Abstract
The cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) is a significant pest in the world and it was identified in Brazil in 2013, causing severe economic losses. Recent studies showed a significant decrease in the susceptibility of H. armigera to diamide insecticides in Brazil. Understanding the genetic basis and mechanisms of the resistance are essential to develop proactive resistance management strategies. A laboratory strain of H. armigera resistant to the phthalic acid diamide flubendiamide (Flub-R) was selected from a field-collected population to characterize the resistance. The resistance ratio of the Flub-R strain was >50,000-fold. The inheritance pattern of the resistance was characterized as an autosomal dominant trait. Flub-R showed no cross-resistance to the anthranilic diamides chlorantraniliprole, cyantraniliprole or cyclaniliprole. Susceptible strain larvae that fed on flubendiamide-treated soybean leaves at field-recommended rates were killed while heterozygotes and Flub-R larvae showed a high survival and no reduction in the leaf consumption, confirming the functional dominance of the resistance. No indication of metabolic resistance was detected. The partial sequencing of ryanodine receptor (RyR) genes covering the transmembrane II to VI did not show any amino acid mutations, indicating the presence of a non-common resistance mechanism to diamide insecticides in the Flub-R strain.
1. Introduction
Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) is a severe agricultural pest in the world and this pest was officially identified in Brazil in 2013 [1,2]. This species is widely distributed in the world [3,4], and besides Brazil, it was reported in many other countries of the Americas such as Argentina [5], Paraguay [6], Uruguay [6,7], and the United States of America [8]. H. armigera has a high capacity of dispersion, reproduction, and a wide range of plant hosts [3,9,10]. These characteristics favor the evolution of resistance due to intense selection pressure with the use of insecticides, mainly under tropical agricultural production systems with successive crop cultivation throughout the year. In the Michigan Resistance Database, 891 cases of H. armigera resistant to 48 different active ingredients were reported in the world [11], however, so far there is no case of resistance to diamide insecticides.
The diamide insecticides act as ryanodine receptor modulators (IRAC MoA group 28). This chemical class is divided into two subclasses: the phthalic acid, including flubendiamide, and the anthranilic acid, including chlorantraniliprole, cyantraniliprole, and cyclaniliprole. The first class discovered was phthalic acid, developed in the 90s by Nihon Nohyaku Co. Ltd. (Tokyo, Japan) Later, in collaboration with Bayer Crop Science, it resulted in the development of flubendiamide, a molecule with high insecticidal activity on lepidopteran pests, launched in the market in 2007 [12]. This insecticide class affects the homeostasis of calcium (Ca2+) into the muscle cells by binding to ryanodine receptors (RyR). The calcium channels are kept open, and uncontrolled calcium efflux from the sarcoplasmic reticulum stores of muscle cells results in paralysis due to muscle contraction, cessation of feeding, and consequently insect death [13,14].
The resistance to diamide insecticides is frequently associated with amino acid alteration in the region C-terminal, which contains the transmembrane I to VI of the ryanodine receptors (RyR) [14,15]. These alterations promote different resistance magnitudes, and they may be associated with one or more mutations in the ryanodine receptor gene [15]. Some of these mutations can confer cross-resistance between diamide subclasses, such as G4946E [16] and I4790M [17,18]. Moreover, other mechanisms can lead to insect resistance to diamides, such as the enzymatic detoxification by cytochromes P450 (P450), carboxylesterases (CE), and glutathione S-transferases (GST) [15]. Resistance to flubendiamide has already been detected in major lepidopteran pests, with cross-resistance to other diamide insecticides in most cases [15,19,20,21,22].
Because of intense use of insecticides to control H. armigera in Brazil, a significant decrease in the susceptibility to diamide insecticides was recently reported [23]. Thus, to evaluate the resistance risk evolution to diamide insecticides and implement Insect Resistance Management (IRM) strategies, we selected a laboratory strain of H. armigera resistant to the phthalic acid diamide flubendiamide from a field-collected population to (i) characterize the genetic basis of the resistance of H. armigera to flubendiamide; (ii) evaluate cross-resistance between flubendiamide and the anthranilic diamides chlorantraniliprole, cyantraniliprole, and cyclaniliprole; (iii) assess functional dominance based on the survival and leaf consumption of larvae of different genotypes (flubendiamide-resistant, heterozygous, and susceptible) on flubendiamide-treated soybean leaves; (iv) evaluate possible resistance mechanisms by conducting synergist tests and sequencing the main region of the ryanodine receptor (RyR) gene responsible for diamide resistance in other insect pests.
2. Materials and Methods
2.1. Insects
The susceptible reference strain (SUS) was obtained in September 2013, a few months after the first report of H. armigera in Brazil. Approximately 1000 insects were collected in the field from a dry bean crop at the municipality of Luiz Eduardo Magalhães, Bahia state, Brazil (12°05′58″ S and 45°47′54″ W). The SUS strain was reared in the absence of selection pressure with insecticides since 2013. The flubendiamide-resistant strain (Flub-R) was selected from a field-collected population in a soybean crop in January 2016 from the same location of our SUS strain. The Flub-R strain was obtained by selecting surviving larvae at the diagnostic dose (2.64 μg a.i. cm−2) of flubendiamide (Belt®, BayerS.A., São Paulo, SP, Brazil 480 g a.i. L−1) [23] for seven generations, and then at 157.89 μg a.i. cm−2 for an additional seven generations.
