Characterization of a Wheat-Dasypyrum breviaristatum Chromosome Addition and Its Derived Progenies Carrying Novel Dasypyrum-Specific Gliadin Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fluorescence In Situ Hybridization (FISH)
2.3. Molecular Marker Analysis
2.4. Gliadin Electrophoresis and Gene Sequences Analysis
2.5. Agronomic Traits and Grain Quality Observation
3. Results
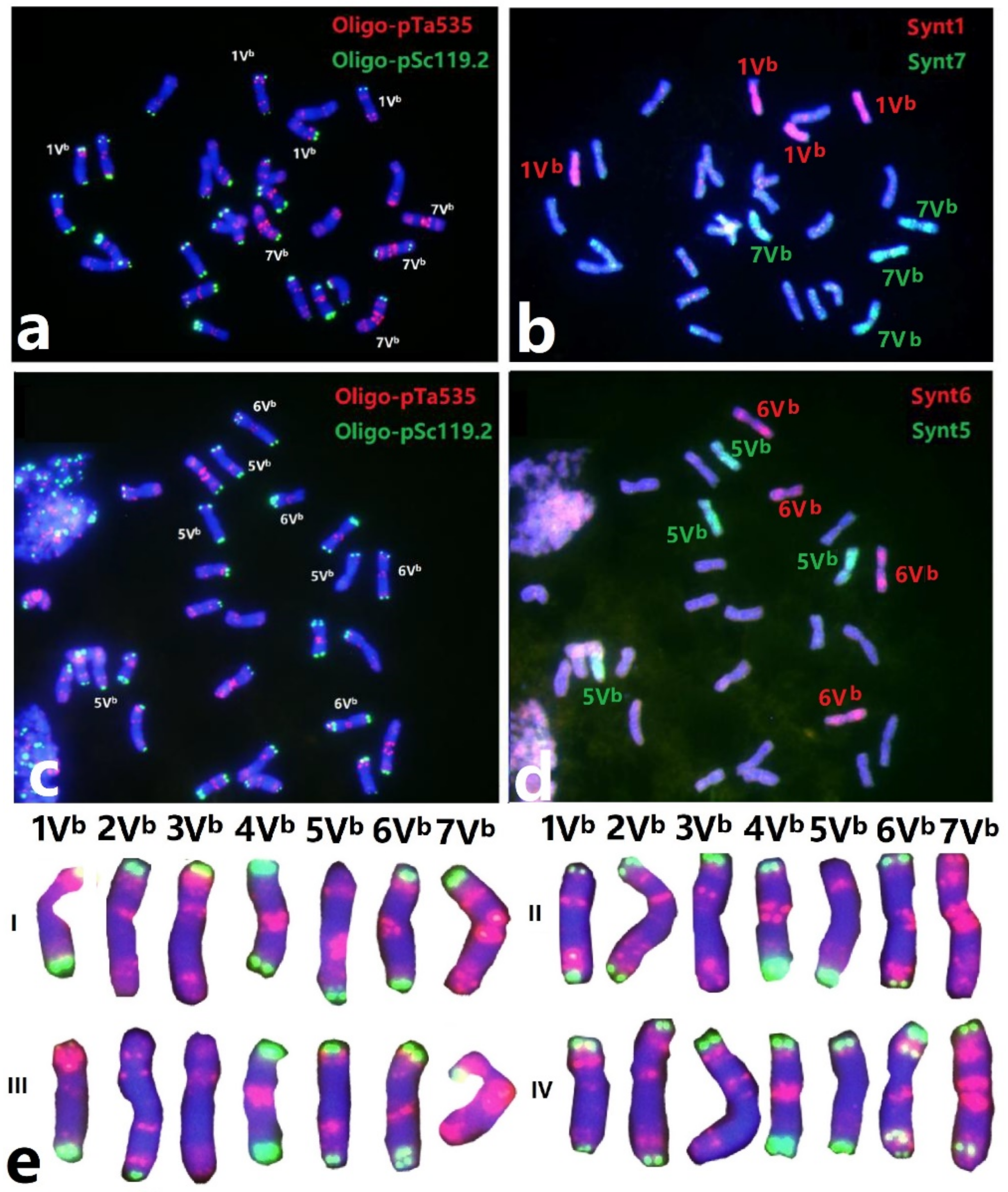
3.1. Karyotyping of Dasypyrum breviaristatum Revealed by Sequential Oligo-Fish Painting
3.2. Characterization of Wheat-Dasypyrum breviaristatum Addition Line D2138
3.3. Transmission of D. breviaristatum 6VbS and 2VbL in D2138 and Wheat Hybrids
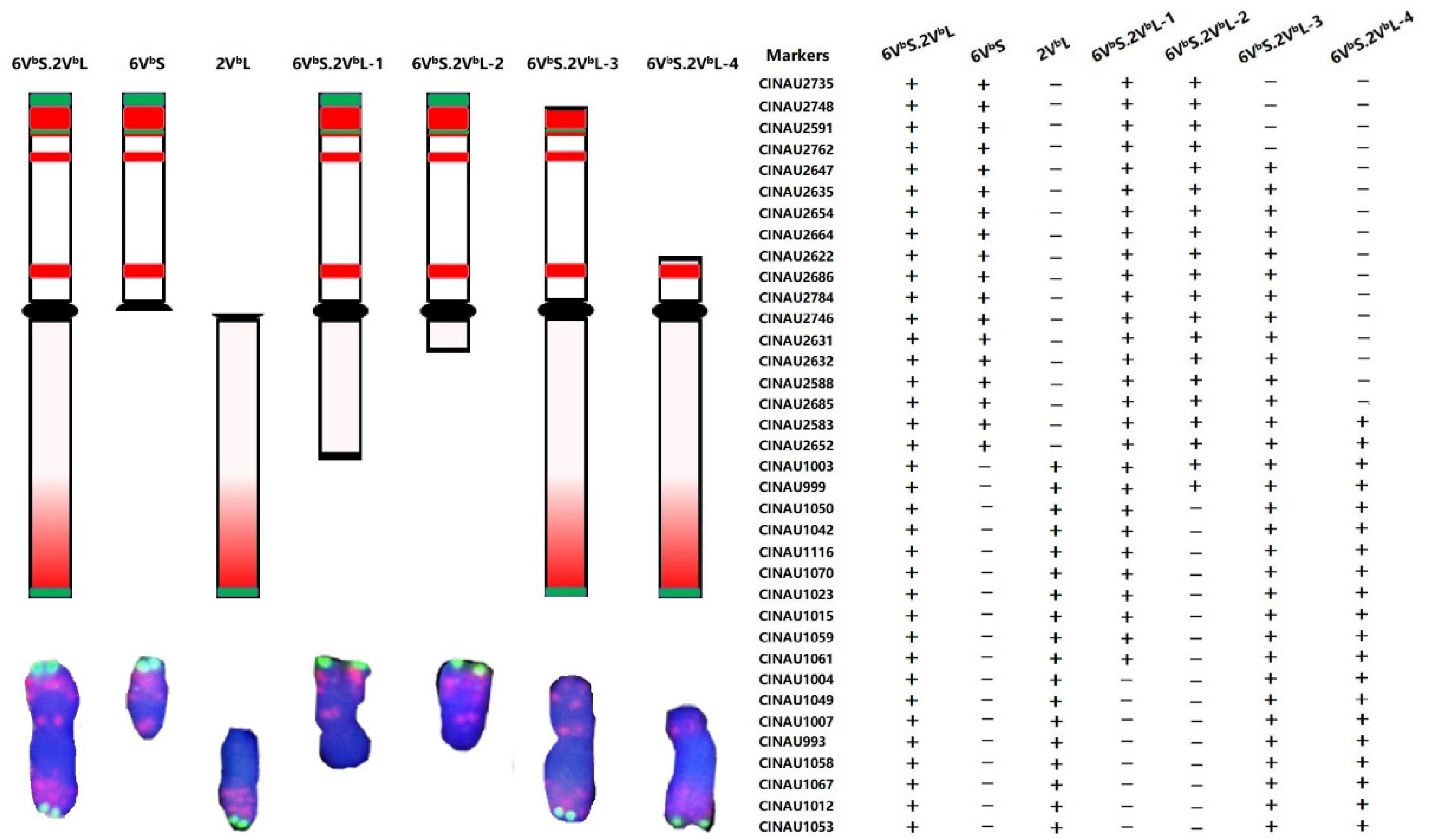
3.4. Identification and Physical Location of 6VbS.2VbL Chromosome Deletions
3.5. Characterization of New Wheat-D. breviaristatum Translocation Lines
3.6. Sequences of α-Gliadin Genes Located on 6VbS
3.7. Plant Agronomic Traits and Grain Quality Observation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frederiksen, S. Taxonomic studies in Dasypyrum (Poaceae). Nord. J. Bot. 1991, 11, 135–142. [Google Scholar] [CrossRef]
- Galasso, I.; Blanco, A.; Katsiotis, A.; Pignone, D.; Heslop-Harrison, H.S. Genomic organization and phylogenetic relationships in the genus Dasypyrum analysed by southern and in situ hybridization of total genomic and cloned DNA probes. Chromosoma 1997, 106, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.R.; Edwards, T.; Johnson, D.A. What does the nr5S DNA multigene family tell us about the genomic relationship between Dasypyrum breviaristatum and D. villosum (Triticeae, Poaceae)? Mol. Genet. Genom. 2014, 289, 553–565. [Google Scholar] [CrossRef] [PubMed]
- De Pace, C.; Vaccino, P.; Cionini, P.G.; Pasquini, M.; Bizzarri, M.; Qualset, C.O. Dasypyrum. In Wild Crop Relatives, Genomic and Breeding Resources, Cereals; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Chapter 4; pp. 185–292. [Google Scholar]
- Chen, P.D.; Qi, L.L.; Zhang, S.Z.; Liu, D.J. Development and molecular cytogenetic analysis of wheat-Haynaldia 6VS/6AL translocation lines specifying resistance to powdery mildew. Theor. Appl. Genet. 1995, 91, 1125–1128. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Zhang, P.; Qian, C.; Bowden, R.L.; Rouse, M.N.; Jin, Y.; Gill, B.S. A novel Robertsonian translocation event leads to transfer of a stem rust resistance gene (Sr52) effective against race Ug99 from Dasypyrum villosum into bread wheat. Theor. Appl. Genet. 2011, 123, 159–167. [Google Scholar] [CrossRef]
