Characterization of the MADS-Box Gene CmFL3 in chrysanthemum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Gene Sequence Analysis
2.3. qRT-PCR and RNA In Situ Hybridization
2.4. Subcellular Localization of CmFL3
2.5. Transactivation Assay
2.6. CmFL3 Genetic Transformation
3. Results
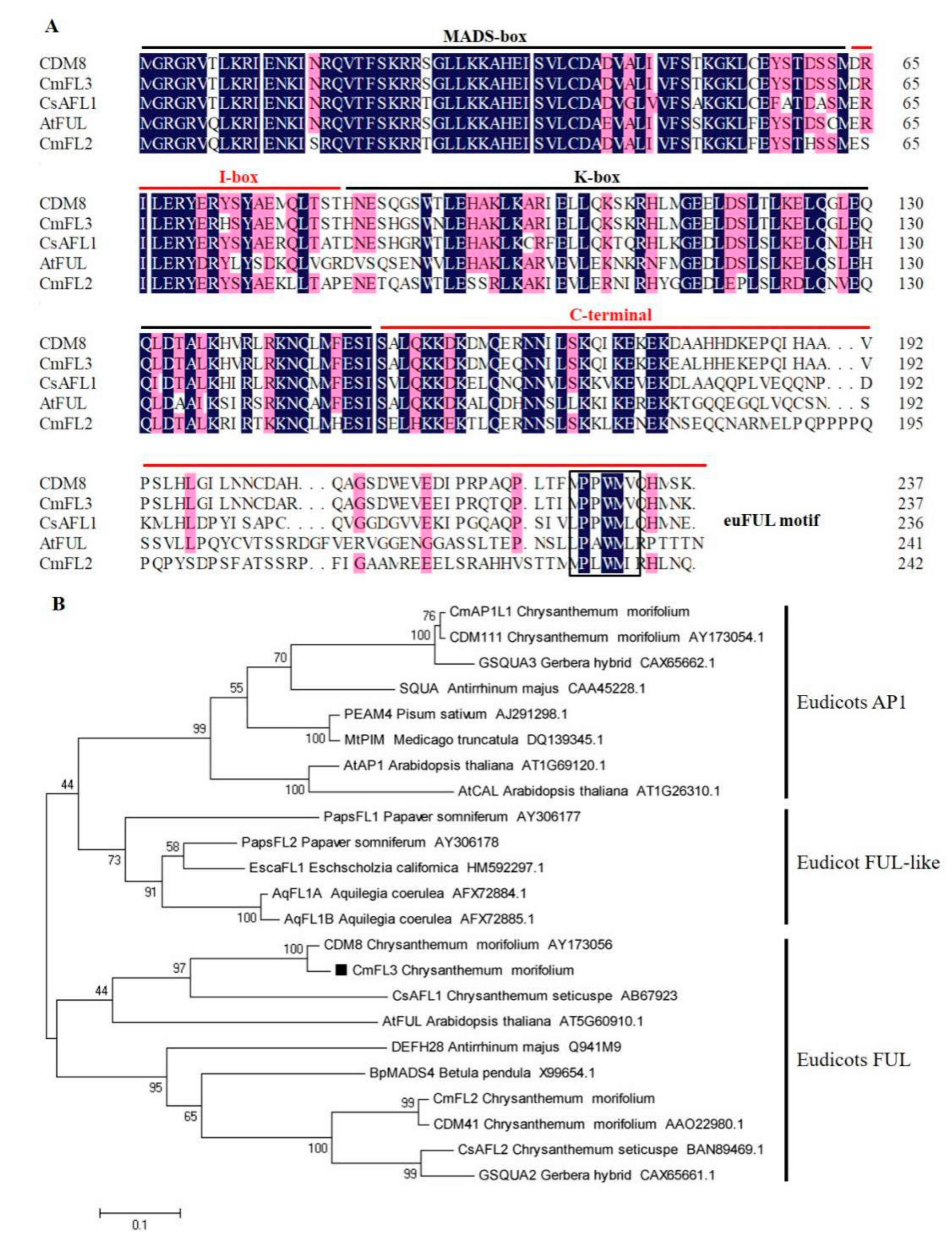
3.1. Sequence Analysis of CmFL3
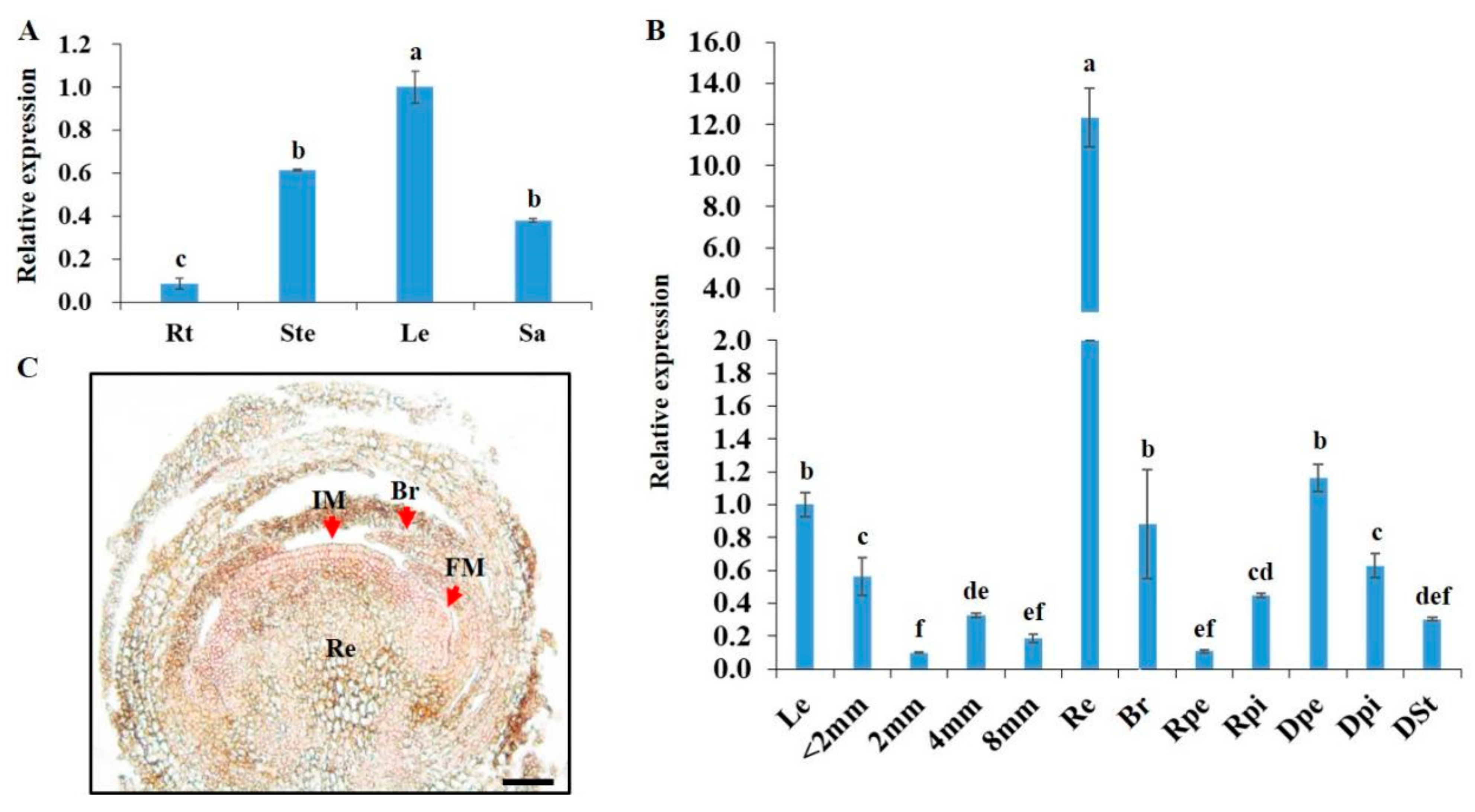
3.2. Expression Patterns of CmFL3
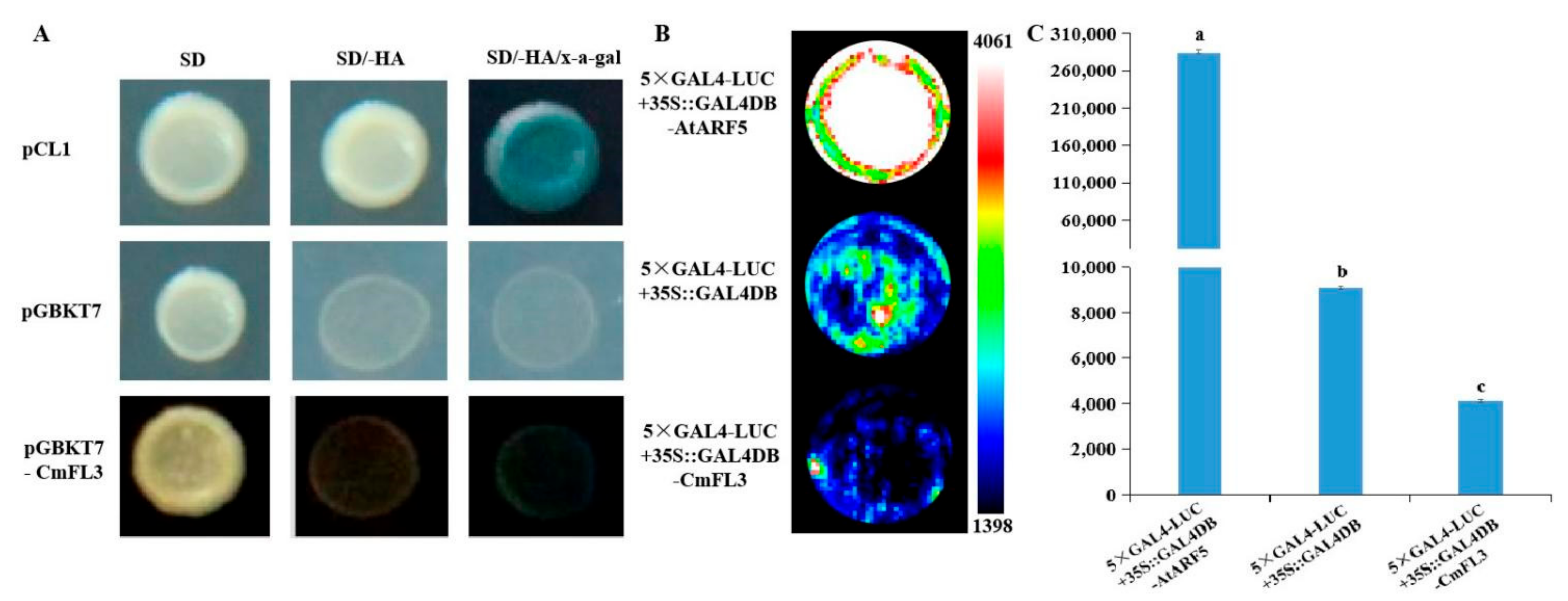
3.3. Subcellular Localization and Transactivation Activity of CmFL3
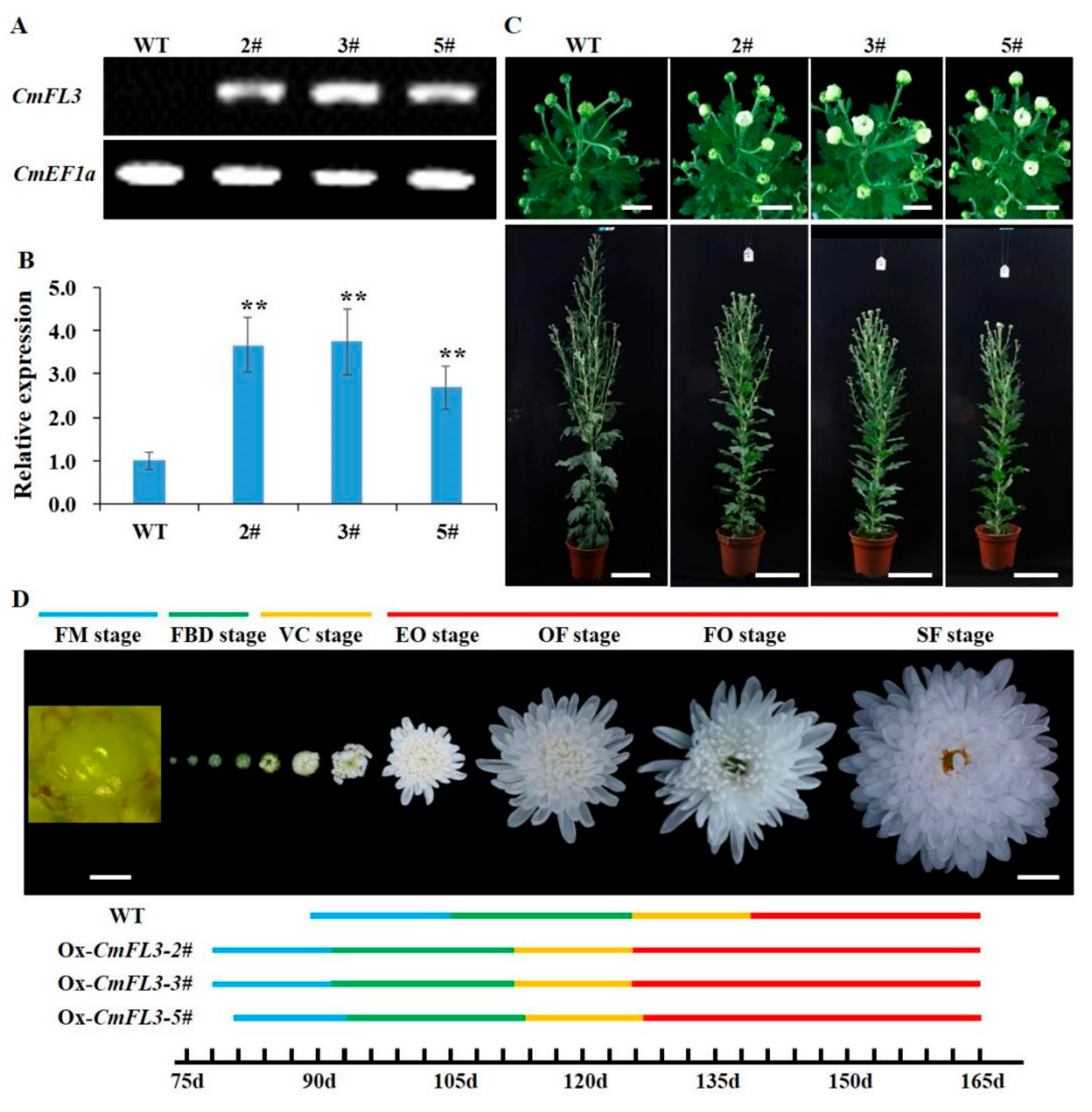
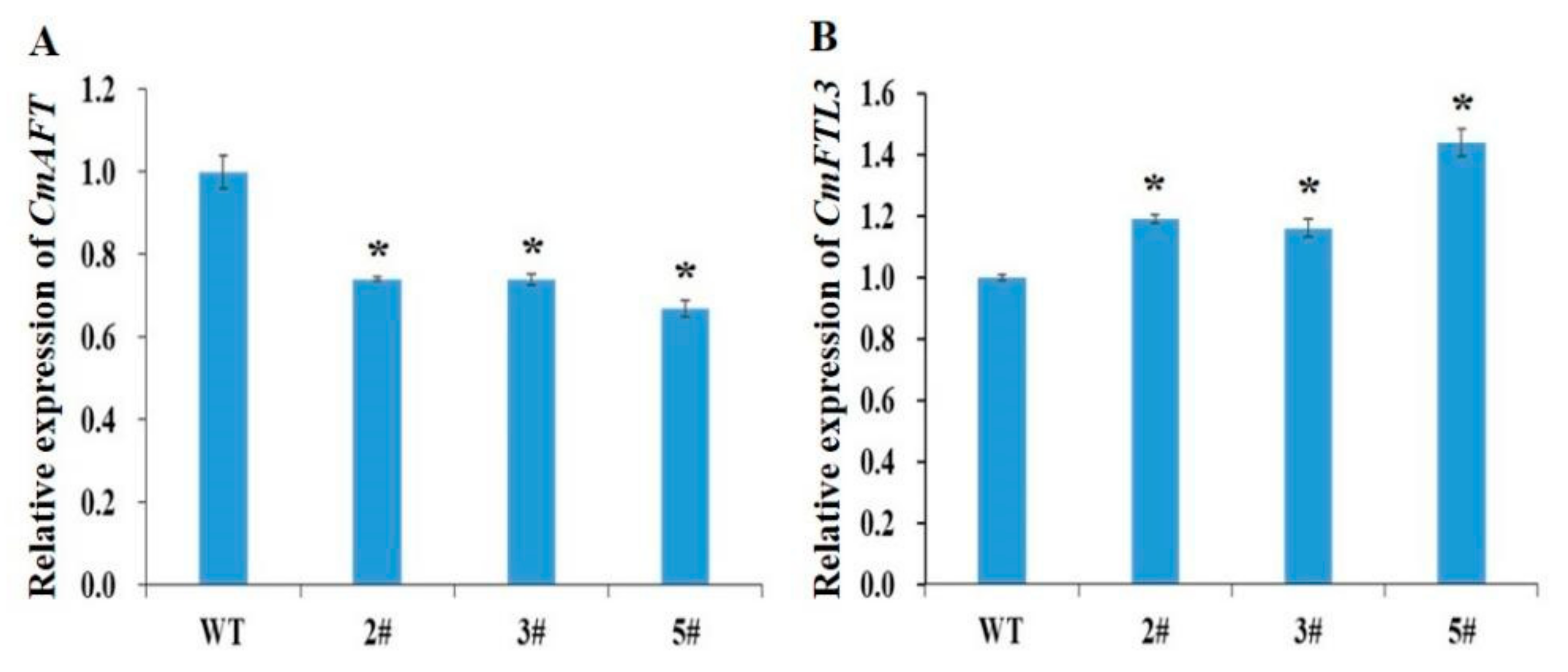
3.4. Overexpression of the CmFL3 Gene Accelerated Flowering in Chrysanthemum
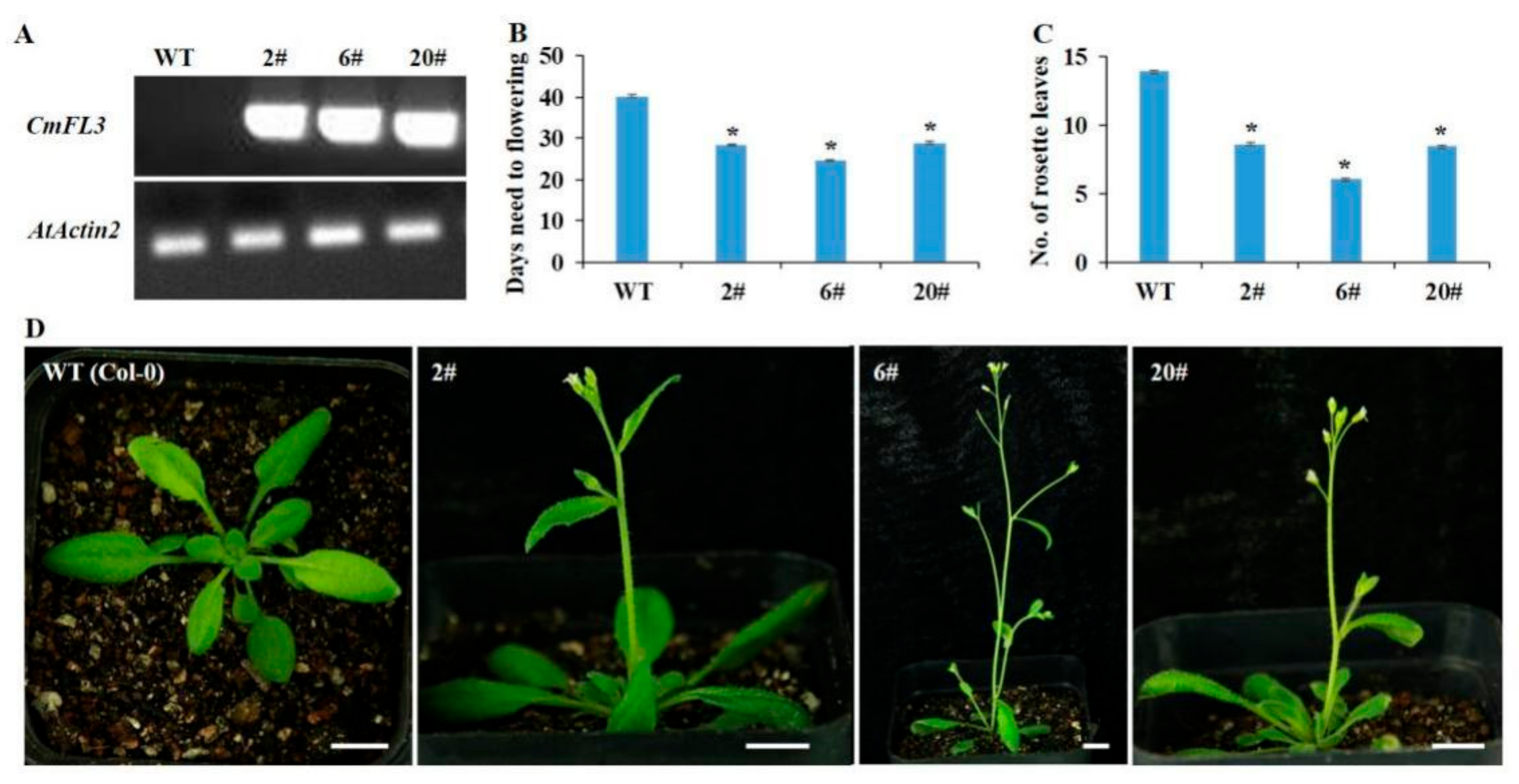
3.5. Ectopic Expression of CmFL3 Induced Early Flowering in Arabidopsis
4. Discussion
4.1. CmFL3 Is One of the FUL Group MADS-Box Genes
4.2. The Function of CmFL3 Is Conserved in the Regulation of Flowering, but Not in Flower Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rounsley, S.D.; Ditta, G.S.; Yanofsky, M.F. Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 1995, 7, 1259–1269. [Google Scholar] [PubMed] [Green Version]
- Ng, M.; Yanofsky, M.F. Function and evolution of the plant MADS-box gene family. Nat. Rev. Genet. 2001, 2, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Theißen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 2003, 29, 464–489. [Google Scholar] [CrossRef]
- Hileman, L.C.; Sundstrom, J.F.; Litt, A.; Chen, M.; Shumba, T.; Irish, V.F. Molecular and phylogenetic analyses of the MADS-box gene family in tomato. Mol. Biol. Evol. 2006, 23, 2245–2258. [Google Scholar] [CrossRef]
- Dornelas, M.C.; Patreze, C.M.; Angenent, G.C.; Immink, R.G. MADS: The missing link between identity and growth? Trends Plant Sci. 2011, 16, 89–97. [Google Scholar] [CrossRef]