Larvae of both strains were fed with an artificial diet based on beans, brewer’s yeast, soy protein, wheat germ, and casein [24]. The adults were maintained in PVC cages closed at the top with fabric for egg-laying. The fabric-containing eggs was replaced every two days. Newly hatched larvae were transferred to 100 mL plastic cups containing an artificial diet. The insects were maintained under controlled conditions (25 ± 1 °C, 70 ± 10% relative humidity, and photophase of 14 h) in all development stages.
2.2. Characterization of Flubendiamide Resistance
Third-instar larvae from SUS and Flub-R strains were subjected to dose–response bioassays with the insecticide flubendiamide (Belt®, Bayer S.A.; 480 g a.i. L−1). Seven to nine logarithmically distributed doses were tested, between 0.003 to 0.088 μg a.i. cm−2 for susceptible strains, and at 15.79 to 5052.63 μg a.i. cm−2 for resistant strains, with larval mortality from 5 to 95%. To obtain these doses the insecticide was diluted in distilled water and added with the surfactant Triton® at 0.1% to obtain a uniform solution spread over the diet surface. The control treatment consisted of distilled water and Triton® only. The ingestion bioassays were performed in 24-well acrylic plates (Costar®, Washington, DC, USA) with 1.25 mL of artificial diet per well (area of 1.9 cm2). An amount of 30 μL of the insecticide solution was added per well by using an electronic repeating pipette. After drying out, one larva was added to each well, and at least four replicates with 24 larvae each were tested per dose. The trays were sealed with their covers and kept under controlled conditions (25 ± 1 °C, 70 ± 10% relative humidity, and photophase of 14 h). Mortality was evaluated at 96 h after transferring the larvae in the bioassay plates by considering the ones that did not show coordinated movements when probing with fine paint brush to be dead.
2.3. Inheritance of Flubendiamide Resistance
Reciprocal crosses between susceptible and resistant strains were performed to generate the heterozygotes, H1 (♀ SUS × ♂ Flub-R) and H2 (♀ Flub-R × ♂ SUS), using 20 individuals of each strain. Dose–mortality bioassays were conducted with the third instar larvae from the F1 progeny. The larvae were exposed to eight doses of flubendiamide spaced on a logarithmic scale, between 15.79 to 5052.63 µg a.i. cm−2 of flubendiamide. Dose–mortality bioassays were subjected as outlined in the characterization of flubendiamide resistance.
2.4. Dominance of Resistance
Dose–mortality data outlined in Section 2.2 were used. According to Stone et.al. method [25], the average degree of dominance is given by the equation:
where the coefficients XF, XR, and XS are logarithms of the lethal dose that kills 50% of larvae (LD50), which were estimated for the heterozygotes, Flub-R, and SUS strains, respectively. For the values of dominance (D): D = 1 indicates completely dominant, 0 < D < 1 indicates incompletely dominant, −1 < D < 0 indicates incompletely recessive, and D = −1 indicates completely recessive. The standard error (SE) of D was calculated based on Lehmann and Roman [26], as described by Robertson et al. [27].
D = (2XF − XR − XS)/(XR − XS)
The dominance of resistance was also analyzed using the method proposed by Bourguet et.al. [28] given by Equation (2), where dominance was calculated to each tested dose.
where MLRR, MLSS, and MLRS represent the mortality of the resistant, susceptible, and heterozygote strains to each dose tested, respectively. The values of effective dominance (DML) are: DML = 1 indicates completely dominant, DML close to 1 indicates incompletely dominant, close to 0 indicates incompletely recessive, and DML = 0 indicates completely recessive.
DML = (MLRS − MLSS)/(MLRR − MLSS)
2.5. Functional Dominance of Resistance and Leaf Consumption
Susceptible (SUS), flubendiamide-resistant (Flub-R) strains, and heterozygotes H1 (♀ SUS × ♂ Flub-R) and H2 (♀ Flub-R × ♂ SUS) were used to assess the functional dominance. The strains were evaluated on untreated or flubendiamide-treated soybean leaves at a concentration of 0.336 μg a.i. mL−1 (recommended field concentration for soybean). The bioassay method was a leaf dip for five seconds. The treated leaf was transferred to a Petri dish, and three third-instar larvae were carefully placed over the treated leaf and then covered with the lid. A Petri dish with three third-instar larvae was a replicate. Each treatment was replicated 10 times, totaling 30 larvae per treatment. After 96 h, the larval survival was evaluated by touching the larvae with a fine paint brush. Larvae with coordinated movements were considered to be alive. Leaf consumption (cm2) was assessed with the average consumption of the three larvae per replicate. The leaves of treatments were digitalized by a scanner, and the consumed area was estimated using ImageJ software [29].
2.6. Cross Resistance
Third-instar larvae from H. armigera-susceptible and flubendiamide-resistant strains were used in dose–mortality bioassays with the commercial insecticides chlorantraniliprole (Premio®, FMC, Campinas, SP, Brazil; 200 g a.i. L−1), cyantraniliprole (Benevia®, FMC; 100 g a.i. L−1) and cyclaniliprole (Goemon®, ISK, Indaiatuba, SP, Brazil; 50 g a.i. L−1) to establish a cross-resistance profile. Susceptible and resistant flubendiamide strains were exposed to several doses of chlorantraniliprole (0.002 to 0.884 μg a.i. cm−2), cyantraniliprole (0.005 to 1.579 μg a.i. cm−2) and cyclaniliprole (0.0003 to 0.03 μg a.i. cm−2). At least four replicates with 24 larvae were tested per dose. Dose–mortality bioassays were obtained as previously described in Section 2.2.