- Lukaszewski, A.J.; Cowger, C. Re-engineering of the Pm21 transfer from Haynaldia villosa to bread wheat by induced homoeologous recombination. Crop Sci. 2017, 57, 2590–2594. [Google Scholar] [CrossRef]
- Zhang, R.; Fan, Y.; Kong, L.; Wang, Z.; Wu, J.; Xing, L.; Cao, A.; Feng, Y. Pm62, an adul-plant powdery mildew resistance gene introgressed from Dasypyrum villosum chromosome arm 2VL into wheat. Theor. Appl. Genet. 2018, 131, 2613–2620. [Google Scholar] [CrossRef]
- Wang, C.L.; Ma, Q.X.; Qi, Z.J.; Zhuang, L.F.; Feng, J.T.; Jiang, D.; Zhou, W. Effects of wheat-Haynaldia villosa T6VS·6AL translocation on grain and flour quality of wheat. J. Triticeae Crops 2009, 29, 787–792. [Google Scholar]
- Cao, A.Z.; Xing, L.P.; Wang, X.Y.; Yang, X.M.; Wang, W.; Sun, Y.L.; Qian, C.; Ni, J. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA 2011, 19, 7727–7732. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Zhu, S.; Zhao, R.; Jiang, Z.; Ji, Y.; Ji, J.; Qiu, D.; Li, H.; Bie, T. Pm21, encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol. Plant 2018, 11, 879–882. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Hu, P.; Liu, J.; Witek, K.; Zhou, S.; Xu, J.; Zhou, W.; Qian, C.; Ni, J. Pm21 from Haynaldia villosa encodes a CC-NBS-LRR protein conferring powdery mildew resistance in wheat. Mol. Plant 2018, 11, 874–878. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Yuan, L.; Lv, Z.; Wang, Q.; Yin, C.; Huang, Z.; Liu, J.; Cao, S.; Zhang, R.; Chen, P.; et al. Long-range assembly of sequences helps to unravel the genome structure and small variation of the wheat-Haynaldia villosa translocated chromosome 6VS.6AL. Plant Biotechnol. J. 2021, 19, 1567–1578. [Google Scholar] [CrossRef]
- Yang, Z.J.; Li, G.R.; Feng, J.; Jiang, H.R.; Ren, Z.L. Molecular cytogenetic characterization and disease resistance observation of wheat-Dasypyrum breviaristatum partial amphiploid and its derivatives. Hereditas 2005, 142, 80–85. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Yan, H.; Zhou, J.; Hu, L.; Lei, M.; Ran, L.; Yang, Z. Molecular and cytogenetic identification of new wheat- D. breviaristatum additions conferring resistance to stem rust and powdery mildew. Breed. Sci. 2011, 61, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Zhao, J.; Li, D.; Yang, E.; Huang, Y.; Liu, C.; Yang, Z. A novel wheat—Dasypyrum breviaristatum substitution line with stripe rust resistance. Cytogenet. Genome Res. 2014, 143, 280–287. [Google Scholar] [CrossRef]