- Immink, R.G.; Ferrario, S.; Busscher-Lange, J.; Kooiker, M.; Busscher, M.; Angenent, G.C. Analysis of the petunia MADS-box transcription factor family. Mol. Genet. Genom. 2003, 268, 598–606. [Google Scholar] [CrossRef]
- Smaczniak, C.; Immink, R.G.; Angenent, G.C.; Kaufmann, K. Developmental and evolutionary diversity of plant MADS-domain factors: Insights from recent studies. Development 2012, 139, 3081–3098. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Shi, X.; Lin, X.; Liu, Y.; Chong, K.; TheiãŸEn, G.; Meng, Z. The ABCs of flower development: Mutational analysis of AP1/FUL-like genes in rice provides evidence for a homeotic (A)-function in grasses. Plant J. 2017, 89, 310–324. [Google Scholar] [CrossRef]
- Huijser, P.; Klein, J.; Lönnig, W.; Meijer, H.; Saedler, H.; Sommer, H. Bracteomania, an inflorescence anomaly, is caused by the loss of function of the MADS-box gene squamosa in Antirrhinum majus. EMBO J. 1992, 11, 1239–1249. [Google Scholar] [CrossRef]
- Bowman, J.L.; Alvarez, J.; Weigel, D.; Meyerowitz, E.M.; Smyth, D.R. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 1993, 119, 721–743. [Google Scholar] [CrossRef]
- Ferrandiz, C.; Gu, Q.; Martienssen, R.; Yanofsky, M.F. Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 2000, 127, 725–734. [Google Scholar] [CrossRef]
- Li, C.; Lin, H.; Chen, A.; Lau, M.; Jernstedt, J.; Dubcovsky, J. Wheat VRN1, FUL2 and FUL3 play critical and redundant roles in spikelet development and spike determinacy. Development 2019, 146, dev175398. [Google Scholar] [CrossRef] [Green Version]
- Litt, A.; Irish, V.F. Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: Implications for the evolution of floral development. Genetics 2003, 165, 821–833. [Google Scholar] [CrossRef]