2.7. Synergist Bioassays
Dose–response bioassays were performed on third-instar larvae of Flub-R strains. Artificial diet–overlay dose assays were conducted in 12-well plates as described in Section 2.2, with a dose near to LD50 of Flub-R strain of 505.28 μg a.i. cm−2. The larvae were topically applied with 1 µL acetonic solutions of piperonyl butoxide (PBO, Sigma-Aldrich, St. Louis, MO, USA, 90%), diethyl maleate (DEM, Sigma-Aldrich, 97%) and S,S,S-tributyl phosphorotrithioate (DEF, Chem Service, West Chester, PA, USA, 97.2%) two hours prior to insecticide exposure. Synergist solutions were prepared in acetone (Labsynth, Diadema, SP, Brazil, 100%) and applied onto the larval pronotum using a micro applicator (Burkard Manufacturing CO, Rickmansworth, UK). Synergists PBO, DEM and DEF were applied at the dose of 1 μg a.i. larva−1. Acetone alone served as solvent control. The highest non-lethal concentration of each synergist was established in preliminary bioassays. Four replicates of approximately 12 larvae were used for each treatment, totaling 48 larvae. After infestation, the larvae were kept under controlled conditions (25 ± 1 °C, 70 ± 10% relative humidity, and photophase of 14 h). Larvae were scored for mortality after 96 h.
2.8. Sequencing Partial RyR
Total RNA was extracted from the thorax of H. armigera adults of the SUS and Flub-R strain. Ribozol™ RNA Extraction Reagent (VWR Life Science, Radnor, PA, USA), according to its protocol, was used for RNA separation, followed by DNase I purification (Thermo Scientific, Waltham, MA, USA). The purified RNA was quantified by spectrophotometer (Epoch, Ellicott City, MD, USA) and its integrity was assessed by 1.5% agarose gel electrophoresis. The RNA was normalized to 500 ng µL−1, and 1 µg total RNA was used in a 20 µL reaction for cDNA synthesis using GoScript™ Reverse Transcriptase and oligo (dT) (Promega, Madison, WI, USA), according to the manufacturer’s instructions.
Primer pairs were designed manually based on the cDNA sequences of the RyR of H. armigera (GenBank KF641862.1 and KJ573634.1) covering the transmembrane II to VI (Table 1). Primer pairs were used to amplify fragments, overlapping each other, and covering the 1553 bp H. armigera RyR fragment. The PCR reaction contained 100 ng of cDNA, 500 nM of each primer, 10 mM Tris-Cl+ 50 mM KCl (PCR buffer 10x, Sinapse), 1.5 mM MgCl2, 0.2 mM dNTP (Sinapse), 1 U of Taq DNA Polymerase (Invitrogen (5 U µL−1)) and MilliQ water to complete 25 µL. The reaction was subjected to cycling conditions of 10 s at 98 °C followed by 35 cycles at 98 °C for 3 s, 57 °C for 35 s, and 72 °C for 50 s, and a final extension step at 72 °C for 3 min in the thermal cycle (SimpliAmp, ThermoFisher, Waltham, MA, USA). The PCR product was verified by 1% agarose gel electrophoresis, purified by an EasyPure PCR purification kit (Trans, Haidian District, BJ, China), and then sent to Sanger-sequencing at the Laboratory of Molecular Biology of Plants (Department of Biological Sciences–ESALQ/USP), using the forward and reverse primers described in Table 1. The 1553 bp of H. armigera partial RyR obtained from the Sanger-sequencing was translated and aligned with the RyR of Plutella xylostella (L.) (Lepidoptera: Plutellidae) (GenBank AET09964) and RyR of H. armigera (GenBank AHB33498) amino acid sequence to find any alteration.

Table 1.
List of primers used in sanger sequencing.
2.9. Data Analysis
The dose–mortality data were corrected with controls (Natural Mortality) by Abbott’s formula [30]. The data corrected were subjected to Probit analysis with MASS package [31] by function glm with a binomial distribution and probit or logit links in R software [32] to estimate the LD50 values. The resistance ratio (RR) was determined by dividing the LD50 value of the Flub-R strain by LD50 value of the SUS strain. The likelihood ratio test (LRT) [31] was performed to verify the variation between strain responses. In addition, parallelism, and equality tests (p < 0.05) were performed to test the equality hypotheses between regression lines of each strain [31].
Functional dominance and leaf consumption data were analyzed using software R [32]. Mortality data were modeled by the GAMLSS package with the gamlss function [33], with a binomial distribution, probit link, and sigma adjustment. Results were submitted to a post-hoc test to verify significant differences in the mean by function emmeans [34] with the Tukey’s test, α = 5% and p < 0.05. The consumption data were submitted to the LM model with package MASS [31]; after this, the residual was tested for normality [35], homoscedasticity [36], and independency [37] by the package lmtest [38]. The ANOVA and Tukey’s test were subjected to verify significant differences in means with α = 5% and p < 0.05. The data describing the mortality of the synergist bioassay was subjected to the analysis of variance (ANOVA) and means were compared using the Tukey’s test, α = 5% and p < 0.05.
3. Results
3.1. Characterization of Flubendiamide Resistance
The LD50 of SUS strain was 0.018 µg a.i. cm−2 of flubendiamide, and the LD50 of Flub-R strain was 871.83 µg a.i. cm−2 of flubendiamide (Table 2). The resistance ratio (RR) of the Flub-R strain was >50,000-fold. The null hypothesis of equality and parallelism was rejected with χ2 (1) = 17.19, p < 0.05 between the slopes and intercepts of the strains.