- Li, G.; Gao, D.; Zhang, H.; Li, J.; Wang, H.; La, S.; Ma, J.; Yang, Z. Molecular cytogenetic characterization of Dasypyrum breviaristatum chromosomes in wheat background revealing the genomic divergence between Dasypyrum species. Mol. Cytogenet. 2016, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, G.; Li, D.; Gao, D.; Zhang, J.; Yang, E.; Yang, Z. Molecular and cytogenetic characterization of new wheat—Dasypyrum breviaristatum derivatives with post-harvest re-growth habit. Genes 2015, 7, 1242–1255. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yu, Z.; Li, B.; Lang, T.; Li, G.; Yang, Z. Characterization of new wheat-Dasypyrum breviaristatum introgression lines with superior gene(s) for spike length and stripe rust resistance. Cytogenet. Genome Res. 2018, 156, 117–125. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Li, B.; Yu, Z.; Li, G.; Zhang, J.; Yang, Z. Molecular cytogenetic characterization of new wheat-Dasypyrum breviaristatum introgression lines for improving grain quality of wheat. Front. Plant Sci. 2018, 9, 365. [Google Scholar] [CrossRef]
- Jiang, J.M.; Gill, B.S. Preferential male transmission of an alien chromosome in wheat. J. Hered. 1998, 89, 87–89. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Z.; Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Tang, S.; Qiu, L.; Tang, Z.; Fu, S. Oligonucleotides and ND-FISH displaying different arrangements of tandem repeats and identification of Dasypyrum villosum chromosomes in wheat backgrounds. Molecules 2018, 22, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, T.; Li, G.; Wang, H.; Yu, Z.; Chen, Q.; Yang, E.; Fu, S.; Tang, Z.; Yang, Z. Physical location of tandem repeats in the wheat genome and application for chromosome identification. Planta 2019, 249, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, H.; Jiang, W.; Jiang, C.; Yuan, W.; Li, G.; Yang, Z. Karyotyping Dasypyrum breviaristatum chromosomes by ND-FISH with multiple oligonucleotide probes reveals the genomic divergence in Dasypyrum. Genome 2021, 64, 789–800. [Google Scholar] [CrossRef]
- Li, G.; Zhang, T.; Yu, Z.; Wang, H.; Yang, E.; Yang, Z. An efficient Oligo-FISH painting system for revealing chromosome rearrangements and polyploidization in Triticeae. Plant J. 2021, 105, 978–993. [Google Scholar] [CrossRef]