- Ruokolainen, S.; Ng, Y.P.; Broholm, S.K.; Albert, V.A.; Elomaa, P.; Teeri, T.H. Characterization of SQUAMOSA-like genes in Gerbera hybrida, including one involved in reproductive transition. BMC Plant Biol. 2010, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Immink, R.G.; Hannapel, D.J.; Ferrario, S.; Busscher, M.; Franken, J.; Campagne, M.L.; Angenent, G.C. A petunia MADS box gene involved in the transition from vegetative to reproductive development. Development 1999, 126, 5117–5126. [Google Scholar] [CrossRef]
- Zhao, K.; Ding, L.; Xia, W.; Zhao, W.; Zhang, X.; Jiang, J.; Chen, F. Characterization of an APETALA1 and a FRUITFUL-like homolog in chrysanthemum. Sci. Hortic. 2020, 272, 109518. [Google Scholar] [CrossRef]
- Chuang, T.H.; Li, K.H.; Li, P.F.; Yang, C.H. Functional analysis of an APETALA1-like MADS box gene from Eustoma grandiflorum in regulating floral transition and formation. Plant Biotechnol. Rep. 2018, 12, 115–125. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yasuno, N.; Sato, Y.; Yoda, M.; Yamazaki, R.; Kimizu, M.; Kyozuka, J. Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell 2012, 24, 1848–1859. [Google Scholar] [CrossRef] [Green Version]
- Berbel, A.; Ferrandiz, C.; Hecht, V.; Dalmais, M.; Lund, O.S.; Sussmilch, F.C.; Madueno, F. VEGETATIVE1 is essential for development of the compound inflorescence in pea. Nat. Commun. 2012, 3, 797. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Takase, T.; Takahashi, S.; Sumitomo, K.; Higuchi, Y.; Hisamatsu, T. Chrysanthemum requires short-day repeats for anthesis: Gradual CsFTL3 induction through a feedback loop under short-day conditions. Plant Sci. 2019, 283, 247–255. [Google Scholar] [CrossRef]
- Higuchi, Y.; Narumi, T.; Oda, A.; Nakano, Y.; Sumitomo, K.; Fukai, S.; Hisamatsu, T. The gated induction system of a systemic floral inhibitor, antiflorigen, determines obligate short-day flowering in chrysanthemums. Proc. Natl. Acad. Sci. USA 2013, 110, 17137–17142. [Google Scholar] [CrossRef] [Green Version]
- Teper-Bamnolker, P.; Samach, A. The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell 2005, 17, 2661–2675. [Google Scholar] [CrossRef] [Green Version]