Table 2.
Dose–mortality of flubendiamide to susceptible (SUS) and resistant (Flub-R) strains of H. armigera and heterozygotes from reciprocal crosses.
3.2. Inheritance of Flubendiamide Resistance
The heterozygote strain H1 (♀ SUS × ♂ Flub-R) showed an LD50 equal to 509.43, 95% CI [412.26–629.48] µg a.i. cm−2 of flubendiamide, and the heterozygote strain H2 (♀ Flub-R × ♂ SUS) showed an LD50 equal to 504.36, 95% CI [403.78–630.00] µg a.i. cm−2 of flubendiamide. The null hypothesis of equality and parallelism was not rejected between the slopes and intercepts of strains H1 and H2 with χ2 (1) = 0.001, p = 0.969. Moreover, the null hypothesis of the likelihood ratio test of LD50 between strains H1 and H2 was not rejected with χ2 (1) = 0.173, p = 0.863. Therefore, this finding confirms the hypothesis of autosomal inheritance of the resistance of H. armigera to flubendiamide and ruled out the hypothesis of sex-linked or mitochondrial inheritance.
3.3. Dominance of Resistance
The analysis of dominance was assessed by two methods. According to Stone’s method [25], the degree of dominance calculated by LD50 and approximate 95% confidence interval for reciprocal crosses were D = 0.903, SE = 0.053 CI (D ± 2 SE = (0.795–1.007)) for H1 (♀ SUS × ♂ Flub-R) and D = 0.899, SE = 0.055 CI (D ± 2 SE = (0.790–1.009)) for H2 (♀ Flub-R × ♂ SUS). Considering the standard errors calculated using Lehmann’s formula [26] for each value of D and the approximate 95% confidential intervals for D (D ± 2 SE), the resistance to flubendiamide is completely dominant. According to Bourguet et al. [28] method, by which dominance for each tested dose was calculated, the dominance values were higher than 0.73 for the higher doses, indicating an incomplete dominance (Figure 1), except at the lowest tested dose, that is, the dose recommended in the field when the dominance is completely dominant (Figure 1). Thus, the dominance values decreased as the flubendiamide dose increased.
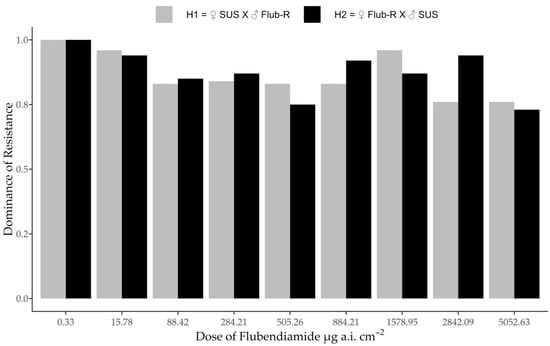
Figure 1.
Dominance of resistance to flubendiamide in H. armigera as a function of dose. Reciprocal crosses represented by H1 (♀ SUS × ♂ Flub-R) and H2 (♀ Flub-R × ♂ SUS).
3.4. Functional Dominance of Resistance and Leaf Consumption
When we compared the survival of strains on untreated leaves, SUS 96.7%, Flub-R 100%, H1 100% and H2 96.7%, there was no significant statistical difference (F(3,36) = [0.002], p = 0.999) (Figure 2), when in treated leaves there were significant statistical differences between strains (F(3,36) = (7.243), p < 0.05). Tukey’s test for pairwise comparisons showed that SUS 10.34% differed from Flub-R 100% t(36) = [15.85], p < 0.05, two-tailed, H1 96.66% t(36) = [13.20], p < 0.05, two-tailed, and H2 100% t(36) = [15.85], p < 0.05, two-tailed) (Figure 2). In terms of the mean leaves’ consumption, the strains in untreated leaves SUS (M = 2.23 cm2), Flub-R (M = 2.41 cm2), H1 (M = 2.40 cm2) and H2 (M = 2.22 cm2) did not show significant statistical differences between strains (F(3,36) = (0.136), p = 0.938) (Figure 3); otherwise in treated leaves there was a significant statistical difference between strains (F(3, 36) = (12.47), p < 0.05). The Tukey’s test for multiple comparisons found that the in treated leaves the strain SUS (M = 0.18 cm2) differed from Flub-R (M = 1.83) (p < 0.05, 95% CI = (0.77–2.54)), H1 (M = 1.80 cm2) (p < 0.05, 95% CI = (0.74–2.50)) and H2 (M = 1.81 cm2) (p < 0.05, 95% CI = (0.75–2.52)) (Figure 3). In summary, the survival of heterozygotes and the leaf consumption of treated leaves are not different from that of the resistant strain. Thus, we demonstrated that the resistance of H. armigera to flubendiamide at a field rate is functionally dominant.
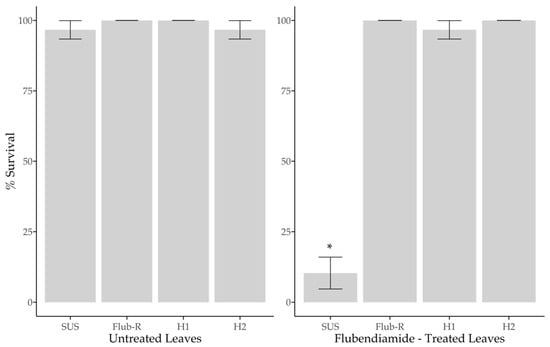
Figure 2.