- Han, F.P.; Lamb, J.C.; Birchler, J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Yang, Z. Oligo-FISH paints in Triticeae. Curr. Protoc. 2022, 2, e364. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, C.; Feng, J.; Li, G.R.; Deng, K.J.; Zhou, J.P.; Ren, Z.L. Studies on genome relationship and species-specific PCR marker for Dasypyrum breviaristatum in Triticeae. Hereditas 2006, 143, 47–54. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, X.; Xiao, J.; Yuan, C.; Wu, Y.; Cao, A.; Xing, L.; Chen, P.; Zhang, S.; Wang, X.; et al. Whole genome development of intron targeting (IT) markers specific for Dasypyrum villosum chromosomes based on next-generation sequencing technology. Mol. Breed. 2017, 37, 115. [Google Scholar] [CrossRef]
- Hu, L.J.; Li, G.R.; Zeng, Z.X.; Chang, Z.J.; Liu, C.; Zhou, J.P.; Yang, Z.J. Molecular cytogenetic identification of a new wheat-Thinopyrum substitution line with stripe rust resistance. Euphytica 2011, 177, 169–177. [Google Scholar] [CrossRef]
- Li, G.R.; Liu, C.; Zeng, Z.X.; Jia, J.Q.; Zhang, T.; Zhou, J.P.; Ren, Z.L.; Yang, Z.J. Identification of α-gliadin genes in Dasypyrum in relation to evolution and breeding. Euphytica 2009, 165, 155–163. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11, Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, L.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Raia, V.; Auricchio, S.; Qian, C.; Ni, J. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 2003, 362, 30–37. [Google Scholar] [CrossRef]
- Schaart, J.G.; Salentijn, E.M.J.; Goryunova, S.V.; Chidzanga, C.; Esselink, D.G.; Gosman, N. Exploring the alpha-gliadin locus: The 33-mer peptide with sixoverlapping coeliac disease epitopes in Triticum aestivum is derived from a subgroup of Aegilops tauschii. Plant J. 2021, 106, 86–94. [Google Scholar] [CrossRef]
- Sun, H.; Song, J.; Lei, J.; Song, X.; Dai, K.; Xiao, J.; Yuan, C.; An, S.; Wang, H.; Wang, X. Construction and application of oligo-based FISH karyotype of Haynaldia villosa. J. Genet. Genom. 2018, 45, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Khlestkina, E.K. Current applications of wheat and wheat–alien precise genetic stocks. Mol. Breed. 2014, 34, 273–281. [Google Scholar] [CrossRef]
- Bolibok-Brągoszewska, H.; Targońska, M.; Bolibok, L.; Kilian, A.; Rakoczy-Trojanowska, M. Genome-wide characterization of genetic diversity and population structure in Secale. BMC Plant Biol. 2014, 14, 184. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Lei, Y.; Zhang, H.; Song, D.; Liu, X.; Cao, Z.; Chu, C.; Zhuang, L.; Qi, Z. Frequent variations in tandem repeats pSc200 and pSc119.2 cause rapid chromosome evolution of open-pollinated rye. Mol. Breed. 2019, 39, 133. [Google Scholar] [CrossRef]
