Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Sieve Analysis
2.2. Organic Matter and Organic Elements Characterization
2.2.1. Thermogravimetry
2.2.2. Elemental Analysis
2.2.3. Total Organic Carbon
2.3. Conductivity and pH of Water Extract
2.4. Structural Characterization
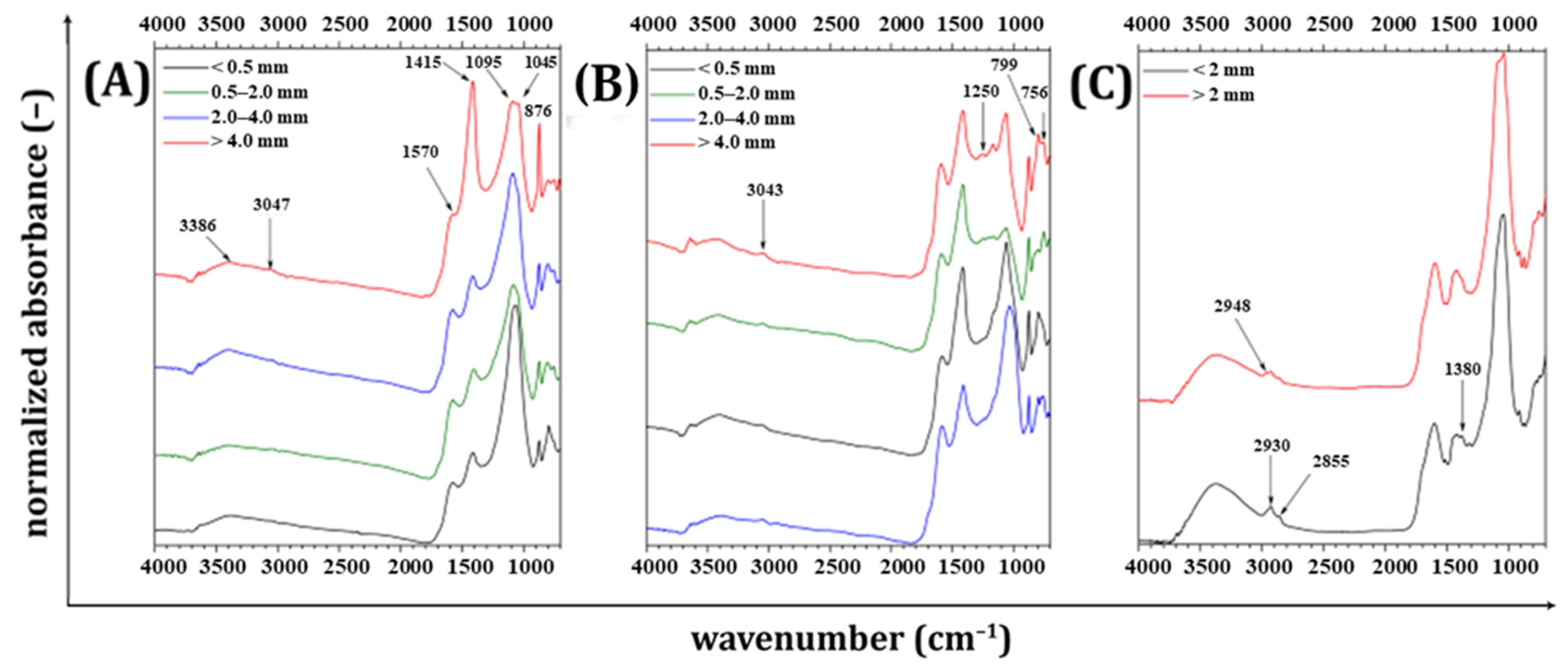
2.4.1. FTIR Spectrometry
2.4.2. 13C NMR Analysis
2.5. Morphological Analysis
2.5.1. SEM Characterization
2.5.2. BET Analysis
2.5.3. Statistical Analysis
3. Results and Discussion
3.1. Organic Matter Content and Elemental Analysis
3.2. pH, Conductivity of Aqueous Extract
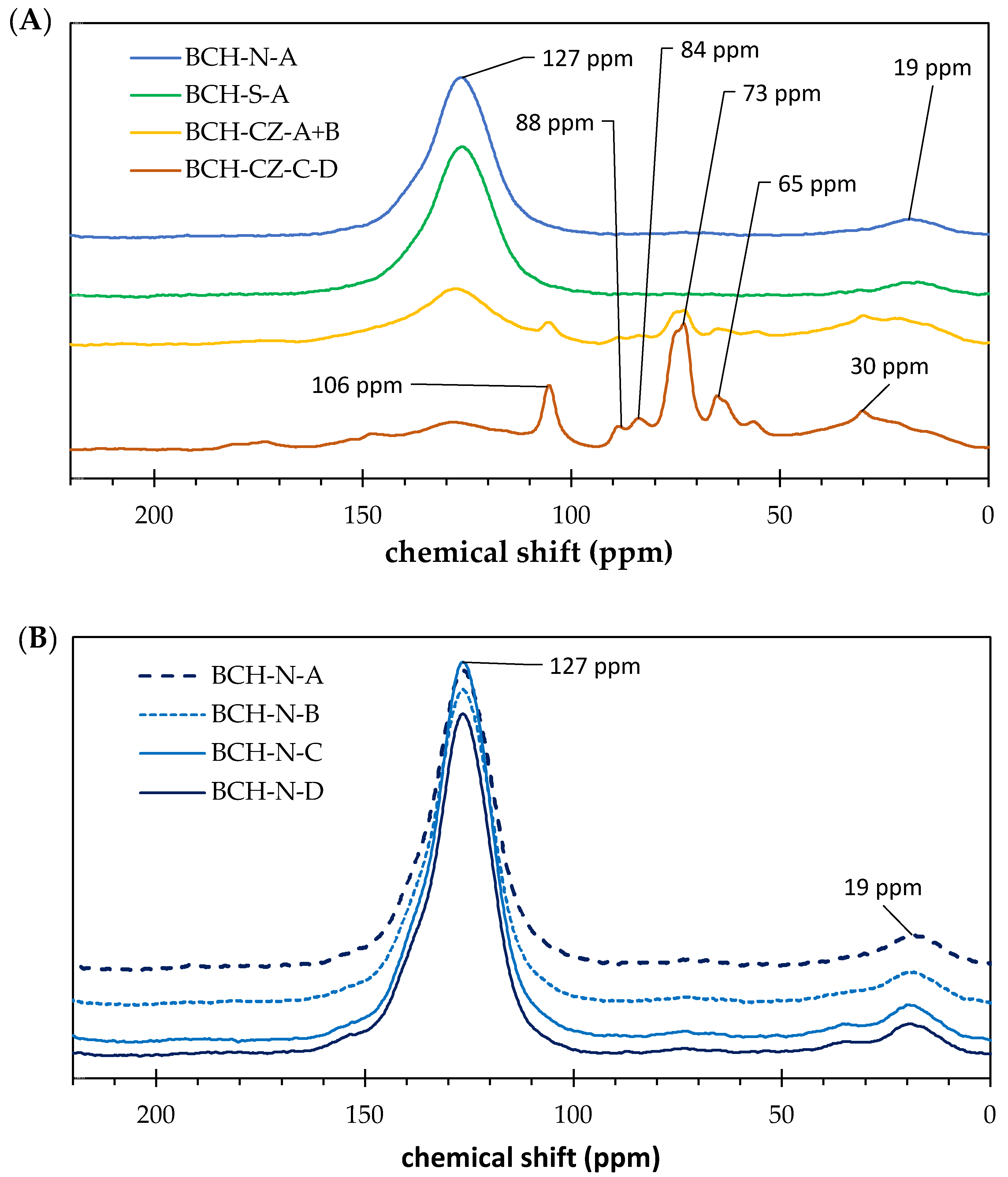
3.3. Structural Analysis
3.4. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeeshan, M.; Ahmad, W.; Hussain, F.; Ahamd, W.; Numan, M.; Shah, M.; Ahmad, I. Phytostabalization of the Heavy Metals in the Soil with Biochar Applications, the Impact on Chlorophyll, Carotene, Soil Fertility and Tomato Crop Yield. J. Clean. Prod. 2020, 255, 120318. [Google Scholar] [CrossRef]
- Yang, C.D.; Lu, S.G. Effects of Five Different Biochars on Aggregation, Water Retention and Mechanical Properties of Paddy Soil: A Field Experiment of Three-Season Crops. Soil Tillage Res. 2021, 205, 104798. [Google Scholar] [CrossRef]
- Feng, W.; Yang, F.; Cen, R.; Liu, J.; Qu, Z.; Miao, Q.; Chen, H. Effects of Straw Biochar Application on Soil Temperature, Available Nitrogen and Growth of Corn. J. Environ. Manag. 2021, 277, 111331. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, G.; Miles, D.; Gao, L.; Jia, Y.; Li, C.; Qu, Z. Impact of Biochar on Greenhouse Gas Emissions and Soil Carbon Sequestration in Corn Grown under Drip Irrigation with Mulching. Sci. Total Environ. 2020, 729, 138752. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.C.