Proportion of larval survival by susceptible (SUS), resistant (Flub-R) and heterozygous H1 (♀ SUS X ♂ Flub-R) and H2 (♀ Flub-R X ♂ SUS) H. armigera in soybean leaves untreated and treated with flubendiamide (0.33 μg a.i. cm−2). * Significant difference between strains according to the Tukey’s test (p < 0.05).
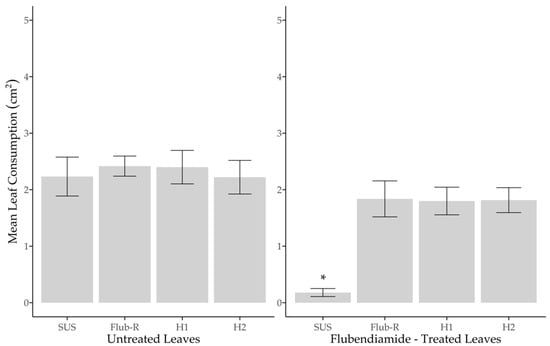
Figure 3.
Mean leaf consumption by susceptible (SUS), resistant (Flub-R) and heterozygous H1 (♀ SUS X ♂ Flub-R) and H2 (♀ Flub-R X ♂ SUS) H. armigera larva in soybean leaves untreated and flubendiamide-treated (0.33 μg a.i. cm−2). * Significant difference between strains according to the Tukey test (p < 0.05).
3.5. Cross Resistance
The flubendiamide-resistant strain (Flub-R) showed no cross-resistance to the anthranilic diamides. The Flub-R showed an LD50 of 0.090, 95% CI [0.077–0.106] µg a.i. cm−2, 0.193, 95% CI [0.158–0.237] µg a.i. cm−2 and 0.004, 95% CI [0.003–0.005] µg a.i. cm−2 for chlorantraniliprole, cyantraniliprole and cyclaniliprole, respectively (Table 3). When compared with the SUS LD50 of each insecticide tested, Flub-R showed low resistance ratio to chlorantraniliprole (4.50-fold), cyantraniliprole (4.94-fold) and cyclaniliprole (1.33-fold) (Table 3).

Table 3.
Dose–mortality data for evaluating cross resistance between flubendiamide and anthranilic diamides in H. armigera.
3.6. Synergist Bioassays
Third instar larvae of Flub-R strain subjected to controls (acetone plus flubendiamide) showed a mortality of 43.8 ± 3.98%. The treatment that larvae were pretreated with, PBO, and then treated with flubendiamide presented a mortality value of 56.2 ± 4.16%. The synergist DEM applied before flubendiamide resulted in a similar mortality compared to the control, which showed 50 ± 3.40%. The treatment with DEF showed mortality values of 45.8 ± 3.98%. The analysis of variance (ANOVA) showed no significant variation among treatments, (F(3,12) = (2), p = 0.168) (Figure 4).
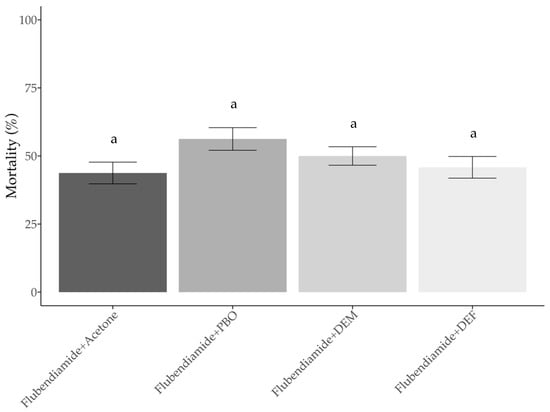
Figure 4.
Effect of synergists (PBO, DEM and DEF) on H. armigera (third-instar larvae) mortality to flubendiamide. Bars fallowed by the same letter do not differ from the treatment control (flubendiamide + acetone) according to the Tukey’s test (p > 0.05).
3.7. Sequencing Partial RyR
The amplified PCR products that overlap each other were sequenced to identify non-synonymous mutations related to diamides resistance. The amplified PCR product translates approximately 470 amino acids from RyR, covering transmembrane domains II to VI. The amplified partial RyR of H. armigera SUS and Flub-R showed a high similarity (91% pairwise identified at amino acid level) to the respective stretch of the P. xylostella (GenBank AET09964). Analysis obtained from the Flub-R strain did not show any change in terms of amino acids (Figure 5) when compared to SUS strain and H. armigera reference sequence from GenBank in National Center for Biotechnology Information (NCBI).
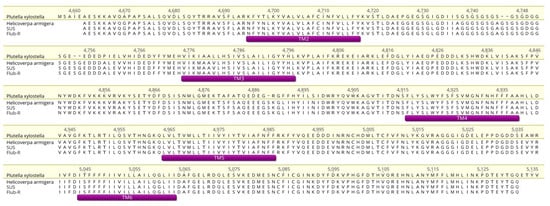
Figure 5.
Multiple alignments of partial ryanodine receptors, spanning transmembrane II to VI. This region harbors the main mutations related to diamide resistance. Alignment was performed based on the H. armigera sequence (GenBank AHB33498). No mutations were found in the flubendiamide-resistant strain (Flub-R). The sites are numbered according to the sequence of P. xylostella (GenBank AET09964).