- Friebe, B.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat-alien translocations conferring resistance to diseases and pests, current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Lukaszewski, A.J. Manipulation of homologous and homoeologous chromosome recombination in wheat. In Plant Cytogenetics, Methods and Protocols; Kianian, S.F., Kianian, P.M.A., Eds.; Springer: New York, NY, USA, 2016; pp. 77–89. [Google Scholar]
- Ren, Z.L.; Lelley, T.; Röbbelen, G. The use of monosomic rye addition lines for transferring rye chromatin into bread wheat. I. The occurrence of translocations. Plant Breed. 1990, 105, 257–264. [Google Scholar] [CrossRef]
- Fu, S.; Yang, M.; Fei, Y.; Tan, F.; Ren, Z.; Yan, B.; Zhang, H.; Tang, Z. Alterations and abnormal mitosis of wheat chromosomes induced by wheat-rye monosomic addition lines. PLoS ONE 2013, 8, e70483. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, H.; Tsunewaki, K. Gametocidal genes in wheat and its relatives. II. Suppressor of the chromosome 3C gametocidal gene of Aegilops triuncialis. Can. J. Genet. Cytol. 1985, 27, 178–185. [Google Scholar] [CrossRef]
- Endo, T.R. Gametocidal chromosomes and their induction of chromosome mutations in wheat. Jpn. J. Genet. 1990, 65, 135–152. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.R. Dissection of the rye genome by the gametocidal system. In The Rye Genome, Compendium of Plant Genomes; Rabanus-Wallace, M.T., Stein, N., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; pp. 77–84. [Google Scholar]
- Tsujimoto, H. Gametocidal genes in wheat and its relatives. IV. Functional relationships between six gametocidal genes. Genome 1995, 38, 283–289. [Google Scholar] [CrossRef]
- Shewry, P.R.; Sabelli, P.A.; Parmar, S.; Lafiandra, D. α-Type prolamins are encoded by genes on chromosomes 4Ha and 6Ha of Haynaldia villosa Schur (syn. Dasypyrum villosum L.). Biochem. Genet. 1991, 29, 207–211. [Google Scholar] [CrossRef]
- De Pace, C.; Snidaro, D.; Ciaffi, M.; Vittori, D.; Ciofo, A.; Cenci, A.; Tanzarella, O.A.; Qualset, C.O.; Scarascia Mugnozza, G.T. Introgression of Dasypyrum villosum chromatin into common wheat improves grain protein quality. Euphytica 2001, 117, 67–75. [Google Scholar] [CrossRef]
- Vaccino, P.; Banfi, R.; Corbellini, M.; De Pace, C. Broadening and improving the wheat genetic diversity for end-use grain quality by introgression of chromatin from the wheat wild relative Dasypyrum villosum. Crop Sci. 2010, 50, 528–540. [Google Scholar] [CrossRef] [Green Version]




| Hybrids | F2 Plants | Disomic 6VbS.2VbL | Monosomic 6VbS.2VbL | Monosomic 2VbL | Monosomic 6VbS | Monosomic iso-2VbL | Monosomic iso-6VbS | Wheat Chromosome Variation |
