Functional Analysis of BcSNX3 in Regulating Resistance to Turnip Mosaic Virus (TuMV) by Autophagy in Pak-choi (Brassica campestris ssp. chinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Gene Cloning and Plasmid Construction
2.3. DUAL Membrane Yeast-Two-Hybrid (mYTH) Assay
2.4. Transient Protein Expression
2.5. Virus-Induced Gene Silencing (VIGS) Experiment
2.6. TuMV Inoculation
2.7. RNA Extraction and qRT-PCR
2.8. Statistical Analysis
3. Results
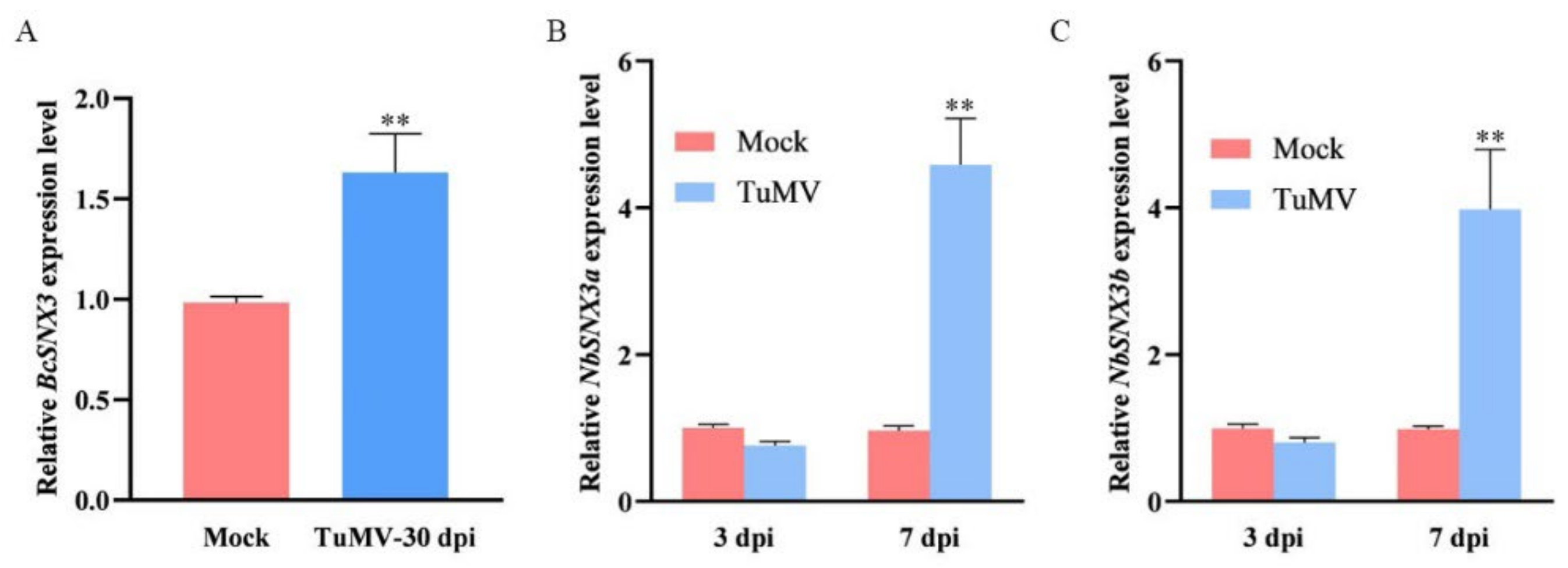
3.1. TuMV Infection Accelerates the Expression Level of SNX3
3.2. Subcellular Localization of BcSNX3
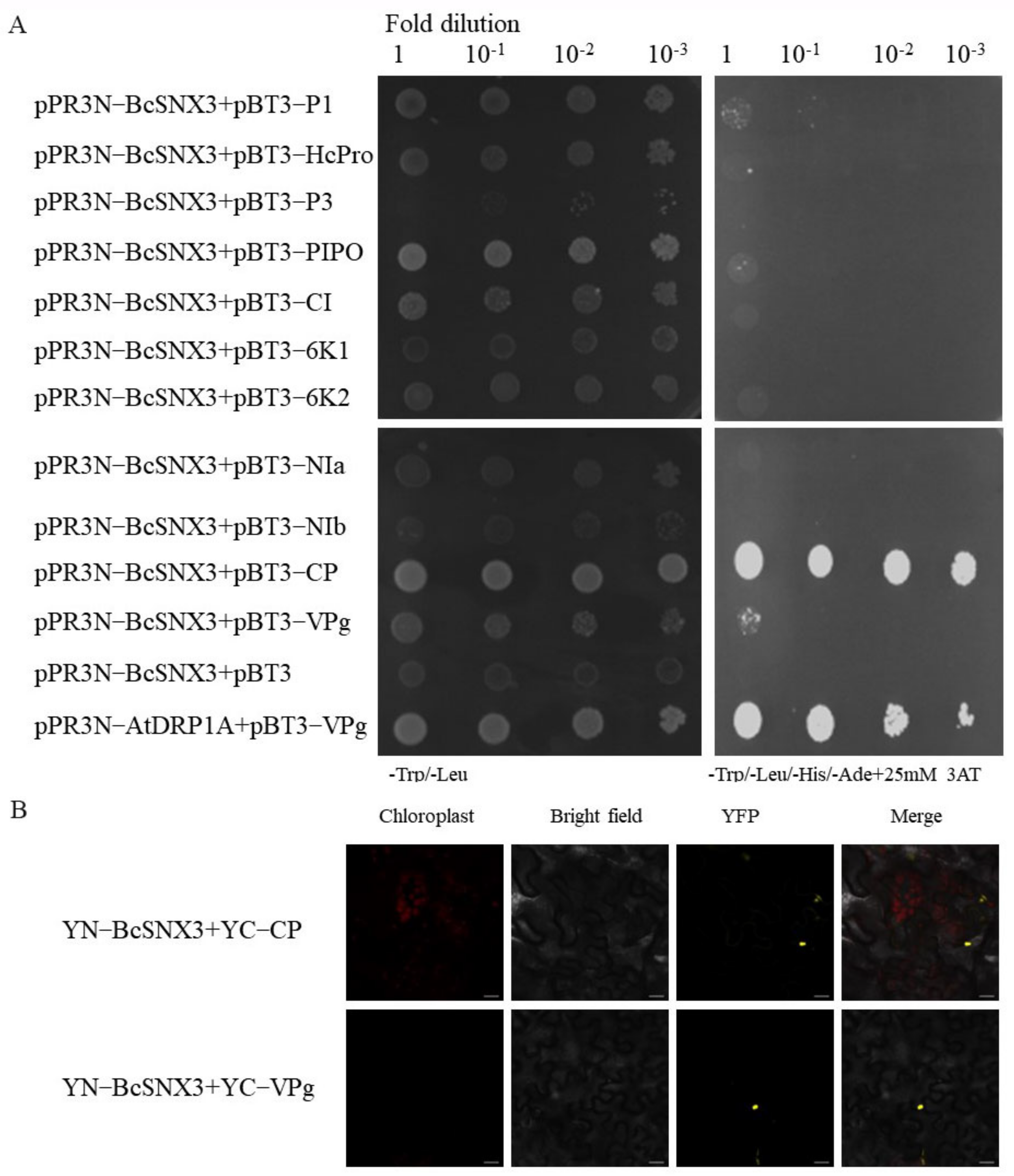
3.3. BcSNX3 Interacts with CP and VPg
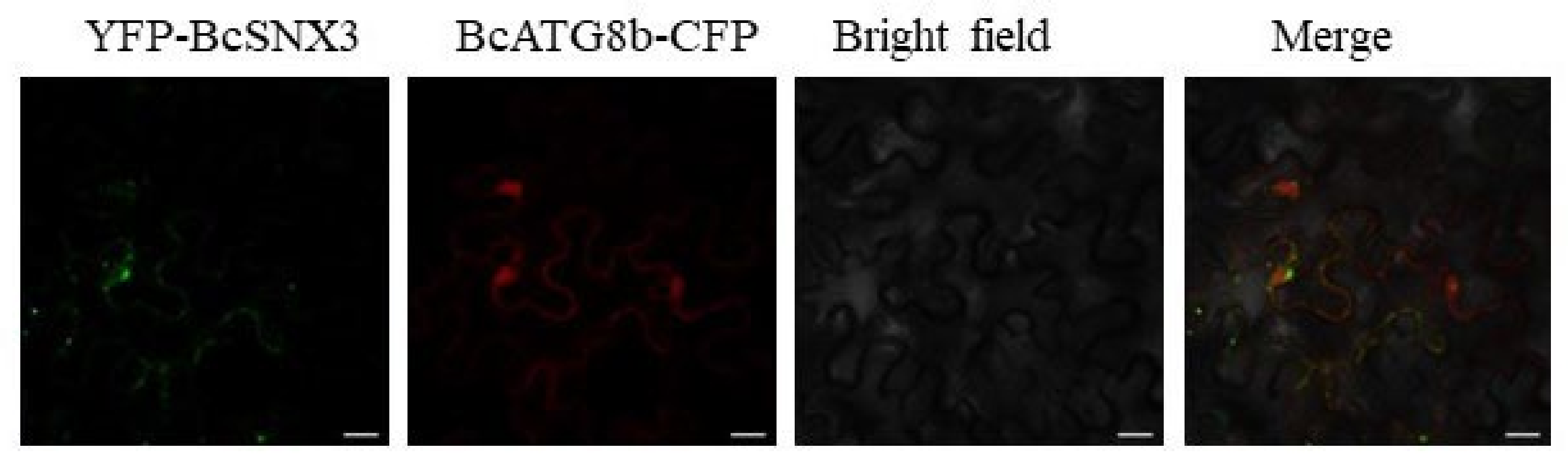
3.4. BcSNX3 Colocalized with the Autophagosome Marker BcATG8b
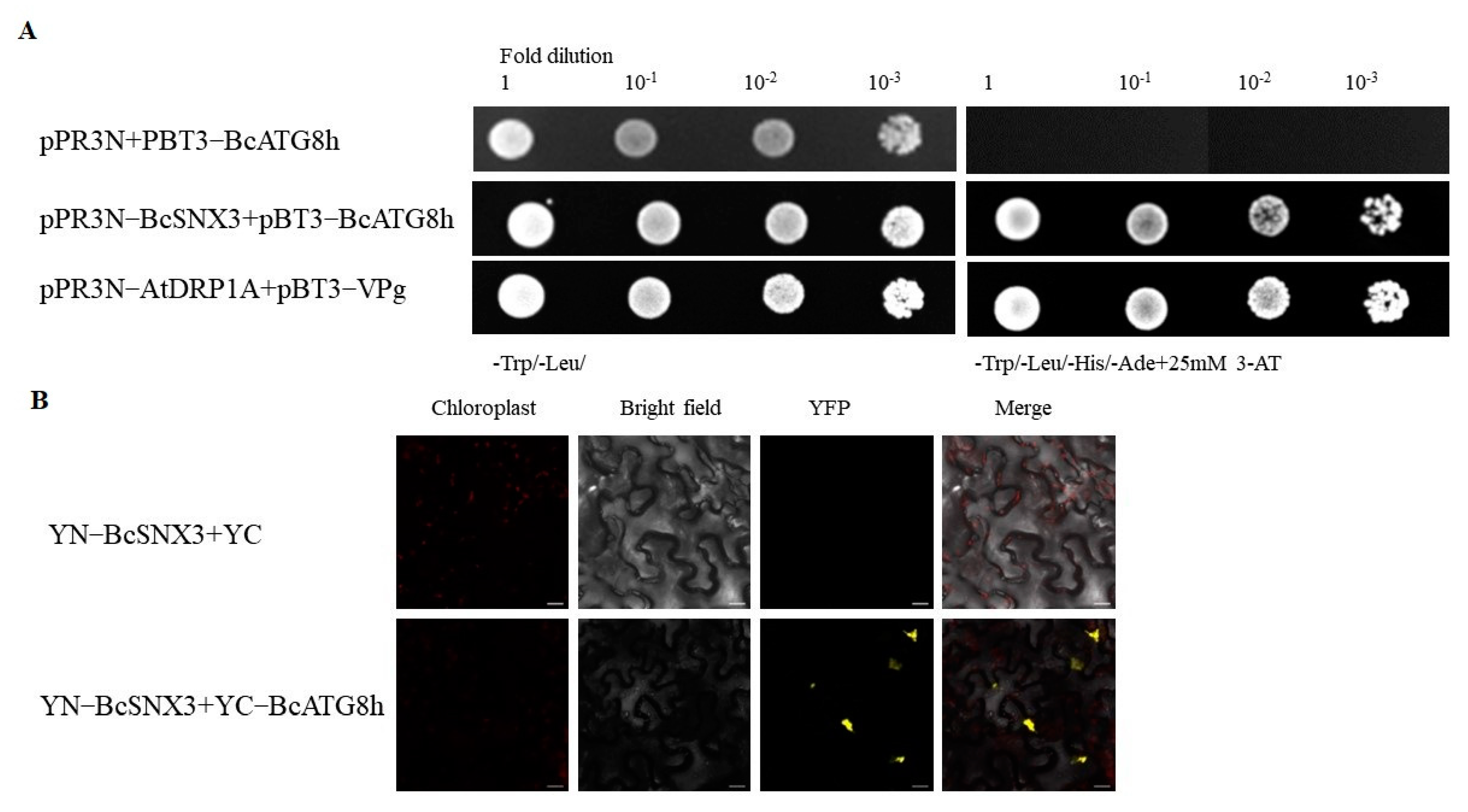
3.5. BcSNX3 Interacts with BcATG8h
3.6. Overexpression of BcSNX3 Inhibits the Expression of Autophagy Components and Promotes TuMV Infection in N. benthamiana
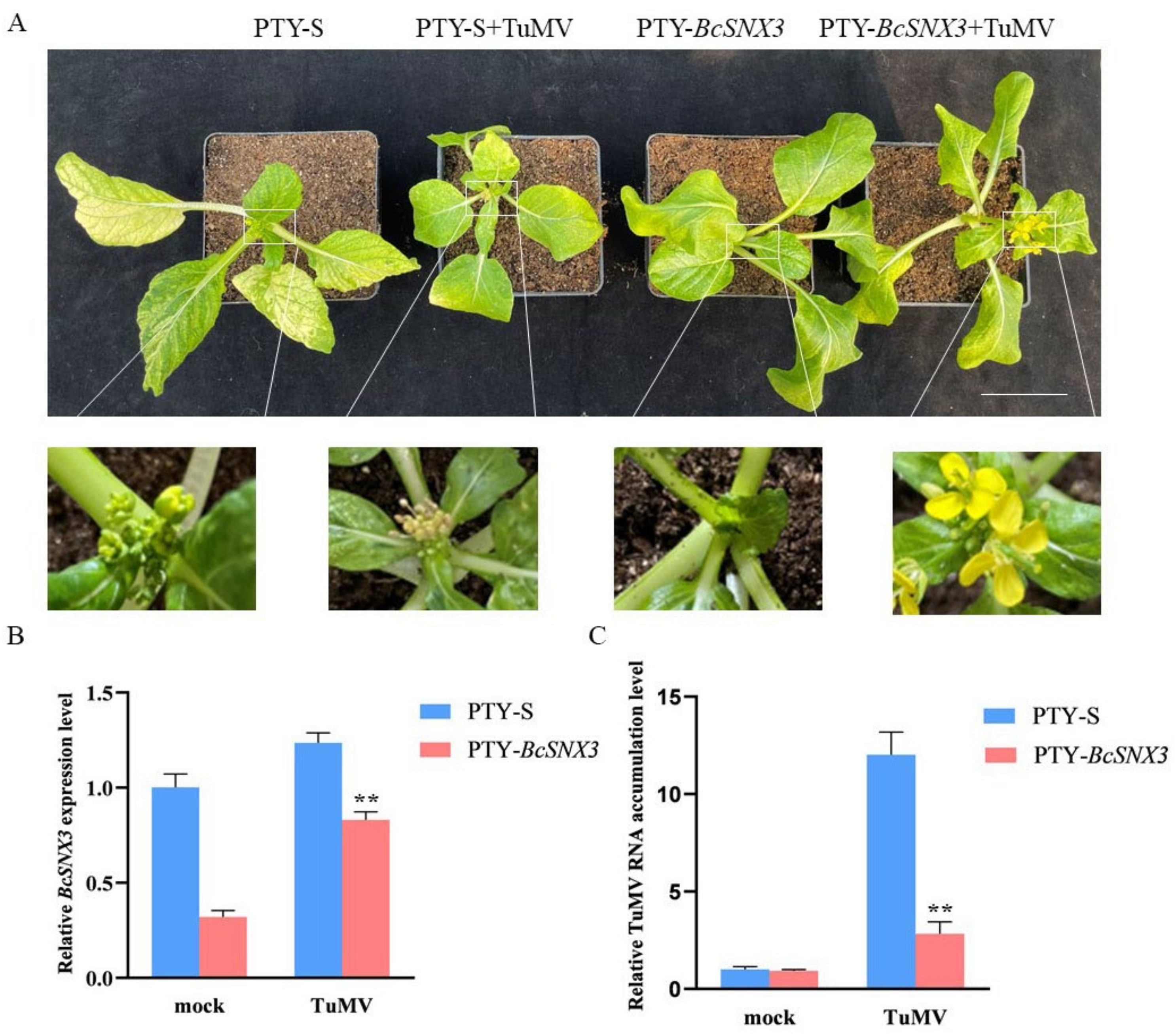
3.7. BcSNX3 Gene Silencing Inhibits TuMV Infection in Pak-choi
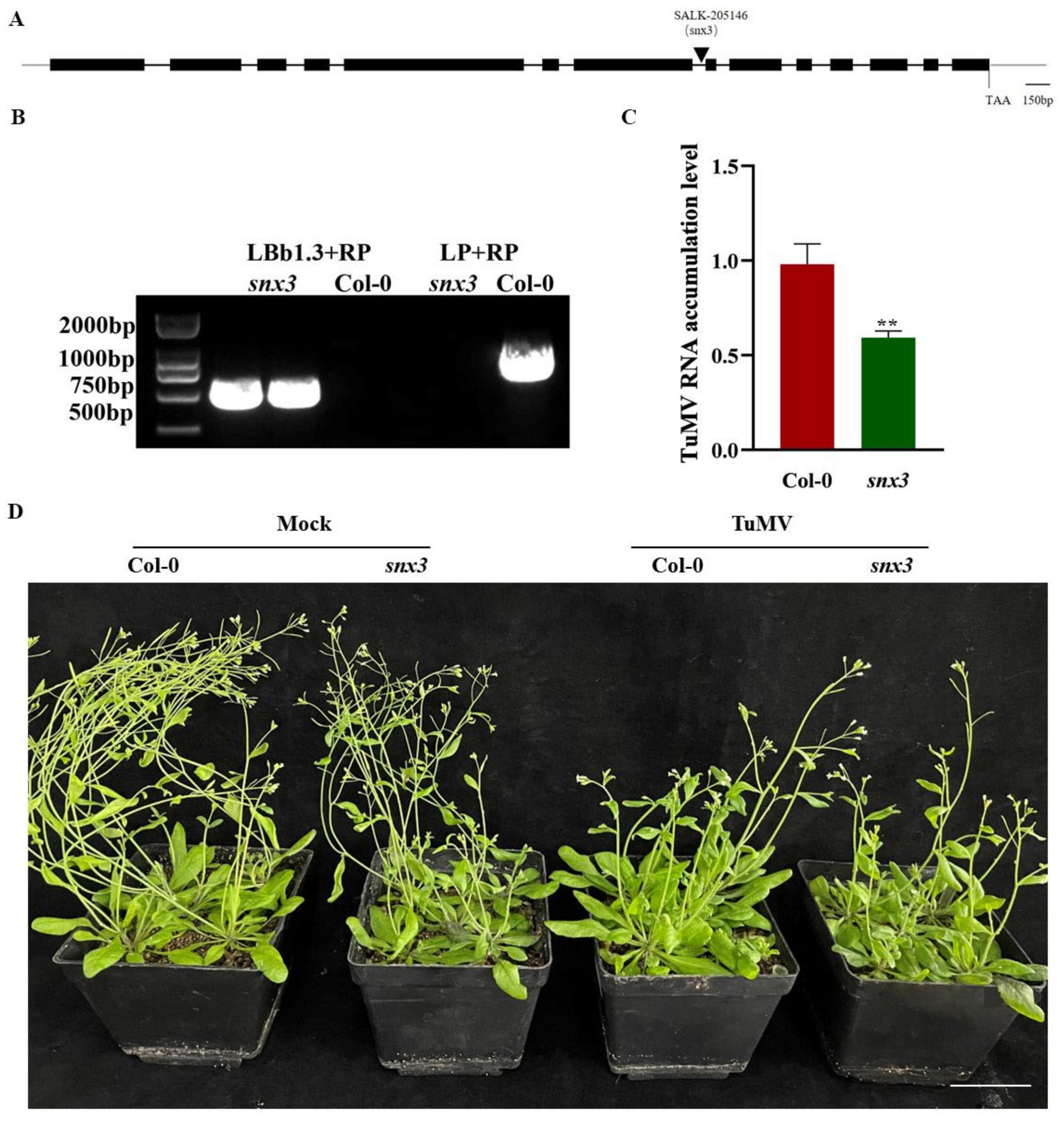
3.8. AtSNX3 Gene Knockout Suppressed TuMV Infection in Arabidopsis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, S.J.; Yu, B.J.; Wang, Y.; Liu, Y.L. Role of plant autophagy in stress response. Protein Cell 2011, 2, 784–791. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by Self-Digestion Molecular Mechanisms and Biological Functions of Autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Bassham, D.C. Function and regulation of macroautophagy in plants. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2009, 1793, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Yu, J.Q.; Chen, Z.X. Role and regulation of autophagy in heat stress responses of tomato plants. Front. Plant Sci. 2014, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yin, L.-L.; Zhou, J.; Xia, X.-J.; Shi, K.; Yu, J.-Q.; Zhou, Y.-H.; Foyer, C.H. Interactions between 2-Cys peroxiredoxins and ascorbate in autophagosome formation during the heat stress response in Solanum lycopersicum. J. Exp. Bot. 2016, 67, 1919–1933. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cai, S.-Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Reiter, R.J.; et al. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal. Res. 2016, 61, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Xiong, Y.; Bassham, D.C. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 2009, 5, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Ismayil, A.; Yang, M.; Liu, Y.L. Role of autophagy during plant-virus interactions. Semin. Cell Dev. Biol. 2020, 101, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shao, L.; Wang, J.; Zhang, Y.; Guo, X.; Peng, Y.; Cao, Y.; Lai, Z. Phosphorylation of ATG18a by BAK1 suppresses autophagy and attenuates plant resistance against necrotrophic pathogens. Autophagy 2021, 17, 2093–2110. [Google Scholar] [CrossRef]
- Niu, E.; Liu, H.; Zhou, H.; Luo, L.; Wu, Y.; Andika, I.B.; Sun, L. Autophagy Inhibits Intercellular Transport of Citrus Leaf Blotch Virus by Targeting Viral Movement Protein. Viruses 2021, 13, 2189. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Zheng, X.; Yang, X.; Chen, Y.; Zhang, T.; Zhao, X.; Wang, S.; Zhao, X.; Song, X.; et al. The plant protein NbP3IP directs degradation of Rice stripe virus p3 silencing suppressor protein to limit virus infection through interaction with the autophagy-related protein NbATG8. New Phytol. 2021, 229, 1036–1051. [Google Scholar] [CrossRef]
