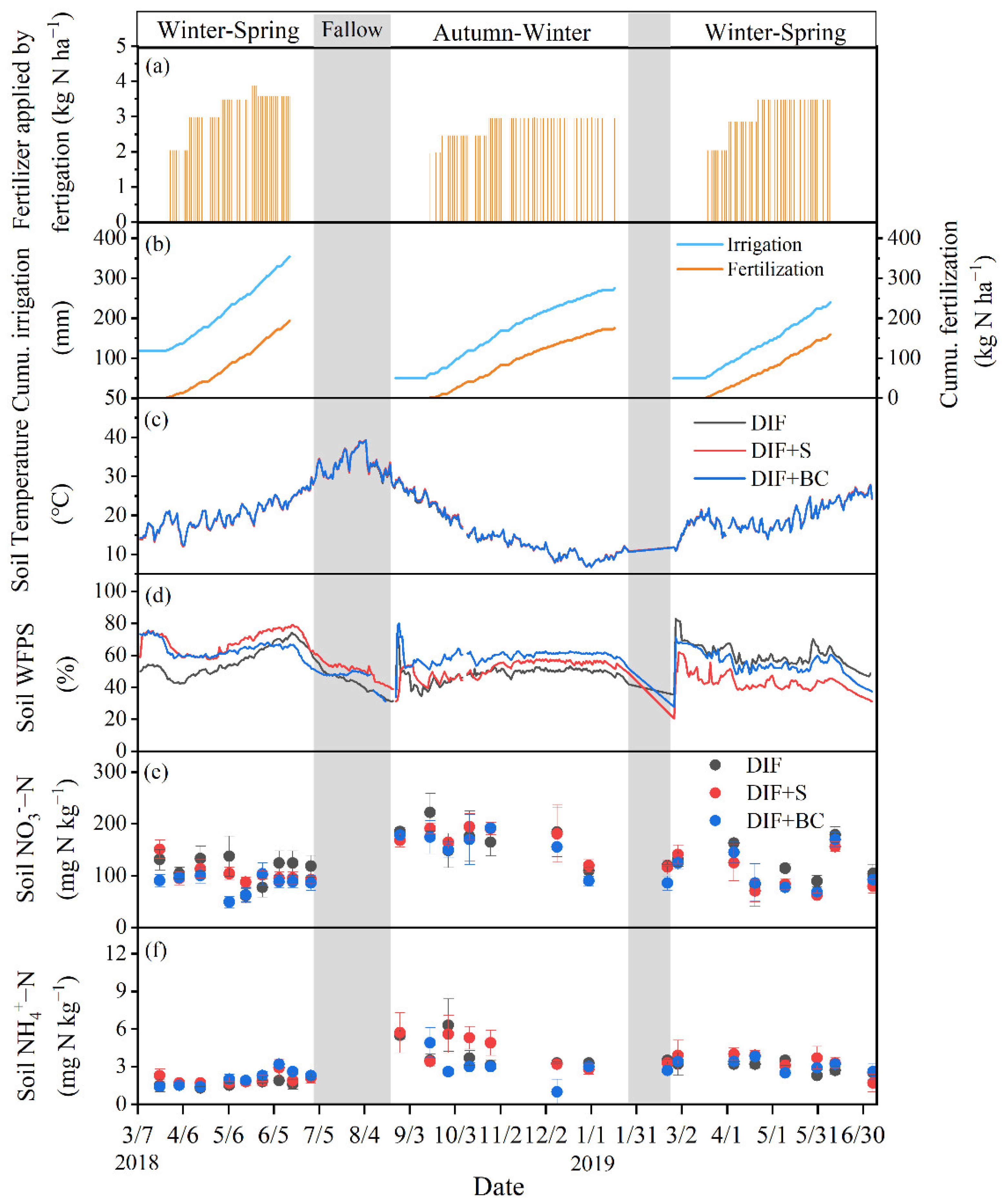
3.1. Soil Environmental Conditions
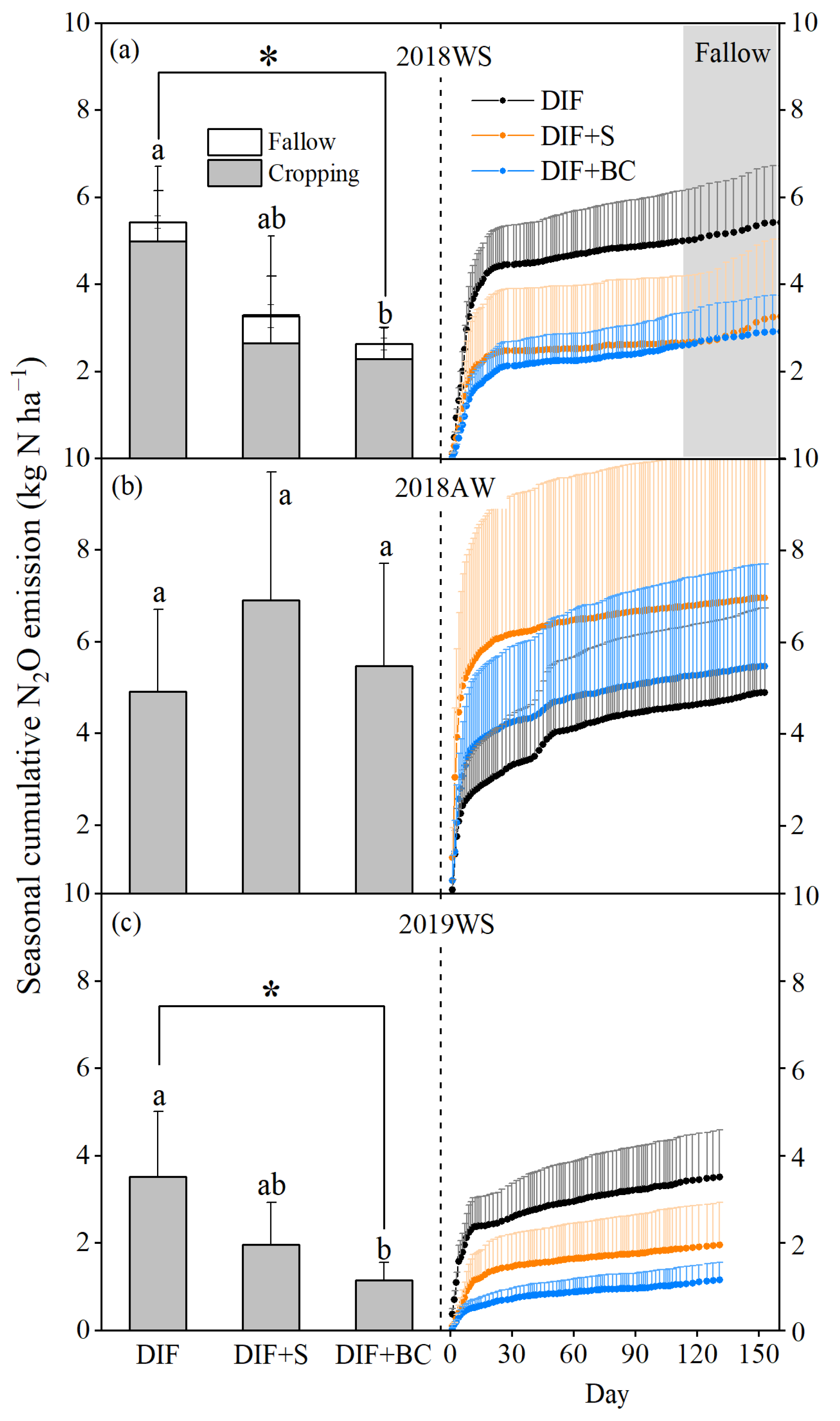
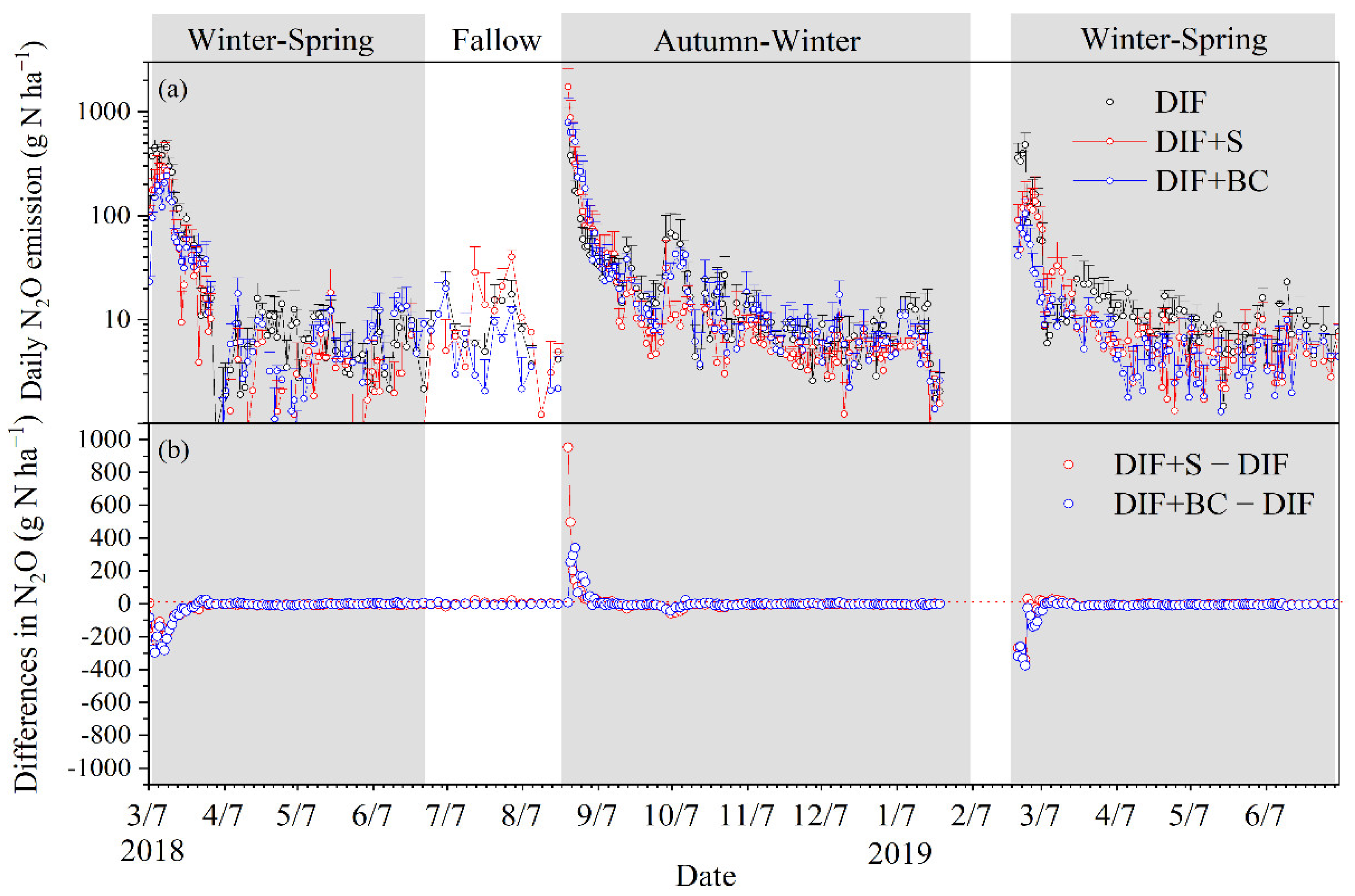
3.2. Seasonal Cumulative N2O Emission and Fluxes
3.3. Yield-Scaled N2O Emission and EFApparent