- Shan, H.; Zhang, N.; Liu, C.; Xu, G.; Zhang, J.; Chen, Z. Patterns of gene duplication and functional diversification during the evolution of the AP1/SQUA subfamily of plant MADS-box genes. Mol. Phylogenetics Evol. 2007, 44, 26–41. [Google Scholar] [CrossRef]
- Mandel, M.A.; Gustafsonbrown, C.; Savidge, B.; Yanofsky, M.F. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 1992, 360, 273–277. [Google Scholar] [CrossRef]
- Irish, V.F.; Sussex, I.M. Function of the apetala-1 gene during Arabidopsis floral development. Plant Cell 1990, 2, 741–753. [Google Scholar]
- Benlloch, R.; d′Erfurth, I.; Ferrandiz, C.; Cosson, V.; Beltrán, J.P.; Cañas, L.A.; Ratet, P. Isolation of mtpim proves Tnt1 a useful reverse genetics tool in Medicago truncatula and uncovers new aspects of AP1-like functions in legumes. Plant Physiol. 2006, 142, 972–983. [Google Scholar] [CrossRef] [Green Version]
- Balanzã, V.; Martínez-Fernández, I.; Sato, S.; Yanofsky, M.F.; Kaufmann, K.; Angenent, G.C.; Ferrã, N.C. Genetic control of meristem arrest and life span in Arabidopsis by a FRUITFULL-APETALA2 pathway. Nat. Commun. 2018, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Ferrandiz, C.; Liljegren, S.J.; Yanofsky, M.F. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 2000, 289, 436–438. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, Y.; Fu, J.; Qi, S.; Ma, H.; Dai, S. Isolation and functional analysis of the ClM8-FRUITFULL-like MADS-box gene from Chrysanthemum lavandulifolium. Sci. Hortic. 2013, 161, 125–133. [Google Scholar] [CrossRef]
- Litt, A. An evaluation of A-function: Evidence from the APETALA1 and APETALA2 gene lineages. Int. J. Plant Sci. 2007, 168, 73–91. [Google Scholar] [CrossRef]