- Tong, X.; Liu, S.-Y.; Zou, J.-Z.; Zhao, J.-J.; Zhu, F.-F.; Chai, L.-X.; Wang, Y.; Han, C.; Wang, X.-B. A small peptide inhibits siRNA amplification in plants by mediating autophagic degradation of SGS3/RDR6 bodies. EMBO J. 2021, 40, e108050. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Tang, D. The autophagy gene, ATG18a, plays a negative role in powdery mildew resistance and mildew-induced cell death in Arabidopsis. Plant Signal Behav. 2011, 6, 1408–1410. [Google Scholar] [CrossRef]
- Patel, S.; Dinesh-Kumar, S.P. Arabidopsis ATG6 is required to limit the pathogen-associated cell death response. Autophagy 2008, 4, 20–27. [Google Scholar] [CrossRef]
- Lu, Y.; He, P.; Zhang, Y.; Ren, Y.; Zhang, L. The emerging roles of retromer and sorting nexins in the life cycle of viruses. Virol. Sin. 2022, 37, 321–330. [Google Scholar] [CrossRef]
- Teasdale, R.D.; Loci, D.; Houghton, F.; Karlsson, L.; Gleeson, P.A. A large family of endosome-localized proteins related to sorting nexin 1. Biochem. J. 2001, 358, 7–16. [Google Scholar] [CrossRef]
- Worby, C.A.; Dixon, J.E. Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 2002, 3, 919–931. [Google Scholar] [CrossRef]
- Heucken, N.; Ivanov, R. The retromer, sorting nexins and the plant endomembrane protein trafficking. J. Cell Sci. 2018, 131, jcs203695. [Google Scholar] [CrossRef]
- Barr, V.A.; Phillips, S.A.; Taylor, S.I.; Haft, C.R. Overexpression of a Novel Sorting Nexin, SNX15, Affects Endosome Morphology and Protein Trafficking. Traffic 2000, 1, 904–916. [Google Scholar] [CrossRef]