3.4. Changes in SOC Content
4. Discussion
4.1. Magnitude and Interrelationships of Soil N2O Emissions and N Productivity
4.2. Different Seasonal Responses of N2O Emissions to Biochar or Straw Incorporation
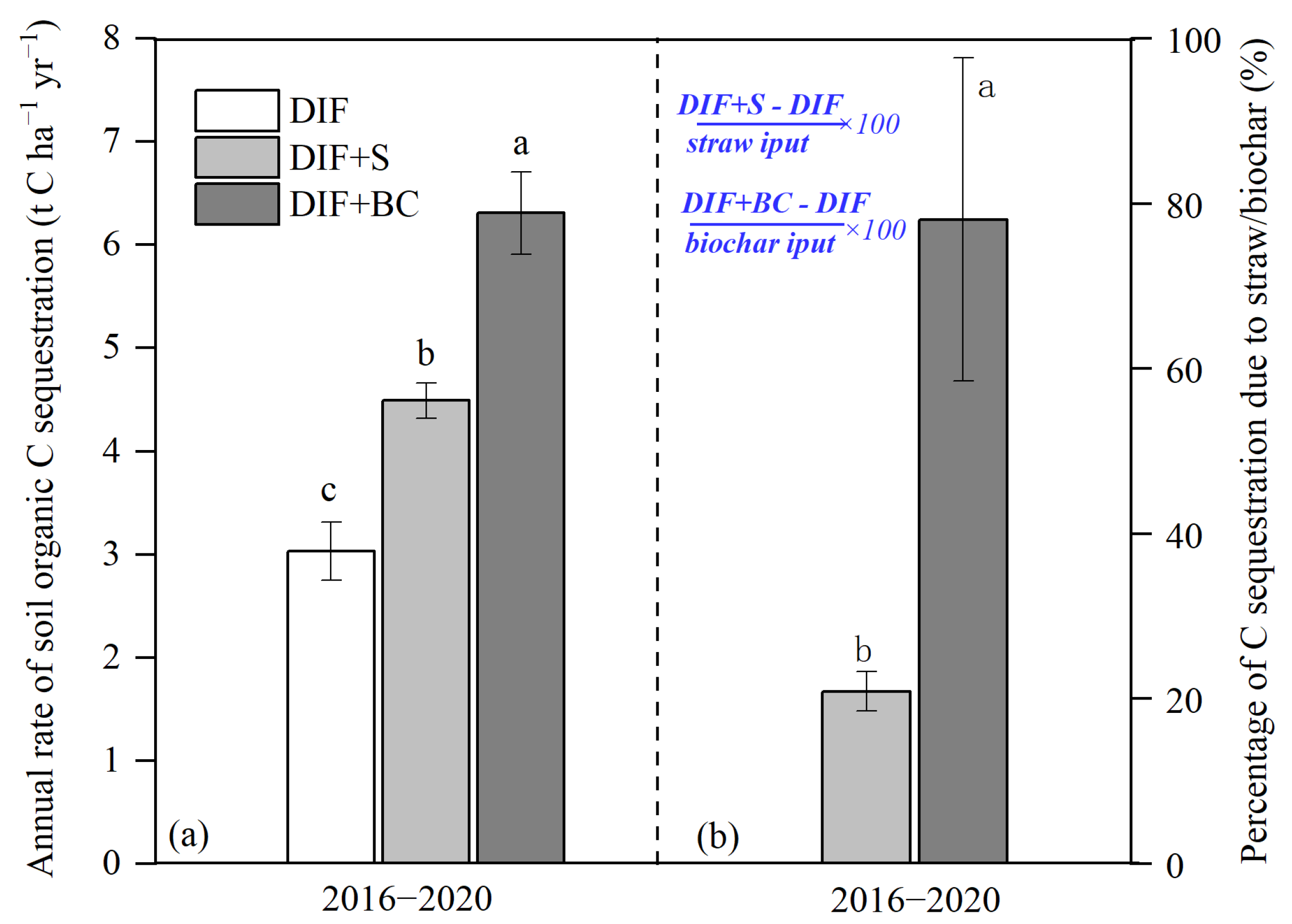
4.3. Changes in Rates of Soil C Sequestration Due to Additional C Inputs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Production: Crop. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 July 2020).