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing Straw, Compost, and Biochar Regarding Their Suitability as Agricultural Soil Amendments to Affect Soil Structure, Nutrient Leaching, Microbial Communities, and the Fate of Pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Kumar, A.; Joseph, S.; Tsechansky, L.; Schreiter, I.J.; Schüth, C.; Taherysoosavi, S.; Mitchell, D.R.G.; Graber, E.R. Mechanistic Evaluation of Biochar Potential for Plant Growth Promotion and Alleviation of Chromium-Induced Phytotoxicity in Ficus Elastica. Chemosphere 2020, 243, 125332. [Google Scholar] [CrossRef]
- Albert, H.A.; Li, X.; Jeyakumar, P.; Wei, L.; Huang, L.; Huang, Q.; Kamran, M.; Shaheen, S.M.; Hou, D.; Rinklebe, J.; et al. Influence of Biochar and Soil Properties on Soil and Plant Tissue Concentrations of Cd and Pb: A Meta-Analysis. Sci. Total Environ. 2021, 755, 142582. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Yang, X.; Zhou, Y.; Zhang, L.; Yang, Y.; Luo, L.; Yan, Q. Responses of Ammonia-Oxidizing Microorganisms to Biochar and Compost Amendments of Heavy Metals-Polluted Soil. J. Environ. Sci. 2021, 102, 263–272. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J.-S. Bioformulation of Biochar as a Potential Inoculant Carrier for Sustainable Agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Hussain, R.; Ravi, K.; Garg, A. Influence of Biochar on the Soil Water Retention Characteristics (SWRC): Potential Application in Geotechnical Engineering Structures. Soil Tillage Res. 2020, 204, 104713. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Wang, X. Machine Learning Prediction of Biochar Yield and Carbon Contents in Biochar Based on Biomass Characteristics and Pyrolysis Conditions. Bioresour. Technol. 2019, 288, 121527. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Chen, D.; Ren, W.; Lin, H.; Zhang, H. Wood-Based Biochar as an Excellent Activator of Peroxydisulfate for Acid Orange 7 Decolorization. Chemosphere 2019, 231, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Muter, O.; Berzins, A.; Strikauska, S.; Pugajeva, I.; Bartkevics, V.; Dobele, G.; Truu, J.; Truu, M.; Steiner, C. The Effects of Woodchip- and Straw-Derived Biochars on the Persistence of the Herbicide 4-Chloro-2-Methylphenoxyacetic Acid (MCPA) in Soils. Ecotoxicol. Environ. Saf. 2014, 109, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shan, S.; Yang, C.; Zhang, C.; Zhou, X.; Ma, Q.; Yrjälä, K.; Zheng, H.; Cao, Y. The Comparison of Dissolved Organic Matter in Hydrochars and Biochars from Pig Manure. Sci. Total Environ. 2020, 720, 137423. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Liu, M.; Ren, H. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Walnut Shell for Ammonium and Phosphate Adsorption from Water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Qian, T.-T.; Wu, P.; Qin, Q.-Y.; Huang, Y.-N.; Wang, Y.-J.; Zhou, D.-M. Screening of Wheat Straw Biochars for the Remediation of Soils Polluted with Zn (II) and Cd (II). J. Hazard. Mater. 2019, 362, 311–317. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Pan, L.; Li, C.; You, F.; Xie, S.; Wang, Y.; Ma, J.; Shang, X. Study of Ciprofloxacin Removal by Biochar Obtained from Used Tea Leaves. J. Environ. Sci. 2018, 73, 20–30. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does Biochar Improve Soil Water Retention? A Systematic Review and Meta-Analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Nemati, M.R.; Simard, F.; Fortin, J.-P.; Beaudoin, J. Potential Use of Biochar in Growing Media. Vadose Zone J. 2015, 14, 1–8. [Google Scholar] [CrossRef]
- EBC (2012–2022). European Biochar Certificate-Guidelines for a Sustainable Production of Biochar; Version 10.0; European Biochar Foundation (EBC): Arbaz, Switzerland; Available online: http://european-biochar.org (accessed on 1 January 2022).