- Rojas, R.; Kametaka, S.; Haft, C.R.; Bonifacino, J.S. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol. Cell Biol. 2007, 27, 1112–1124. [Google Scholar] [CrossRef]
- Cullen, P.J.; Korswagen, H.C. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat. Cell Biol. 2011, 14, 29–37. [Google Scholar] [CrossRef]
- Harterink, M.; Port, F.; Lorenowicz, M.J.; McGough, I.J.; Silhankova, M.; Betist, M.C.; van Weering, J.R.T.; van Heesbeen, R.G.H.P.; Middelkoop, T.C.; Basler, K.; et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat. Cell Biol. 2011, 13, 914–923. [Google Scholar] [CrossRef]
- Lorenowicz, M.J.; Macurkova, M.; Harterink, M.; Middelkoop, T.C.; De Groot, R.; Betist, M.C.; Korswagen, H.C. Inhibition of late endosomal maturation restores Wnt secretion in Caenorhabditis elegans vps-29 retromer mutants. Cell. Signal. 2014, 26, 19–31. [Google Scholar] [CrossRef]
- Lu, D.; Li, Y.; Liu, Q.-R.; Wu, Q.; Zhang, H.; Xie, P.; Wang, Q. Wls promotes the proliferation of breast cancer cells via Wnt signaling. Med. Oncol. 2015, 32, 140. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Huang, J.; Dong, Q. Wnt signaling regulation of stem-like properties in human lung adenocarcinoma cell lines. Med. Oncol. 2015, 32, 157. [Google Scholar] [CrossRef]
- Yang, M.; Han, Y.-M.; Han, Q.; Rong, X.-Z.; Liu, X.-F.; Ln, X.-Y. KCTD11 inhibits progression of lung cancer by binding to β-catenin to regulate the activity of the Wnt and Hippo pathways. J. Cell Mol. Med. 2021, 25, 9411–9426. [Google Scholar] [CrossRef]
- Liang, C.; Li, C.; Wu, J.; Zhao, M.; Chen, D.; Liu, C.; Chu, J.; Zhang, W.; Hwang, I.; Wang, M. SORTING NEXIN2 Proteins Mediate Stomatal Movement and Response to Drought Stress by Modulating Trafficking and Protein Levels of the ABA Exporter ABCG25. Plant J. 2022, 110, 1603–1618. [Google Scholar] [CrossRef]