- Li, T. Development Strategy of Greenhouse Horticulture in China; China Science Publishing & Media Ltd.: Beijing, China, 2020. [Google Scholar]
- Li, J.; Liu, H.; Wang, H.; Luo, J.; Zhang, X.; Liu, Z.; Zhang, Y.; Zhai, L.; Lei, Q.; Ren, T.; et al. Managing Irrigation and Fertilization for the Sustainable Cultivation of Greenhouse Vegetables. Agric. Water Manag. 2018, 210, 354–363. [Google Scholar] [CrossRef]
- Lv, H.; Lin, S.; Wang, Y.; Lian, X.; Zhao, Y.; Li, Y.; Du, J.; Wang, Z.; Wang, J.; Butterbach-Bahl, K. Drip Fertigation Significantly Reduces Nitrogen Leaching in Solar Greenhouse Vegetable Production System. Environ. Pollut. 2019, 245, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Qasim, W.; Xia, L.; Lin, S.; Wan, L.; Zhao, Y.; Butterbach-Bahl, K. Global Greenhouse Vegetable Production Systems Are Hotspots of Soil N2O Emissions and Nitrogen Leaching: A Meta-Analysis. Environ. Pollut. 2021, 272, 116372. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, J.; Wang, S.; Cai, H.; Chen, Z.; Zhou, J. Excessive Nutrient Balance Surpluses in Newly Built Solar Greenhouses over Five Years Leads to High Nutrient Accumulations in Soil. Agric. Ecosyst. Environ. 2020, 288, 106717. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, Y.; Wang, Y.; Wan, L.; Wang, J.; Butterbach-Bahl, K.; Lin, S. Conventional Flooding Irrigation and over Fertilization Drives Soil PH Decrease Not Only in the Top- but Also in Subsoil Layers in Solar Greenhouse Vegetable Production Systems. Geoderma 2020, 363, 114156. [Google Scholar] [CrossRef]
- Ni, B.; Zhang, W.; Xu, X.; Wang, L.; Bol, R.; Wang, K.; Hu, Z.; Zhang, H.; Meng, F. Exponential Relationship between N2O Emission and Fertilizer Nitrogen Input and Mechanisms for Improving Fertilizer Nitrogen Efficiency under Intensive Plastic-Shed Vegetable Production in China: A Systematic Analysis. Agric. Ecosyst. Environ. 2021, 312, 107353. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Lv, H.; Bachelor, W.D.; Lian, X.; Wang, Z.; Hu, K. Simulating Nitrate and DON Leaching to Optimize Water and N Management Practices for Greenhouse Vegetable Production Systems. Agric. Water Manag. 2020, 241, 106377. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, W.; Dong, J.; He, S.; Chen, F.; Bi, M.; Wang, Q.; Li, J.; Liang, B. Irrigation Amount Dominates Soil Mineral Nitrogen Leaching in Plastic Shed Vegetable Production Systems. Agric. Ecosyst. Environ. 2021, 317, 107474. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Gao, X.; Guan, X.; Zhang, Y.; Shi, X.; Chen, X. Nitrate Leaching from Open-Field and Greenhouse Vegetable Systems in China: A Meta-Analysis. Environ. Sci. Pollut. Res. 2018, 25, 31007–31016. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Gao, X.; Guan, X.; Zhang, W.; Zhang, Y.; Shi, X.; Chen, X. Nitrous Oxide Emissions in Chinese Vegetable Systems: A Meta-Analysis. Environ. Pollut. 2018, 239, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yan, G.; Wang, R.; Zheng, X.; Liu, C.; Butterbach-Bahl, K. Drip Irrigation or Reduced N-Fertilizer Rate Can Mitigate the High Annual N2O + NO Fluxes from Chinese Intensive Greenhouse Vegetable Systems. Atmos. Environ. 2019, 212, 183–193. [Google Scholar] [CrossRef]
- Zhao, Y.; Lv, H.; Qasim, W.; Wan, L.; Wang, Y.; Lian, X.; Liu, Y.; Hu, J.; Wang, Z.; Li, G.; et al. Drip Fertigation with Straw Incorporation Significantly Reduces N2O Emission and N Leaching While Maintaining High Vegetable Yields in Solar Greenhouse Production. Environ. Pollut. 2021, 273, 116521. [Google Scholar] [CrossRef]
- Philibert, A.; Loyce, C.; Makowski, D. Quantifying Uncertainties in N2O Emission Due to N Fertilizer Application in Cultivated Areas. PLoS ONE 2012, 7, e50950. [Google Scholar] [CrossRef] [PubMed]
- Loveland, P. Is There a Critical Level of Organic Matter in the Agricultural Soils of Temperate Regions: A Review. Soil Tillage Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Wang, Y.; Dannenmann, M.; Lin, S.; Lv, H.; Li, G.; Lian, X.; Wang, Z.; Wang, J.; Butterbach-Bahl, K. Improving Soil Respiration While Maintaining Soil C Stocks in Sunken Plastic Greenhouse Vegetable Production Systems–Advantages of Straw Application and Drip Fertigation. Agric. Ecosyst. Environ. 2021, 316, 107464. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, S.; Li, J.; Liang, B.; Ding, X. Partial Substitution of Rice Husks for Manure in Greenhouse Vegetable Fields: Insight from Soil Carbon Stock and Aggregate Stability. Land Degrad. Dev. 2021, 32, 3962–3972. [Google Scholar] [CrossRef]