- Gramzow, L.; Theissen, G. A hitchhiker′s guide to the MADS world of plants. Genome Biol. 2010, 11, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shchennikova, A.V.; Shulga, O.A.; Immink, R.; Skryabin, K.G.; Angenent, G.C. Identification and characterization of four chrysanthemum MADS-box genes, belonging to the APETALA1/FRUITFULL and SEPALLATA3 subfamilies. Plant Physiol. 2004, 134, 1632–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandel, M.A.; Yanofsky, M.F. The Arabidopsis AGL8 MADS box gene is expressed in inflorescence meristems and is negative regulated by APETALAL1. Plant Cell 1995, 7, 1763–1771. [Google Scholar] [PubMed] [Green Version]
- Gu, Q.; Ferrándiz, C.; Yanofsky, M.F.; Martienssen, R. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 1998, 125, 1509–1517. [Google Scholar] [CrossRef]
- Melzer, S.; Lens, F.; Gennen, J.; Vanneste, S.; Rohde, A.; Beeckman, T. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 2008, 40, 1489–1492. [Google Scholar] [CrossRef] [Green Version]
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of flowering in Arabidopsis. Cell 2010, 141, 550.e1–550.e2. [Google Scholar] [CrossRef] [Green Version]
- Shulga, O.A.; Mitiouchkina, T.Y.; Shchennikova, A.V.; Skryabin, K.G.; Dolgov, S.V. Overexpression of AP1-like genes from Asteraceae induces early-flowering in transgenic Chrysanthemum plants. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 553–560. [Google Scholar] [CrossRef]
- Oda, A.; Narumi, T.; Li, T.; Kando, T.; Higuchi, Y.; Sumitomo, K.; Hisamatsu, T. CsFTL3, a chrysanthemum FLOWERING LOCUS T-like gene, is a key regulator of photoperiodic flowering in chrysanthemums. J. Exp. Bot. 2012, 63, 1461–1477. [Google Scholar] [CrossRef] [Green Version]
- Shulga, O.; Shchennikova, A.; Angenent, G.; Skryabin, K. MADS-box genes controlling inflorescence morphogenesis in sunflower. Russ. J. Dev. Biol. 2008, 39, 2–5. [Google Scholar] [CrossRef]