- Nishimura, Y.; Takiguchi, S.; Yoshioka, K.; Nakabeppu, Y.; Itoh, K. Silencing of SNX1 by siRNA stimulates the ligand-induced endocytosis of EGFR and increases EGFR phosphorylation in gefitinib-resistant human lung cancer cell lines. Int. J. Oncol. 2012, 41, 1520–1530. [Google Scholar] [CrossRef]
- Wu, G.; Cui, X.; Dai, Z.; He, R.; Li, Y.; Yu, K.; Bernards, M.; Chen, X.; Wang, A. A plant RNA virus hijacks endocytic proteins to establish its infection in plants. Plant J. 2020, 101, 384–400. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, C.; Hou, X. Cloning and Functional Analysis of BcMYB101 Gene Involved in Leaf Development in Pak Choi (Brassica rapa ssp. Chinensis). Int. J. Mol. Sci. 2020, 21, 2750. [Google Scholar] [CrossRef]
- Yu, J.; Yang, X.D.; Wang, Q.; Gao, L.W.; Yang, Y.; Xiao, D.; Liu, T.K.; Li, Y.; Hou, X.L.; Zhang, C.W. Efficient virus-induced gene silencing in Brassica rapa using a turnip yellow mosaic virus vector. Biol. Plant. 2018, 62, 826–834. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Song, X.; Sun, F.; Xiao, D.; Wei, Y.; Hou, X.; Zhang, C. Genome-wide association study of turnip mosaic virus resistance in non-heading Chinese cabbage. 3 Biotech 2020, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, R.; Brumbarova, T.; Blum, A.; Jantke, A.-M.; Fink-Straube, C.; Bauer, P. SORTING NEXIN1 is required for modulating the trafficking and stability of the Arabidopsis IRON-REGULATED TRANSPORTER1. Plant Cell 2014, 26, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Pourcher, M.; Santambrogio, M.; Thazar, N.; Thierry, A.-M.; Fobis-Loisy, I.; Miège, C.; Jaillais, Y.; Gaude, T. Analyses of sorting nexins reveal distinct retromer-subcomplex functions in development and protein sorting in Arabidopsis thaliana. Plant Cell 2010, 22, 3980–3991. [Google Scholar] [CrossRef]