- Sierra, J.; Causeret, F.; Chopin, P. A Framework Coupling Farm Typology and Biophysical Modelling to Assess the Impact of Vegetable Crop-Based Systems on Soil Carbon Stocks. Application in the Caribbean. Agric. Syst. 2017, 153, 172–180. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng-Hua, L.; Zhong-Jian, P. Effect of Greenhouse Soil Management on Soil Aggregation and Organic Matter in Northeast China. Catena 2015, 133, 412–419. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Wang, F.; Shang, M. Design and Applied Research of an Intelligent Cooling Vetiliation Fan for Greenhouse. Shandong Agric. Sci. 2018, 50, 147–150. [Google Scholar]
- Dahri, I.A.; Tagar, A.A.; Adamowski, J.; Leghari, N.; Shah, A.R.; Soomro, S.A. Influence of Straw Incorporation-to-Planting Interval on Soil Physical Properties and Maize Performance. Int. Agrophysics 2018, 32, 341–348. [Google Scholar] [CrossRef]
- Huang, R.; Liu, J.; He, X.; Xie, D.; Ni, J.; Xu, C.; Zhang, Y.; Ci, E.; Wang, Z.; Gao, M. Reduced Mineral Fertilization Coupled with Straw Return in Field Mesocosm Vegetable Cultivation Helps to Coordinate Greenhouse Gas Emissions and Vegetable Production. J. Soils Sediments 2020, 20, 1834–1845. [Google Scholar] [CrossRef]
- Lou, Y.; Xu, M.; Wang, W.; Sun, X.; Liang, C. Soil Organic Carbon Fractions and Management Index after 20 Yr of Manure and Fertilizer Application for Greenhouse Vegetables: Soil Organic C and Fertilizer Use. Soil Use Manag. 2011, 27, 163–169. [Google Scholar] [CrossRef]
- Luan, H.; Gao, W.; Huang, S.; Tang, J.; Li, M.; Zhang, H.; Chen, X.; Masiliūnas, D. Organic Amendment Increases Soil Respiration in a Greenhouse Vegetable Production System through Decreasing Soil Organic Carbon Recalcitrance and Increasing Carbon-Degrading Microbial Activity. J. Soils Sediments 2020, 20, 2877–2892. [Google Scholar] [CrossRef]
- Poulton, P.; Johnston, J.; Macdonald, A.; White, R.; Powlson, D. Major Limitations to Achieving “4 per 1000” Increases in Soil Organic Carbon Stock in Temperate Regions: Evidence from Long-Term Experiments at Rothamsted Research, United Kingdom. Glob. Change Biol. 2018, 24, 2563–2584. [Google Scholar] [CrossRef] [Green Version]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous Oxide Emissions from Soils: How Well Do We Understand the Processes and Their Controls? Phil. Trans. R. Soc. B 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wu, F.; Jia, Z.; Wang, S.; Cai, Y.; Chang, S.X. Wheat Straw and Its Biochar Differently Affect Soil Properties and Field-Based Greenhouse Gas Emission in a Chernozemic Soil. Biol. Fertil. Soils 2020, 56, 1023–1036. [Google Scholar] [CrossRef]
- Guenet, B.; Gabrielle, B.; Chenu, C.; Arrouays, D.; Balesdent, J.; Bernoux, M.; Bruni, E.; Caliman, J.-P.; Cardinael, R.; Chen, S.; et al. Can N2O Emissions Offset the Benefits from Soil Organic Carbon Storage? Glob. Chang. Biol. 2021, 27, 237–256. [Google Scholar] [CrossRef]
- Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of Soil-Borne Diseases of Organic Vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, S.; Liu, Y.; Li, G.; Wang, J.; Butterbach-Bahl, K. Application of Mixed Straw and Biochar Meets Plant Demand of Carbon Dioxide and Increases Soil Carbon Storage in Sunken Solar Greenhouse Vegetable Production. Soil Use Manag. 2020, 36, 439–448. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Whitaker, J. The Effect of Biochar Addition on N2O and CO2 Emissions from a Sandy Loam Soil–The Role of Soil Aeration. Soil Biol. Biochem. 2012, 51, 125–134. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Munkholm, L.J.; Peltre, C.; Hauggaard-Nielsen, H.; Jensen, L.S. The Effect of Straw and Wood Gasification Biochar on Carbon Sequestration, Selected Soil Fertility Indicators and Functional Groups in Soil: An Incubation Study. Geoderma 2016, 269, 99–107. [Google Scholar] [CrossRef]
- Kang, S.-W.; Kim, S.-H.; Park, J.-H.; Seo, D.-C.; Ok, Y.S.; Cho, J.-S. Effect of Biochar Derived from Barley Straw on Soil Physicochemical Properties, Crop Growth, and Nitrous Oxide Emission in an Upland Field in South Korea. Env. Sci. Pollut. Res. 2018, 25, 25813–25821. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Ahmed, B.; Singh, V.; Mandzhieva, S.; Sushkova, S.; Bauer, T.; Verma, K.; Shan, S.; van Hullebusch, E.; et al. Nano-Biochar: A Novel Solution for Sustainable Agriculture and Environmental Remediation. Environ. Res. 2022, 210, 112891. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, Soil and Land-Use Interactions That Reduce Nitrate Leaching and N2O Emissions: A Meta-Analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s Role in Mitigating Soil Nitrous Oxide Emissions: A Review and Meta-Analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in Climate Change Mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, F.; Li, J.; Duan, Z.; Yang, F.; Li, X.; Lu, J.; Li, F. Effects of Maize Straw-Derived Biochar Application on Soil Temperature, Water Conditions and Growth of Winter Wheat. Eur. J. Soil Sci. 2019, 70, 1280–1289. [Google Scholar] [CrossRef]