4. Discussion
In this study, a very high resistance ratio of H. armigera to flubendiamide (>50,000-fold) was obtained after laboratory selection of a field-collected population in the Western Bahia State, Brazil in 2016. So far, there have been no records of resistance of H. armigera populations to diamide insecticides in Brazil. However, previous studies conducted by Pereira et al. [23] have already indicated significant reductions in susceptibility to diamide insecticides in H. armigera populations throughout the crop seasons in Brazil, with survival rates increasing from 0% in the 2014 crop season up to 51.99% in the 2018 crop season based on diagnostic dose bioassays. A high resistance ratio to flubendiamide was also reported for Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) [19] and Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) [39] collected from same region. This region is characterized by an intensive agriculture and climatic conditions that favor the development of many pest generations during the entire year and the high use of insecticides to pest control may have led to the selection of resistant alleles.
The inheritance pattern of H. armigera resistant to flubendiamide was defined as an autosomal and completely dominant trait. This finding eliminates the hypothesis of sex-linked and maternal influence on the resistance of H. armigera to flubendiamide. The resistance of S. frugiperda [19], T. absoluta [39] and P. xylostella (L.) [40] to diamide insecticides was characterized as autosomal and incompletely recessive. The resistance of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) was autosomal and incompletely recessive to anthranilic diamides and completely recessive to flubendiamide [16]. Different from these works, our Flub-R strain showed a dominant trait. The dominance level has a great impact on pest control, given that the resistance allele tends to be fixed in the population when inheritance is dominant. The completely dominant trait contributes to the rapid evolution of resistance due to the survival of heterozygotes. These heterozygotes are the main carriers of resistance alleles in the field [41,42].
The heterozygote larvae that fed on soybean leaves treated with flubendiamide at the field-recommended rates did not differ in leaf consumption and survival when compared to the resistant strain. These results indicate that the resistance at field rates of flubendiamide is completely dominant trait. In fact, dominance is characterized as the relationship between phenotypes and genotypes (homozygous resistant, homozygous susceptible and heterozygous) that can vary according to environmental conditions [28]. Thus, the use of doses below those that are field-recommended promotes the maintenance of heterozygotes in the area, increasing the frequency of resistance alleles in the population. Therefore, it is necessary to implement proactive Insect Resistance Management (IRM) strategies to delay the evolution of resistance and, consequently, the fixation of resistance alleles in the populations [41].
Our results showed the potential risk of the development of diamide resistance under applied field conditions. However, it is important to note that the selected strain with flubendiamide did not show cross-resistance to chlorantraniliprole, cyantraniliprole or cyclaniliprole (anthranilic diamides). This result is different from other published studies showing cross-resistance between diamide insecticides due to a mutation in the ryanodine receptor genes [16,17,20,43]. Synergists inhibiting detoxification enzyme families such as cytochromes P450 (P450), carboxylesterases (CE), and glutathione S-transferases (GST) did not increase flubendiamide efficacy in the Flub-R strain, suggesting the absence of metabolic resistance. Some studies show that detoxifying enzymes could be associated with resistance to diamide insecticides [44,45,46], but they promote low, or no resistance levels compared to mutations at the target site [15].
The partial sequencing of the ryanodine receptor genes of H. armigera-resistant strains to flubendiamide showed no mutations in the region that covers the transmembrane regions II to VI. This C-terminal region was formerly described to contain target site mutations [15,47]. The primary lepidopteran resistance mechanism to diamides is the mutation in RyR, which can promote very high resistance ratios [15]. Some mutations such as G4946E and I4790M are responsible for cross-resistance between flubendiamide, chlorantraniliprole, and cyantraniliprole [16,17,20,43], as observed in S. frugiperda [48] and P. xylostella [49]. Otherwise, S. exigua resistance to diamides was related to InDels, which showed a 29-bp insertion close to the intron associated with the resistance mutation [50]. The fact that our sequencing did not find mutations in the main region could explain the lack of cross-resistance between the diamides tested. Our results indicate that resistance of H. armigera to flubendiamide in Brazil may be related to a non-common mutation in another region of the ryanodine receptor gene or a different mechanism of resistance. We are conducting studies such as genotype–phenotype association to investigate the possible mechanisms responsible for H. armigera resistance to flubendiamide and we hope to clarify this question.
This is the first report of resistance of H. armigera to flubendiamide. Our results reveal the high risk of resistance evolution of H. armigera to flubendiamide and the importance of implementing resistance management strategies to preserve the lifetime of this insecticide to control H. armigera in Brazil.
5. Conclusions
Our results show the potential risk for the rapid evolution of flubendiamide resistance in H. armigera based on a completely dominant trait. In addition, no cross-resistance was found between flubendiamide (phthalic acid diamide), chlorantraniliprole, cyantraniliprole or cyclaniliprole (anthranilic diamides) in H. armigera. We did not find any reported mutations in transmembrane regions II to VI of the ryanodine receptor genes, nor indication of metabolic resistance in the flubendiamide-resistant strain of H. armigera.
Author Contributions
Conceptualization, D.A.-N., D.A. and C.O.; Data curation, D.A.-N., D.A. and A.S.C.; Formal analysis, D.A.; Funding acquisition, C.O.; Investigation, D.A.-N.; Methodology, D.A.-N., D.A., A.S.C. and C.O.; Project administration, C.O.; Resources, C.O.; Software, D.A.; Supervision, A.S.C. and C.O.; Validation, D.A.-N., D.A., S.S.-S., A.S.C. and C.O.; Visualization, D.A.-N., D.A., R.M.P., M.B. and T.M.G.; Writing—original draft, D.A.-N. and D.A.; Writing—review & editing, A.S.C. and C.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by São Paulo Research Foundation (FAPESP), grant number 2019/18282-9, National Council for Scientific and Technological Development (CNPq), grants 314160/2020-5 and 142590/2019-3, and Coordination of Superior Level Staff Improvement-Brazil (CAPES)-Finance Code 001.