- Campos, P.; Miller, A.Z.; Prats, S.A.; Knicker, H.; Hagemann, N.; de la Rosa, J.M. Biochar Amendment Increases Bacterial Diversity and Vegetation Cover in Trace Element-Polluted Soils: A Long-Term Field Experiment. Soil Biol. Biochem. 2020, 150, 108014. [Google Scholar] [CrossRef]
- Wang, L.; Yang, K.; Gao, C.; Zhu, L. Effect and Mechanism of Biochar on CO2 and N2O Emissions under Different Nitrogen Fertilization Gradient from an Acidic Soil. Sci. Total Environ. 2020, 747, 141265. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Nawaz, S.; Alghamdi, H.A.; Alrumman, S.; Yan, W.; Nawaz, M.Z. Effects of Manure-Based Biochar on Uptake of Nutrients and Water Holding Capacity of Different Types of Soils. Case Stud. Therm. Eng. 2020, 2, 100036. [Google Scholar] [CrossRef]
- Zaccheo, P.; Crippa, L.; Cattivello, C. Liming power of different particle fractions of biochar. Acta Hortic. 2014, 1034, 363–368. [Google Scholar] [CrossRef]
- Angst, T.E.; Sohi, S.P. Establishing Release Dynamics for Plant Nutrients from Biochar. GCB Bioenergy 2013, 5, 221–226. [Google Scholar] [CrossRef]
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant Nutrient Availability and PH of Biochars and Their Fractions, with the Possible Use as a Component in a Growing Media. Agronomy 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Cybulak, M.; Sokołowska, Z.; Boguta, P.; Tomczyk, A. Influence of PH and Grain Size on Physicochemical Properties of Biochar and Released Humic Substances. Fuel 2019, 240, 334–338. [Google Scholar] [CrossRef]
- Liao, W.; Thomas, S. Biochar Particle Size and Post-Pyrolysis Mechanical Processing Affect Soil PH, Water Retention Capacity, and Plant Performance. Soil Syst. 2019, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Sonnenerde_Datenblatt_Bio. Available online: https://cdn-pf.niceshops.com/upload/file/sonnenerde_datenblatt_bio_pflanzenkohle_v2021_06.pdf (accessed on 13 January 2022).
- EBC-Pflanzenkohle-Analytik-PK-B31.Pdf. Available online: https://www.novocarbo.com/wp-content/uploads/2021/08/EBC-Pflanzenkohle-Analytik-PK-B31.pdf (accessed on 13 January 2022).
- Agrouhel_Pribal_Letak.Pdf. Available online: https://www.agrouhel.cz/wp-content/uploads/2017/09/agrouhel_pribal_letak.pdf (accessed on 27 May 2022).
- Dommergues, Y.R.; Ganry, F. Biological Nitrogen Fixation and Soil Fertility Maintenance. In Management of Nitrogen and Phosphorus Fertilizers in Sub-Saharan Africa; Springer: Dordrecht, The Netherlands, 1986; pp. 95–115. [Google Scholar]
- Gezahegn, S.; Sain, M.; Thomas, S. Variation in Feedstock Wood Chemistry Strongly Influences Biochar Liming Potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.M.; Lima, I.M.; Gaskin, J.W.; Steiner, C.; Ahmedna, M.; Watts, D.W.; Schomberg, H.H. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on a Loamy Sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and Quantification of Biochar Alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical Characterization of Rice Straw-Derived Biochar for Soil Amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of Magnesium in Carbon Partitioning and Alleviating Photooxidative Damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Wu, M.; Feng, Q.; Sun, X.; Wang, H.; Gielen, G.; Wu, W. Rice (Oryza Sativa L.) Plantation Affects the Stability of Biochar in Paddy Soil. Sci. Rep. 2015, 5, 10001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Fang, Y.; Johnston, C.T. A Fourier-Transform Infrared Study of Biochar Aging in Soils. Soil Sci. Soc. Am. J. 2016, 80, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar Surface Functional Groups as Affected by Biomass Feedstock, Biochar Composition and Pyrolysis Temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Lu, S.; Zong, Y. Pore Structure and Environmental Serves of Biochars Derived from Different Feedstocks and Pyrolysis Conditions. Environ. Sci. Pollut. Res. 2018, 25, 30401–30409. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wei, L.L.; Sun, J.N.; Shao, H.B.; Chang, S.X. What Is More Important for Enhancing Nutrient Bioavailability with Biochar Application into a Sandy Soil: Direct or Indirect Mechanism? Ecol. Eng. 2013, 52, 119–124. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, B.; Budai, A.; Jeng, A.; Hao, X.; Wei, D.; Zhang, Y.; Rasse, D. Study of Biochar Properties by Scanning Electron Microscope–Energy Dispersive X-Ray Spectroscopy (SEM-EDX). Commun. Soil Sci. Plant Anal. 2016, 47, 593–601. [Google Scholar] [CrossRef]
- Méndez, A.; Terradillos, M.; Gascó, G. Physicochemical and Agronomic Properties of Biochar from Sewage Sludge Pyrolysed at Different Temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Mohanty, P.; Nanda, S.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Evaluation of the Physiochemical Development of Biochars Obtained from Pyrolysis of Wheat Straw, Timothy Grass and Pinewood: Effects of Heating Rate. J. Anal. Appl. Pyrolysis 2013, 104, 485–493. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Chen, W.; Yang, H.; Chen, H. The Structure Evolution of Biochar from Biomass Pyrolysis and Its Correlation with Gas Pollutant Adsorption Performance. Bioresour. Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef] [PubMed]


| Biochar Producer | Feedstock | Pyrolysis Conditions | Fraction Description | Label |
|---|---|---|---|---|
| Sonnenerde GmbH | corn and sunflower peels, fruit sludge | 20 min, max. 650 °C | <0.5 mm | BCH-S-A |
| 0.5–2.0 mm | BCH-S-B | |||
| 2.0–4.0 mm | BCH-S-C | |||
| >4.0 mm | BCH-S-D | |||
| NovoCarbo GmbH | softwood woodcut | 10 min, max. 720 °C | <0.5 mm | BCH-N-A |
| 0.5–2.0 mm | BCH-N-B | |||
| 2.0–4.0 mm | BCH-N-C | |||
| >4.0 mm | BCH-N-D | |||
| Biouhel.cz s.r.o | corn digestate, wheat straw, grass biomass | 20–30 min, 450–470 °C | <2.0 mm | BCH-CZ-A+B |
| >2.0 mm | BCH-CZ-C+D |
| Sample Label | worg (wt.%) | winorg (wt.%) | TOC (wt.%) | Elemental Composition (wt.%) | H/C * | |||
|---|---|---|---|---|---|---|---|---|
| C | H | O | N | |||||
| BCH-S-A | 67.9 | 32.1 | 54.4 | 59.97 ± 0.63 | 0.81 ± 0.09 | 5.39 ± 0.28 | 1.70 ± 0.11 | 0.16 |