- Obia, A.; Mulder, J.; Hale, S.E.; Nurida, N.L.; Cornelissen, G. The Potential of Biochar in Improving Drainage, Aeration and Maize Yields in Heavy Clay Soils. PLoS ONE 2018, 13, e0196794. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Wang, G.; Li, G.; Zhu, K. Implication of O2 Dynamics for Both N2O and CH4 Emissions from Soil during Biological Soil Disinfestation. Sci. Rep. 2021, 11, 6590. [Google Scholar] [CrossRef]
- Bai, S.H.; Reverchon, F.; Xu, C.-Y.; Xu, Z.; Blumfield, T.J.; Zhao, H.; van Zwieten, L.; Wallace, H.M. Wood Biochar Increases Nitrogen Retention in Field Settings Mainly through Abiotic Processes. Soil Biol. Biochem. 2015, 90, 232–240. [Google Scholar] [CrossRef]
- Teutscherova, N.; Houška, J.; Navas, M.; Masaguer, A.; Benito, M.; Vazquez, E. Leaching of Ammonium and Nitrate from Acrisol and Calcisol Amended with Holm Oak Biochar: A Column Study. Geoderma 2018, 323, 136–145. [Google Scholar] [CrossRef]
- Duan, P.; Zhang, X.; Zhang, Q.; Wu, Z.; Xiong, Z. Field-Aged Biochar Stimulated N2O Production from Greenhouse Vegetable Production Soils by Nitrification and Denitrification. Sci. Total Environ. 2018, 642, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, B.; Chen, Z.; Xie, Z.; Xiong, Z. Effects of Biochar Application on Vegetable Production and Emissions of N2O and CH4. Soil Sci. Plant Nutr. 2012, 58, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Huang, W.; Elsgaard, L.; Yang, B.; Li, Z.; Yang, H.; Lu, Y. Optimal Biochar Amendment Rate Reduced the Yield-Scaled N2O Emissions from Ultisols in an Intensive Vegetable Field in South China. Sci. Total Environ. 2020, 723, 138161. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and Denitrification in Soils: When, How Much and Why Does Biochar Reduce N2O Emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, G.L.; Livingston, G.P. Use of chamber systems to measure trace gas fluxes. In Agricultural Ecosystem Effects on Trace Gases and Global Climate Change; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1993; pp. 63–78. ISBN 978-0-89118-321-1. [Google Scholar]
- Jastrow, J.D.; Miller, R.M.; Boutton, T.W. Carbon Dynamics of Aggregate-Associated Organic Matter Estimated by Carbon-13 Natural Abundance. Soil Sci. Soc. Am. J. 1996, 60, 801–807. [Google Scholar] [CrossRef]
- Jia, J.; Sun, L.; Kong, X.; Yan, X.; Xiong, Z. Annual N2O and CH4 Emissions from Intensively Managed Vegetable Fields in Nanjing, China. Soil Sci. Plant Nutr. 2012, 58, 91–103. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Müller, C.; Cai, Z. The Mechanisms of High N2O Emissions from Greenhouse Vegetable Field Soils: Soil. Clean-Soil Air Water 2017, 45, 1600210. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Berube, P.M.; Urakawa, H.; de la Torre, J.R.; Stahl, D.A. Ammonia Oxidation Kinetics Determine Niche Separation of Nitrifying Archaea and Bacteria. Nature 2009, 461, 976–979. [Google Scholar] [CrossRef]
- Verhoeven, E.; Decock, C.; Barthel, M.; Bertora, C.; Sacco, D.; Romani, M.; Sleutel, S.; Six, J. Nitrification and Coupled Nitrification-Denitrification at Shallow Depths Are Responsible for Early Season N2O Emissions under Alternate Wetting and Drying Management in an Italian Rice Paddy System. Soil Biol. Biochem. 2018, 120, 58–69. [Google Scholar] [CrossRef]
- Qasim, W.; Zhao, Y.; Wan, L.; Lv, H.; Lin, S.; Gettel, G.M.; Butterbach-Bahl, K. The Potential Importance of Soil Denitrification as a Major N Loss Pathway in Intensive Greenhouse Vegetable Production Systems. Plant Soil 2022, 471, 157–174. [Google Scholar] [CrossRef]
- Burger, M.; Jackson, L.E.; Lundquist, E.J.; Louie, D.T.; Miller, R.L.; Rolston, D.E.; Scow, K.M. Microbial Responses and Nitrous Oxide Emissions during Wetting and Drying of Organically and Conventionally Managed Soil under Tomatoes. Biol. Fertil. Soils 2005, 42, 109–118. [Google Scholar] [CrossRef]
- Vico, A.; Sáez, J.A.; Pérez-Murcia, M.D.; Martinez-Tomé, J.; Andreu-Rodríguez, J.; Agulló, E.; Bustamante, M.A.; Sanz-Cobena, A.; Moral, R. Production of Spinach in Intensive Mediterranean Horticultural Systems Can Be Sustained by Organic-Based Fertilizers without Yield Penalties and with Low Environmental Impacts. Agric. Syst. 2020, 178, 102765. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Dannenmann, M. Denitrification and Associated Soil N2O Emissions Due to Agricultural Activities in a Changing Climate. Curr. Opin. Environ. Sustain. 2011, 3, 389–395. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, B.; Hu, W.; Weindorf, D.C.; Yang, L. Environmental Assessment of Closed Greenhouse Vegetable Production System in Nanjing, China. J. Soils Sediments 2013, 13, 1418–1429. [Google Scholar] [CrossRef]
- Khalil, M.I.; Baggs, E.M. CH4 Oxidation and N2O Emissions at Varied Soil Water-Filled Pore Spaces and Headspace CH4 Concentrations. Soil Biol. Biochem. 2005, 37, 1785–1794. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, G.; Zheng, X.; Wang, R.; Liu, C.; Butterbach-Bahl, K. Straw Return Reduces Yield-Scaled N2O plus NO Emissions from Annual Winter Wheat-Based Cropping Systems in the North China Plain. Sci. Total Environ. 2017, 590, 174–185. [Google Scholar] [CrossRef]