- Higuchi, Y.; Hisamatsu, T. CsTFL1; a constitutive local repressor of flowering; modulates floral initiation by antagonising florigen complex activity in chrysanthemum. Plant Sci. 2015, 237, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Ren, L.; Zhou, M.; Han, X.; Ding, L.; Jiang, J. CmBBX8 accelerates flowering by targeting CmFTL1 directly in summer chrysanthemum. Plant Biotechnol. J. 2020, 18, 1562–1572. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Ding, L.; Jiang, J.; Shentu, Y.; Zhao, W.; Zhao, K.; Chen, F. Overexpression of the CmJAZ1-like gene delays flowering in Chrysanthemum morifolium. Hortic. Res. 2021, 8, 87. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Ding, L.; Song, A.; Shen, F.; Jiang, J. Transcriptomic and hormone analyses reveal mechanisms underlying petal elongation in Chrysanthemum morifolium ‘Jinba’. Plant Mol. Biol. 2017, 93, 593–606. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood evolutionary distance and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gu, C.; Chen, S.; Liu, Z.; Hong, S.; Luo, H.; Guan, Z.; Chen, F. Reference gene selection for quantitative real-time PCR in chrysanthemum subjected to biotic and abiotic stress. Mol. Biotechnol. 2011, 49, 192–197. [Google Scholar] [CrossRef]
- Ding, L.; Song, A.; Zhang, X.; Li, S.; Su, J.; Xia, W.; Chen, F. The core regulatory networks and hub genes regulating flower development in Chrysanthemum morifolium. Plant Bolecular Biol. 2020, 103, 669–688. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Song, A.; Chen, S.; Shan, H.; Luo, H.; Fang, W. Ambient temperature enhanced freezing tolerance of Chrysanthemum dichrum CdICE1 Arabidopsis via miR398. BMC Biol. 2013, 11, 121. [Google Scholar] [CrossRef] [Green Version]
- Song, A.; Lou, W.; Jiang, J.; Chen, S.; Sun, Z.; Guan, Z.; Uversky, V.N. An isoform of eukaryotic initiation factor 4E from Chrysanthemum morifolium interacts with Chrysanthemum virus B coat protein. PLoS ONE 2013, 8, e57229. [Google Scholar] [CrossRef] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guan, Y.; Ding, L.; Li, P.; Zhao, W.; Jiang, J.; Chen, F. The CmTCP20 gene regulates petal elongation growth in Chrysanthemum morifolium. Plant Sci. 2019, 280, 248–257. [Google Scholar] [CrossRef]