| BCH-S-B | 72.9 | 27.1 | 61.8 | 67.66 ± 1.02 | 0.94 ± 0.23 | 2.33 ± 0.44 | 2.00 ± 0.08 | 0.16 |
| BCH-S-C | 79.0 | 21.0 | 61.0 | 71.72 ± 1.48 | 2.00 ± 0.14 | 2.93 ± 0.56 | 2.31 ± 0.08 | 0.33 |
| BCH-S-D | 77.4 | 22.6 | 59.6 | 65.66 ± 0.54 | 1.61 ± 0.26 | 7.05 ± 0.29 | 3.09 ± 0.09 | 0.29 |
| BCH-N-A | 71.4 | 28.6 | 61.2 | 63.40 ± 0.13 | 1.57 ± 0.06 | 4.69 ± 0.12 | 1.78 ± 0.18 | 0.30 |
| BCH-N-B | 82.6 | 17.4 | 71.3 | 74.48 ± 0.65 | 1.63 ± 0.02 | 4.54 ± 0.26 | 1.92 ± 0.12 | 0.26 |
| BCH-N-C | 91.1 | 8.9 | 78.6 | 84.34 ± 1.23 | 2.62 ± 0.35 | 2.39 ± 0.56 | 1.73 ± 0.10 | 0.37 |
| BCH-N-D | 81.2 | 18.8 | 69.8 | 73.27 ± 0.66 | 2.64 ± 0.12 | 3.97 ± 0.29 | 1.34 ± 0.08 | 0.43 |
| BCH-CZ-A+B | 70.3 | 29.7 | 41.6 | 47.89 ± 0.51 | 4.54 ± 0.16 | 15.06 ± 0.28 | 2.36 ± 0.14 | 1.13 |
| BCH-CZ-C+D | 77.2 | 22.8 | 48.2 | 56.40 ± 0.54 | 3.38 ± 0.39 | 15.29 ± 0.37 | 3.10 ± 0.18 | 0.71 |
| Sample Label | pH H2O (–) | pH CaCl2 (–) | Conductivity (mS/cm) |
|---|---|---|---|
| BCH-S-A | 10.04 ± 0.14 | 9.29 ± 0.06 | 3.053 ± 0.006 |
| BCH-S-B | 10.07 ± 0.02 | 9.26 ± 0.04 | 2.973 ± 0.040 |
| BCH-S-C | 10.05 ± 0.10 | 9.15 ± 0.02 | 2.417 ± 0.049 |
| BCH-S-D | 10.02 ± 0.02 | 9.14 ± 0.02 | 2.260 ± 0.010 |
| BCH-N-A | 9.24 ± 0.21 | 8.41 ± 0.02 | 0.830 ± 0.006 |
| BCH-N-B | 9.65 ± 0.08 | 8.44 ± 0.04 | 1.046 ± 0.008 |
| BCH-N-C | 9.37 ± 0.02 | 8.34 ± 0.07 | 0.969 ± 0.013 |
| BCH-N-D | 9.35 ± 0.01 | 8.24 ± 0 04 | 0.887 ± 0.003 |
| BCH-CZ-A+B | 8.56 ± 0.06 | 7.96 ± 0.04 | 3.447 ± 0.025 |
| BCH-CZ-C+D | 7.16 ± 0.04 | 6.76 ± 0.03 | 3.227 ± 0.051 |
| Sample Label | 185–220 ppm | 160–185 ppm | 90–160 ppm | 60–90 ppm | 45–60 ppm | 0–45 ppm |
|---|---|---|---|---|---|---|
| Carbonyl-C | Carboxyl-C | Aryl-C | O-alkyl-C | N-alkyl-C/Methoxyl-C | Alkyl-C | |
| BCH-S-A | 0.5 | 1.5 | 76.5 | 1.4 | 0.4 | 6.9 |
| BCH-S-B | 0.0 | 0.1 | 81.9 | 0.2 | 0.1 | 5.4 |
| BCH-S-C | 0.0 | 0.3 | 80.4 | 0.2 | 0.0 | 7.1 |
| BCH-S-D | 0.0 | 1.2 | 78.6 | 1.4 | 0.3 | 7.1 |
| BCH-N-A | 0.1 | 0.3 | 77.9 | 1.2 | 0.0 | 7.2 |
| BCH-N-B | 0.7 | 0.1 | 76.8 | 0.8 | 0.1 | 7.9 |
| BCH-N-C | 0.0 | 0.1 | 78.6 | 0.1 | 0.0 | 7.1 |
| BCH-N-D | 0.0 | 0.1 | 79.4 | 0.2 | 0.0 | 5.1 |
| BCH-CZ-A+B | 0.0 | 2.4 | 48.5 | 13.5 | 4.9 | 24.3 |
| BCH-CZ-C+D | 0.4 | 2.9 | 30.1 | 34.8 | 7.2 | 21.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalina, M.; Sovova, S.; Hajzler, J.; Kubikova, L.; Trudicova, M.; Smilek, J.; Enev, V. Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential. Agronomy 2022, 12, 1768. https://doi.org/10.3390/agronomy12081768
Kalina M, Sovova S, Hajzler J, Kubikova L, Trudicova M, Smilek J, Enev V. Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential. Agronomy. 2022; 12(8):1768. https://doi.org/10.3390/agronomy12081768
Chicago/Turabian StyleKalina, Michal, Sarka Sovova, Jan Hajzler, Leona Kubikova, Monika Trudicova, Jiri Smilek, and Vojtech Enev. 2022. "Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential" Agronomy 12, no. 8: 1768. https://doi.org/10.3390/agronomy12081768