- Fan, C.; Chen, H.; Li, B.; Xiong, Z. Biochar Reduces Yield-Scaled Emissions of Reactive Nitrogen Gases from Vegetable Soils across China. Biogeosciences 2017, 14, 2851–2863. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-I.; Park, H.-J.; Jeong, Y.-J.; Seo, B.-S.; Kwak, J.-H.; Yang, H.I.; Xu, X.; Tang, S.; Cheng, W.; Lim, S.-S.; et al. Biochar-Induced Reduction of N2O Emission from East Asian Soils under Aerobic Conditions: Review and Data Analysis. Environ. Pollut. 2021, 291, 118154. [Google Scholar] [CrossRef]
- Bamminger, C.; Poll, C.; Marhan, S. Offsetting Global Warming-Induced Elevated Greenhouse Gas Emissions from an Arable Soil by Biochar Application. Glob. Change Biol. 2018, 24, e318–e334. [Google Scholar] [CrossRef]
- Lin, Y.; Ding, W.; Liu, D.; He, T.; Yoo, G.; Yuan, J.; Chen, Z.; Fan, J. Wheat Straw-Derived Biochar Amendment Stimulated N2O Emissions from Rice Paddy Soils by Regulating the AmoA Genes of Ammonia-Oxidizing Bacteria. Soil Biol. Biochem. 2017, 113, 89–98. [Google Scholar] [CrossRef]
- Zavalloni, C.; Alberti, G.; Biasiol, S.; Vedove, G.D.; Fornasier, F.; Liu, J.; Peressotti, A. Microbial Mineralization of Biochar and Wheat Straw Mixture in Soil: A Short-Term Study. Appl. Soil Ecol. 2011, 50, 45–51. [Google Scholar] [CrossRef]
- Akiyama, H.; Yamamoto, A.; Uchida, Y.; Hoshino, Y.T.; Tago, K.; Wang, Y.; Hayatsu, M. Effect of Low C/N Crop Residue Input on N2O, NO, and CH4 Fluxes from Andosol and Fluvisol Fields. Sci. Total Environ. 2020, 713, 136677. [Google Scholar] [CrossRef] [PubMed]
- Harter, J.; Krause, H.-M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O Emissions from Biochar-Amended Soil to the Structure and Function of the N-Cycling Microbial Community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The Mechanisms of Biochar Interactions with Microorganisms in Soil. Env. Geochem Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, H.; Zheng, H.; Liu, G.; Chen, L.; Xing, B. Reduced Nitrification and Abundance of Ammonia-Oxidizing Bacteria in Acidic Soil Amended with Biochar. Chemosphere 2015, 138, 576–583. [Google Scholar] [CrossRef]
- Cui, P.; Fan, F.; Yin, C.; Song, A.; Huang, P.; Tang, Y.; Zhu, P.; Peng, C.; Li, T.; Wakelin, S.A.; et al. Long-Term Organic and Inorganic Fertilization Alters Temperature Sensitivity of Potential N2O Emissions and Associated Microbes. Soil Biol. Biochem. 2016, 93, 131–141. [Google Scholar] [CrossRef]
- He, T.; Yuan, J.; Luo, J.; Wang, W.; Fan, J.; Liu, D.; Ding, W. Organic Fertilizers Have Divergent Effects on Soil N2O Emissions. Biol. Fertil. Soils 2019, 55, 685–699. [Google Scholar] [CrossRef]
- Brettar, I.; Sanchez-Perez, J.-M.; Tremolieres, M. Nitrate Elimination by Denitrification in Hardwood Forest Soils of the Upper Rhine FLoodplain–Correlation with Redox Potential and Organic Matter. Hydrobiologia 2002, 469, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Chapman, S.J.; Li, Y.; Yao, H. Straw Amendment to Paddy Soil Stimulates Denitrification but Biochar Amendment Promotes Anaerobic Ammonia Oxidation. J. Soils Sediments 2017, 17, 2428–2437. [Google Scholar] [CrossRef]
- Wei, Z.; Shan, J.; Chai, Y.; Well, R.; Yan, X.; Senbayram, M. Regulation of the Product Stoichiometry of Denitrification in Intensively Managed Soils. Food Energy Secur. 2020, 9, e251. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Rennhofer, H.; Tintner, J.; Watzinger, A.; Soja, G.; Lichtenegger, H.; Zehetner, F. Changes in Biochar Physical and Chemical Properties: Accelerated Biochar Aging in an Acidic Soil. Carbon 2017, 115, 209–219. [Google Scholar] [CrossRef]
- Yang, Y.; Meehan, B.; Shah, K.; Surapaneni, A.; Hughes, J.; Fouché, L.; Paz-Ferreiro, J. Physicochemical Properties of Biochars Produced from Biosolids in Victoria, Australia. Int. J. Environ. Res. Public Health 2018, 15, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancinelli, R.; Marinari, S.; Brunetti, P.; Radicetti, E.; Campiglia, E. Organic Mulching, Irrigation and Fertilization Affect Soil CO2 Emission and C Storage in Tomato Crop in the Mediterranean Environment. Soil Tillage Res. 2015, 152, 39–51. [Google Scholar] [CrossRef]
- Farina, R.; Testani, E.; Campanelli, G.; Leteo, F.; Napoli, R.; Canali, S.; Tittarelli, F. Potential Carbon Sequestration in a Mediterranean Organic Vegetable Cropping System. A Model Approach for Evaluating the Effects of Compost and Agro-Ecological Service Crops (ASCs). Agric. Syst. 2018, 162, 239–248. [Google Scholar] [CrossRef]
- Smith, P. Soil Carbon Sequestration and Biochar as Negative Emission Technologies. Glob. Chang. Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef]