- Oda, A.; Higuchi, Y.; Hisamatsu, T. Constitutive expression of CsGI alters critical night length for flowering by changing the photo-sensitive phase of anti-florigen induction in chrysanthemum. Plant Sci. 2020, 293, 110417. [Google Scholar] [CrossRef]
- Balanzà, V.; Martínez-Fernández, I.; Ferrándiz, C. Sequential action of FRUITFULL as a modulator of the activity of the floral regulators SVP and SOC1. J. Exp. Bot. 2014, 65, 1193–1203. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, Y.; Wang, F.; Guo, Y.; Duan, X.; Sun, J.; An, H. Functional conservation and diversification of APETALA1/FRUITFULL genes in Brachypodium distachyon. Physiol. Plant. 2016, 157, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.J.; Wei, H.; Wang, Y.; Wang, H.M.; Yang, R.F.; Zhang, X.B. Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (Oryza sativa L.). Plant Mol. Biol. Report. 2012, 30, 1461–1469. [Google Scholar] [CrossRef]
- Urbanus, S.L.; Folter, S.D.; Shchennikova, A.V.; Kaufmann, K.; Immink, R.G.; Angenent, G.C. In planta localisation patterns of MADS domain proteins during floral development in Arabidopsis thaliana. BMC Plant Biol. 2009, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Higuchi, Y.; Sumitomo, K.; Hisamatsu, T. Flowering retardation by high temperature in chrysanthemums: Involvement of FLOWERING LOCUS T-like 3 gene repression. J. Exp. Bot. 2013, 64, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, H.; Ren, L.; Chen, S.; Chen, F.; Jiang, J. CmFTL2 is involved in the photoperiod- and sucrose-mediated control of flowering time in chrysanthemum. Hortic. Res. 2017, 4, 17001. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Ju, Y.; Seo, P.J.; Lee, J.H.; Park, C.M. The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J. 2012, 69, 577–588. [Google Scholar] [CrossRef]