Data Availability Statement
Not applicable.
Acknowledgments
This research was conducted in partial fulfillment by the senior authors D.A. and D.A.-N for the Doctoral Degree in Entomology at University of São Paulo. We thank Brazilian Insecticide Resistance Action Committee (IRAC-BR) for providing Helicoverpa armigera populations for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Czepak, C.; Albernaz, K.C.; Vivan, L.M.; Guimarães, H.O.; Carvalhais, T. First reported occurrence of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil. Pesq. Agropecu. Trop. 2013, 43, 110–113. [Google Scholar]
- Tay, W.T.; Soria, M.F.; Walsh, T.; Thomazoni, D.; Silvie, P.; Behere, G.T.; Anderson, C.; Downes, S. A brave new world for an old world pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS ONE 2013, 8, e80134. [Google Scholar]
- Fitt, G.P. The ecology of Heliothis species in relation to agroecosystems. Ann. Rev. Entomol. 1989, 66, 17–52. [Google Scholar]
- Tay, W.T.; Gordon, K.H.J. Going global—Genomic insights into insect invasions. Curr. Opin. Insect Sci. 2019, 31, 123–130. [Google Scholar]
- Murúa, M.G.; Scalora, F.S.; Navarro, F.R.; Cazado, L.E.; Casmuz, A.; Villagrán, M.E.; Lobos, E.; Gastaminza, G. First record of Helicoverpa armigera (Lepidoptera: Noctuidae) in Argentina. Fla. Entomol. 2014, 97, 854–856. [Google Scholar]
- Arnemann, J.A.; James, W.J.; Walsh, T.K.; Guedes, J.V.C.; Smagghe, G.; Castiglioni, E.; Tay, W.T. Mitochondrial DNA COI characterization of Helicoverpa armigera (Lepidoptera: Noctuidae) from Paraguay and Uruguay. Gen. Mol. Res. 2016, 15, gmr8292. [Google Scholar]
- Castiglioni, E.; Perini, C.R.; Chiaravalle, W.; Arnemann, J.A.; Ugalde, G.; Guedes, J.V.C. Primer registro de ocurrencia de Helicoverpa armigera (Hübner, 1808) (Lepidoptera: Noctuidae) en soja, en Uruguay. Agrociencia 2016, 20, 31–35. Available online: http://www.scielo.edu.uy/scielo.php?script=sci_arttext&pid=S2301-15482016000100005&nrm=iso (accessed on 30 May 2022).
- Gilligan, T.M.; Goldstein, P.Z.; Timm, A.E.; Farris, R.; Ledezma, L.; Cunningham, A.P. Identification of Heliothine (Lepidoptera: Noctuidae) larvae intercepted at U.S. ports of entry from the new world. J. Econ. Entomol. 2019, 112, 603–615. [Google Scholar]
- Cunningham, J.P.; Zalucki, M.P. Understanding Heliothine (Lepidoptera: Heliothinae) pests: What is a host plant? J. Econ. Entomol. 2014, 107, 881–896. [Google Scholar]
- Widmer, M.W.; Schofield, P. Heliothis: Dispersal and Migration; Tropical Development and Research Institute: London, UK, 1983; Volume 2, p. 41. [Google Scholar]
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database; Michigan State University: East Lansing, MI, USA, 2022; Available online: http://www.pesticideresistance.org (accessed on 27 February 2022).
- Jeanguenat, A. The story of a new insecticidal chemistry class: The diamides. Pest Manag. Sci. 2013, 69, 7–14. [Google Scholar]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar]
- Kato, K.; Kiyonaka, S.; Sawaguchi, Y.; Tohnishi, M.; Masaki, T.; Yasokawa, N.; Mizuno, Y.; Mori, E.; Inoue, K.; Hamachi, I.; et al. Molecular characterization of flubendiamide sensitivity in the lepidopterous ryanodine receptor Ca2+ release channel. Biochemistry 2009, 48, 10342–10352. [Google Scholar]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Davies, T.G.E.; Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar]
- Zuo, Y.; Wang, H.; Xu, Y.; Huang, J.; Wu, S.; Wu, Y.; Yang, Y. CRISPR/Cas9 mediated G4946E substitution in the ryanodine receptor of Spodoptera exigua confers high levels of resistance to diamide insecticides. Insect Biochem. Mol. Biol. 2017, 89, 79–85. [Google Scholar]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; García-Vidal, L.; Martínez-Aguirre, M.R.; Bielza, P.; Morou, E.; et al. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar]
- Troczka, B.J.; Williamson, M.S.; Field, L.M.; Davies, T.G.E. Rapid selection for resistance to diamide insecticides in Plutella xylostella via specific amino acid polymorphisms in the ryanodine receptor. Neuro Toxicol. 2017, 60, 224–233. [Google Scholar]
- Bolzan, A.; Padovez, F.E.; Nascimento, A.R.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar]
- Troczka, B.; Zimmer, C.T.; Elias, J.; Schorn, C.; Bass, C.; Davies, T.G.E.; Field, L.M.; Williamson, M.S.; Slater, R.; Nauen, R. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem. Mol. Biol. 2012, 42, 873–880. [Google Scholar]
- Zuo, Y.; Ma, H.; Lu, W.; Wang, X.; Wu, S.; Nauen, R.; Wu, Y.; Yang, Y. Identification of the ryanodine receptor mutation I4743M and its contribution to diamide insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2020, 27, 791–800. [Google Scholar]