- Jatav, H.S.; Rajput, V.D.; Minkina, T.; Singh, S.K.; Chejara, S.; Gorovtsov, A.; Barakhov, A.; Bauer, T.; Sushkova, S.; Mandzhieva, S.; et al. Sustainable Approach and Safe Use of Biochar and Its Possible Consequences. Sustainability 2021, 13, 10362. [Google Scholar] [CrossRef]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of Long-term Straw Return on Soil Organic Carbon Storage and Sequestration Rate in North China Upland Crops: A Meta-analysis. Glob. Chang. Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Gupta, R.K.; Panwar, A.S.; Mahajan, N.C.; Singh, R.; Mandal, A. Effect of Tillage and Straw Return on Carbon Footprints, Soil Organic Carbon Fractions and Soil Microbial Community in Different Textured Soils under Rice–Wheat Rotation: A Review. Rev. Environ. Sci. Biotechnol. 2020, 19, 103–115. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar Amendment Techniques for Upland Rice Production in Northern Laos. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable Biochar to Mitigate Global Climate Change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Characteristics of experimental site | |
| Site | Wuqin District, Tianjin |
| Coordinates | 39°25′35′′ N, 116°57′18′′ E |
| Climate | Warm temperate monsoon climate |
| Mean annual precipitation (mm) | 532 (1981–2020) |
| Mean annual air temperature (°C) | 13.5 (1981–2020) |
| Annual sunshine duration (h) | 2392 (Meteorological Observation Station) |
| Characteristics of experimental soil | |
| Soil type | Aquic cambisols (FAO classification) |
| Soil texture | Silty loam |
| Particle fraction (%) | |
| Sand (20–2000 µm) | 30 ± 1.40 |
| Silt (2–20 µm) | 62 ± 1.50 |
| Clay (<2 µm) | 8 ± 0.26 |
| Bulk density (g cm−3) | 1.34 ± 0.02 |
| Soil organic carbon (g kg−1) | 17.3 ± 1.31 |
| pH | 8.6 ± 0.05 |
| Electric conductivity (µs cm−1) | 370 ± 21 |
| Total C | Total N | δ13C | Incorporation Rate (kg C or N ha−1 Season−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DIF | DIF+S | DIF+BC | |||||||
| Organic Matter | % | % | ‰ | C | N | C | N | C | N |
| Chicken manure | 13.2 ± 0.06 | 1.45 ± 0.01 | −21.83 ± 0.061 | 1821 | 200 | 1821 | 200 | 1821 | 200 |
| Maize straw | 41.6 ± 0.10 | 0.96 ± 0.01 | −14.18 ± 0.055 | - | - | 3500 | 80.8 | - | - |
| Biochar | 54.8 ± 0.10 | 1.08 ± 0.01 | −24.37 ± 0.019 | - | - | - | - | 2100 | 41.4 |
| 2018WS | 2018AW | 2019WS | 2018–2019 | |
|---|---|---|---|---|
| Yield-scaled N2O emission (g N2O-N Mg−1) | ||||
| DIF | 51.8 ± 8.2 a | 63.6 ± 14 a | 46.7 ± 15 a | 56.6 ± 9.4 a |
| DIF+S | 33.0 ± 7.7 ab | 75.3 ± 26 a | 25.5 ± 12 ab | 52.4 ± 17 a |
| DIF+BC | 24.6 ± 1.1 b | 64.8 ± 13 a | 13.0 ± 0.91 b | 40.5 ± 5.4 a |
| Apparent N2O emission factor (%) | ||||
| DIF | 1.38 ± 0.22 a | 1.30 ± 0.28 a | 1.46 ± 0.48 a | 1.22 ± 0.16 a |
| DIF+S | 0.83 ± 0.19 ab | 1.61 ± 0.55 a | 0.81 ± 0.38 ab | 1.00 ± 0.29 a |
| DIF+BC | 0.67 ± 0.03 b | 1.46 ± 0.30 a | 0.48 ± 0.032 b | 0.82 ± 0.041a |
| Tomato yield (Mg fresh fruit ha−1) | ||||
| DIF | 105 ± 2.0 a | 76.7 ± 3.9 a | 75.3 ± 3.3 b | 167 ± 4.5 b |
| DIF+S | 99.4 ± 13 a | 80.3 ± 5.2 a | 76.8 ± 5.6 ab | 176 ± 2.3 a |
| DIF+BC | 107 ± 5.6 a | 84.5 ± 12 a | 88.3 ± 2.8 a | 182 ± 15 a |
| PFPN (kg fresh fruit kg−1 N) | ||||
| DIF | 266 ± 5.0 a | 204 ± 10 a | 210 ± 9.2 b | 222 ± 3.3 a |
| DIF+S | 252 ± 34 a | 213 ± 14 a | 214 ± 16 ab | 224 ± 19 a |
| DIF+BC | 271 ± 14 a | 225 ± 33 a | 246 ± 7.8 a | 242 ± 19 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Lin, S.; Lv, H.; Wang, Y.; Hu, J.; Li, G.; Butterbach-Bahl, K. Increasing the Environmental Sustainability of Greenhouse Vegetable Production by Combining Biochar Application and Drip Fertigation—Effects on Soil N2O Emissions and Carbon Sequestrations. Agronomy 2022, 12, 1661. https://doi.org/10.3390/agronomy12071661
Zhao Y, Lin S, Lv H, Wang Y, Hu J, Li G, Butterbach-Bahl K. Increasing the Environmental Sustainability of Greenhouse Vegetable Production by Combining Biochar Application and Drip Fertigation—Effects on Soil N2O Emissions and Carbon Sequestrations. Agronomy. 2022; 12(7):1661. https://doi.org/10.3390/agronomy12071661
Chicago/Turabian StyleZhao, Yiming, Shan Lin, Haofeng Lv, Yafang Wang, Jing Hu, Guoyuan Li, and Klaus Butterbach-Bahl. 2022. "Increasing the Environmental Sustainability of Greenhouse Vegetable Production by Combining Biochar Application and Drip Fertigation—Effects on Soil N2O Emissions and Carbon Sequestrations" Agronomy 12, no. 7: 1661. https://doi.org/10.3390/agronomy12071661
APA StyleZhao, Y., Lin, S., Lv, H., Wang, Y., Hu, J., Li, G., & Butterbach-Bahl, K. (2022). Increasing the Environmental Sustainability of Greenhouse Vegetable Production by Combining Biochar Application and Drip Fertigation—Effects on Soil N2O Emissions and Carbon Sequestrations. Agronomy, 12(7), 1661. https://doi.org/10.3390/agronomy12071661