- Cotton, S.; Grangeon, R.; Thivierge, K.; Mathieu, I.; Ide, C.; Wei, T.; Wang, A.; Laliberté, J.-F. Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments, and are each derived from a single viral genome. J. Virol. 2009, 83, 10460–10471. [Google Scholar] [CrossRef]
- Huang, T.-S.; Wei, T.; Laliberté, J.-F.; Wang, A. A host RNA helicase-like protein, AtRH8, interacts with the potyviral genome-linked protein, VPg, associates with the virus accumulation complex, and is essential for infection. Plant Physiol. 2010, 152, 255–266. [Google Scholar] [CrossRef]
- Léonard, S.; Viel, C.; Beauchemin, C.; Daigneault, N.; Fortin, M.G.; Laliberté, J.-F. Interaction of VPg-Pro of Turnip mosaic virus with the translation initiation factor 4E and the poly(A)-binding protein in planta. J. Gen. Virol. 2004, 85, 1055–1063. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, Q.; Qiu, S.; Li, S.; Li, M.; Zheng, H.; Wu, G.; Lu, Y.; Peng, J.; Rao, S.; et al. Turnip mosaic virus impairs perinuclear chloroplast clustering to facilitate viral infection. Plant Cell Environ. 2021, 44, 3681–3699. [Google Scholar] [CrossRef]
- Dai, Z.; He, R.; Bernards, M.A.; Wang, A. The cis-expression of the coat protein of turnip mosaic virus is essential for viral intercellular movement in plants. Mol. Plant Pathol. 2020, 21, 1194–1211. [Google Scholar] [CrossRef]
- Nomura, K.; Ohshima, K.; Anai, T.; Uekusa, H.; Kita, N. RNA Silencing of the Introduced Coat Protein Gene of Turnip mosaic virus Confers Broad-Spectrum Resistance in Transgenic Arabidopsis. Phytopathology 2004, 94, 730–736. [Google Scholar] [CrossRef][Green Version]