- Douris, V.; Papapostolou, K.M.; Ilias, A.; Roditakis, E.; Kounadi, S.; Riga, M.; Nauen, R.; Vontas, J. Investigation of the contribution of RyR target-site mutations in diamide resistance by CRISPR/Cas9 genome modification in Drosophila. Insect Biochem. Mol. Biol. 2017, 87, 127–135. [Google Scholar]
- Pereira, R.M.; Abbade-Neto, D.; Amado, D.; Durigan, M.R.; Franciscatti, R.A.; Mocheti, M.; Omoto, C. Baseline susceptibility and frequency of resistance to diamide insecticides in Helicoverpa armigera (Lepidoptera: Noctuidae) populations in Brazil. Crop Prot. 2020, 137, 105266. [Google Scholar]
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 1976, 69, 487–488. [Google Scholar]
- Stone, B.F. A formula for determining degree of dominance in cases of monofactorial inheritance of resistance to chemicals. Bull. World Health Organ 1968, 38, 325–326. [Google Scholar]
- Lehmann, E.L.; Romano, J.P. Testing Statistical Hypotheses, 3rd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bourguet, D.; Genissel, A.; Raymond, M. Insecticide resistance and dominance levels. J. Econ. Entomol. 2000, 93, 1588–1595. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Rigby, R.A.; Stasinopoulos, D.M. Generalized additive models for location, scale and shape. J. R. Stat. Soc. 2005, 54, 507–554. [Google Scholar]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Soft. 2016, 69, 1–33. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality. Biometrika 1965, 52, 591–611. [Google Scholar]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1937, 160, 268–282. [Google Scholar]
- Durbin, J.; Watson, G.S. Testing for serial correlation in least squares regression. Biometrika 1971, 58, 1. [Google Scholar]
- Zeileis, A.; Hothorn, T. Diagnostic checking in regression relationships. R News 2002, 2, 7–10. [Google Scholar]
- Silva, J.E.; Ribeiro, L.M.S.; Vinasco, N.; Guedes, R.N.C.; Siqueira, H.Á.A. Field-evolved resistance to chlorantraniliprole in the tomato pinworm Tuta absoluta: Inheritance, cross-resistance profile, and metabolism. J. Pest Sci. 2019, 92, 1421–1431. [Google Scholar]
- Wang, X.; Khakame, S.K.; Ye, C.; Yang, Y.; Wu, Y. Characterization of field-evolved resistance to chlorantraniliprole in the diamondback moth, Plutella xylostella, from China. Pest Manag. Sci. 2013, 69, 661–665. [Google Scholar]
- Roush, R.T.; Mckenzie, J.A. Ecological genetics of insecticide and acaricide resistance. Ann. Rev. Entomol. 1987, 32, 361–380. [Google Scholar]
- Tabashnik, B.E.; Croft, B.A. Managing pesticide resistance in crop-arthropod complexes: Interactions between biological and operational factors 1. Environ. Entomol. 1982, 11, 1137–1144. [Google Scholar]
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of Tuta absoluta resistance to diamide insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar]
- Li, X.; Li, R.; Zhu, B.; Gao, X.; Liang, P. Overexpression of cytochrome P450 CYP6BG1 may contribute to chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 1386–1393. [Google Scholar]
- Zhao, J.; Xu, L.; Sun, Y.; Song, P.; Han, Z. UDP-Glycosyltransferase genes in the striped rice stem borer, Chilo suppressalis (Walker), and their contribution to chlorantraniliprole resistance. Int. J. Mol. Sci. 2019, 20, 1064. [Google Scholar]
- Yin, F.; Lin, Q.; Wang, X.; Li, Z.; Feng, X.; Shabbir, M.Z. The glutathione S-transferase (PxGST2L) may contribute to the detoxification metabolism of chlorantraniliprole in Plutella xylostella (L.). Ecotoxicology 2021, 30, 1007–1016. [Google Scholar]
- Ma, R.; Haji-Ghassemi, O.; Ma, D.; Jiang, H.; Lin, L.; Yao, L.; Samurkas, A.; Li, Y.; Wang, Y.; Cao, P.; et al. Structural basis for diamide modulation of ryanodine receptor. Nat. Chem. Biol. 2020, 16, 1246–1254. [Google Scholar]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a ryanodine receptor target-site mutation in diamide insecticide resistant fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2019, 76, 47–54. [Google Scholar]
- Jouraku, A.; Kuwazaki, S.; Miyamoto, K.; Uchiyama, M.; Kurokawa, T.; Mori, E.; Mori, M.X.; Mori, Y.; Sonoda, S. Ryanodine receptor mutations (G4946E and I4790K) differentially responsible for diamide insecticide resistance in diamondback moth, Plutella xylostella L. Insect Biochem. Mol. Biol. 2020, 118, 103308. [Google Scholar]
- Kim, J.; Nam, H.Y.; Kwon, M.; Choi, J.H.; Cho, S.R.; Kim, G.-H. Novel diamide resistance-linked mutation in Korean Spodoptera exigua and a LAMP assay based on a mutation-associated intronic InDel. J. Pest Sci. 2021, 94, 1017–1029. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).