|---|---|---|---|---|---|---|---|---|
| D2138 × CM62 | 270 | 1 | 56 | 24 | 48 | 6 | 6 | 45 |
| D2138 × MY11 | 262 | 4 | 52 | 24 | 23 | 3 | - | 79 |
| D2138 × JM22 | 286 | 1 | 46 | 12 | 11 | 7 | 2 | 96 |
| Total | 818 | 6 | 154 | 60 | 81 | 17 | 8 | 220 |
| Lines | PH (cm) | TPP | SL | TKW (g) | GPC (%) | WGC (%) | ZEL (mL) | GH | ABS |
|---|---|---|---|---|---|---|---|---|---|
| MY11 | 84.2 | 3.5 | 9.2 | 33.6 | 14.4 | 34.5 | 45.5 | 59.4 | 56.9 |
| CM62 | 95.3 | 4.0 | 11.8 | 59.8 | 12.1 | 29.1 | 26.6 | 54.2 | 52.4 |
| JM22 | 68.8 | 8.8 | 9.7 | 27.6 | 14.3 | 40.5 | 68.9 | 41.1 | 59.6 |
| D2138 | 91.2 * | 10.0 * | 10.3 * | 33.6 | 17.5 * | 44.2 * | 67.3 * | 52.8 | 61.4 * |
| D2138/MY11 | |||||||||
| −6VbS | 85.5 | 9.0 | 10.6 | 31.1 | 15.3 | 39.5 | 57.1 * | 50.4 | 61.9 |
| +6VbS | 91.4 * | 11.3 | 9.7 | 32.8 * | 16.1 * | 43.5 * | 50.7 | 58.1 * | 62.4 |
| D2138/CM62 | |||||||||
| −6VbS | 93.1 | 9.4 | 11.3 | 49.7 * | 14.9 | 38.1 | 44.8 | 53.1 | 61.8 |
| +6VbS | 94.9 | 11.3 | 11.6 | 48.2 | 16.4 * | 39.2 * | 46.7 * | 59.9 * | 60.9 |
| D2138/JM22 | |||||||||
| −6VbS | 72.2 | 7.4 | 10.0 * | 31.7 * | 14.9 | 39.6 | 54.8 | 51.6 | 62.3 |
| +6VbS | 84.7 * | 7.8 | 9.2 | 30.9 | 15.58 * | 39.8 | 53.4 | 57.2 * | 63.7 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Jiang, W.; Liu, M.; Wang, H.; Yang, E.; Yang, Z.; Li, G. Characterization of a Wheat-Dasypyrum breviaristatum Chromosome Addition and Its Derived Progenies Carrying Novel Dasypyrum-Specific Gliadin Genes. Agronomy 2022, 12, 1673. https://doi.org/10.3390/agronomy12071673
Jiang C, Jiang W, Liu M, Wang H, Yang E, Yang Z, Li G. Characterization of a Wheat-Dasypyrum breviaristatum Chromosome Addition and Its Derived Progenies Carrying Novel Dasypyrum-Specific Gliadin Genes. Agronomy. 2022; 12(7):1673. https://doi.org/10.3390/agronomy12071673
Chicago/Turabian StyleJiang, Chengzhi, Wenxi Jiang, Min Liu, Hongjin Wang, Ennian Yang, Zujun Yang, and Guangrong Li. 2022. "Characterization of a Wheat-Dasypyrum breviaristatum Chromosome Addition and Its Derived Progenies Carrying Novel Dasypyrum-Specific Gliadin Genes" Agronomy 12, no. 7: 1673. https://doi.org/10.3390/agronomy12071673
APA StyleJiang, C., Jiang, W., Liu, M., Wang, H., Yang, E., Yang, Z., & Li, G. (2022). Characterization of a Wheat-Dasypyrum breviaristatum Chromosome Addition and Its Derived Progenies Carrying Novel Dasypyrum-Specific Gliadin Genes. Agronomy, 12(7), 1673. https://doi.org/10.3390/agronomy12071673