- Lyu, S.W.; Gao, L.W.; Zhang, R.J.; Zhang, C.W.; Hou, X.L. Correlation Analysis of Expression Profile and Quantitative iTRAQ-LC-MS/MS Proteomics Reveals Resistance Mechanism Against TuMV in Chinese Cabbage (Brassica rapa ssp. pekinensis). Front. Genet. 2020, 11, 963. [Google Scholar] [CrossRef]
- Michaeli, S.; Clavel, M.; Lechner, E.; Viotti, C.; Wu, J.; Dubois, M.; Hacquard, T.; Derrien, B.; Izquierdo, E.; Lecorbeiller, M.; et al. The viral F-box protein P0 induces an ER-derived autophagy degradation pathway for the clearance of membrane-bound AGO1. Proc. Natl. Acad. Sci. USA 2019, 116, 22872–22883. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, H.; Li, X.; Wei, Y.; Shi, H. Functional Analysis of MaWRKY24 in Transcriptional Activation of Autophagy-Related Gene 8f/g and Plant Disease Susceptibility to Soil-Borne Fusarium oxysporum f. sp. cubense. Pathogens 2019, 8, 264. [Google Scholar] [CrossRef]
- Hayward, A.P.; Tsao, J.; Dinesh-Kumar, S.P. Autophagy and plant innate immunity Defense through degradation. Semin. Cell Dev. Biol. 2009, 20, 1041–1047. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. Atg8, a Ubiquitin-like Protein Required for Autophagosome Formation, Mediates Membrane Tethering and Hemifusion. Cell 2007, 130, 165–178. [Google Scholar] [CrossRef]
- Xie, Z.; Nair, U.; Klionsky, D.J. Atg8 controls phagophore expansion during autophagosome formation. Mol. Biol. Cell 2008, 19, 3290–3298. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Li, Y.; Wu, G.; Hou, X.; Zhou, X.; Wang, A. Beclin1 restricts RNA virus infection in plants through suppression and degradation of the viral polymerase. Nat. Commun. 