- Mcgarry, R.C.; Kragler, F. Phloem-mobile signals affecting flowers: Applications for crop breeding. Trends Plant Sci. 2013, 18, 198–206. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Q.; Liu, Y.; Cheng, P.; Cheng, H.; Liu, W. Strigolactone represses the synthesis of melatonin; thereby inducing floral transition in Arabidopsis thaliana in an FLC-dependent manner. J. Pineal Res. 2019, 67, e12582. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Ferrándiz, C.; Madueño, F.; Parcy, F. How floral meristems are built. Plant Mol. Biol. 2006, 60, 855–870. [Google Scholar] [CrossRef]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y. FD; a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef]
- Li, C.; Dubcovsky, J. Wheat FT protein regulates VRN1 transcription through interactions with FDL2. Plant J. 2010, 55, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Koh, J.; Yoo, M.J.; Kong, H.; Hu, Y.; Ma, H. Expression of floral MADS-box genes in basal angiosperms: Implications for the evolution of floral regulators. Plant J. 2005, 43, 724–744. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Li, S.; Jia, D.; Xing, X.; Wang, H.; Song, A.; Jiang, J.; Chen, S.; Chen, F.; Ding, L. Characterization of the MADS-Box Gene CmFL3 in chrysanthemum. Agronomy 2022, 12, 1716. https://doi.org/10.3390/agronomy12071716
Zhao K, Li S, Jia D, Xing X, Wang H, Song A, Jiang J, Chen S, Chen F, Ding L. Characterization of the MADS-Box Gene CmFL3 in chrysanthemum. Agronomy. 2022; 12(7):1716. https://doi.org/10.3390/agronomy12071716
Chicago/Turabian StyleZhao, Kunkun, Song Li, Diwen Jia, Xiaojuan Xing, Haibin Wang, Aiping Song, Jiafu Jiang, Sumei Chen, Fadi Chen, and Lian Ding. 2022. "Characterization of the MADS-Box Gene CmFL3 in chrysanthemum" Agronomy 12, no. 7: 1716. https://doi.org/10.3390/agronomy12071716