2018, 9, 1268. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Tang, Z.; Zhang, L.; Dai, Z.; Lyu, S.; Li, Y.; Hou, X.; Bernards, M.; Wang, A. A plant RNA virus activates selective autophagy in a UPR-dependent manner to promote virus infection. New Phytol. 2020, 228, 622–639. [Google Scholar] [CrossRef]
- Dong, X.; Yang, Y.; Zou, Z.; Zhao, Y.; Ci, B.; Zhong, L.; Bhave, M.; Wang, L.; Kuo, Y.-C.; Zang, X.; et al. Sorting nexin 5 mediates virus-induced autophagy and immunity. Nature 2021, 589, 456–461. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhang, C.; Lyu, S.; Fang, Z.; Zhu, H.; Hou, X. Functional Analysis of BcSNX3 in Regulating Resistance to Turnip Mosaic Virus (TuMV) by Autophagy in Pak-choi (Brassica campestris ssp. chinensis). Agronomy 2022, 12, 1757. https://doi.org/10.3390/agronomy12081757
Zhang R, Zhang C, Lyu S, Fang Z, Zhu H, Hou X. Functional Analysis of BcSNX3 in Regulating Resistance to Turnip Mosaic Virus (TuMV) by Autophagy in Pak-choi (Brassica campestris ssp. chinensis). Agronomy. 2022; 12(8):1757. https://doi.org/10.3390/agronomy12081757
Chicago/Turabian StyleZhang, Rujia, Changwei Zhang, Shanwu Lyu, Zhiyuan Fang, Hongfang Zhu, and Xilin Hou. 2022. "Functional Analysis of BcSNX3 in Regulating Resistance to Turnip Mosaic Virus (TuMV) by Autophagy in Pak-choi (Brassica campestris ssp. chinensis)" Agronomy 12, no. 8: 1757. https://doi.org/10.3390/agronomy12081757
APA StyleZhang, R., Zhang, C., Lyu, S., Fang, Z., Zhu, H., & Hou, X. (2022). Functional Analysis of BcSNX3 in Regulating Resistance to Turnip Mosaic Virus (TuMV) by Autophagy in Pak-choi (Brassica campestris ssp. chinensis). Agronomy, 12(8), 1757. https://doi.org/10.3390/agronomy12081757







