Physiological Factors Limiting Leaf Net Photosynthetic Rate in C3 Crops like Rice and Approaches for Improving It
Abstract
:1. Introduction
2. Physiological Factors Limiting Leaf Net Photosynthetic Rate in C3 Crops
2.1. Effects of Rubisco on Leaf Net Photosynthetic Rate
2.2. Effects of Leaf Nitrogen Content on Leaf Net Photosynthetic Rate
2.3. Effects of Stomatal Conductance on Leaf Net Photosynthetic Rate
2.4. Effects of Mesophyll Conductance on Leaf Net Photosynthetic Rate
2.5. Effects of the Rate of Sugar Utilization on Leaf Net Photosynthetic Rate
2.6. Response of Leaf Photosynthesis to Changing Environments
3. Approaches for Improving Leaf Net Photosynthetic Rate in C3 Crops
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harley, P.C.; Loreto, F.; Di Marco, G.; Sharkey, T.D. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 1992, 98, 1429–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, T.D. Photosynthesis in intact leaves of C3 crops: Physics, physiology and rate limitations. Bot. Rev. 1985, 51, 53–105. [Google Scholar] [CrossRef]
- Farquhar, G.D. Models of integrated photosynthesis of cells and leaves. Philos. Trans. R. Soc. B Biol. Sci. 1989, 323, 357–367. [Google Scholar]
- Sage, R.F. A model describing the regulation of ribulose-1,5-bisphosphate carboxylase, electron transport, and triose phosphate use in response to light intensity and CO2 in C3 crops. Plant Physiol. 1990, 94, 1728–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harley, P.C.; Sharkey, T.D. An improved model of C3 photosynthesis at high CO2: Reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynth. Res. 1991, 28, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [PubMed] [Green Version]
- Makino, A.; Shimada, T.; Takumi, S.; Kaneko, K.; Matsuoka, M.; Shimamoto, K.; Nakano, H.; Miyao-Tokutomi, M.; Mae, T.; Yamamoto, N. Does decrease in ribulose-1,5-bisphosphate carboxylase by antisense rbcS lead to a higher N-use efficiency of photosynthesis under conditions of saturating CO2 and light in rice plants? Plant Physiol. 1997, 114, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Spreitzer, R.J.; Salvucci, M.E. Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, A.; Farquhar, G.D. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 1985, 165, 397–406. [Google Scholar] [CrossRef]
- Tcherkez, G.G.B.; Farquhar, G.D.; Andrews, T.J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. USA 2006, 103, 7246–7251. [Google Scholar] [CrossRef] [Green Version]
- Iñiguez, C.; Capó-Bauçà, S.; Niinemets, Ü.; Stoll, H.; Aguiló-Nicolau, P.; Galmes, J. Evolutionary trends in RuBisCO kinetics and their co-evolution with CO2 concentrating mechanisms. Plant J. 2020, 101, 897–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Gao, Y.; Xu, X.; Shen, Q.; Guo, S. Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J. Exp. Bot. 2009, 60, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Nagai, T.; Makino, A. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 2011, 34, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. Int. J. Exp. Plant Biol. 2012, 193–194, 70–84. [Google Scholar] [CrossRef]
- Adachi, S.; Nakae, T.; Uchida, M.; Soda, K.; Takai, T.; Oi, T.; Hirasawa, T. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. J. Exp. Bot. 2013, 64, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Makino, A.; Sakashita, H.; Hidema, J.; Mae, T.; Ojima, K.; Osmond, B. Distinctive responses of ribulose-1,5-bisphosphate carboxylase and carbonic anhydrase in wheat leaves to nitrogen nutrition and their possible relationships to CO2-transfer resistance. Plant Physiol. 1992, 100, 1737–1743. [Google Scholar] [CrossRef] [Green Version]
- Makino, A.; Nakano, H.; Mae, T. Responses of ribulose-1,5-bisphosphate carboxylase, cytochrome J and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiol. 1994, 105, 173–179. [Google Scholar] [CrossRef]
- Makino, A. Biochemistry of C3-photosynthesis in high CO2. J. Plant Res. 1994, 107, 79–84. [Google Scholar] [CrossRef]
- Sage, R.F.; Santrucek, J.; Grise, D.J. Temperature effects on the photosynthetic response of C3 crops to long-term CO2 enrichment. Vegetatio 1995, 121, 67–77. [Google Scholar] [CrossRef]
- Medlyn, B.E. The optimal allocation of nitrogen within the C3 photosynthetic system at elevated CO2. Aust. J. Plant Physiol. 1996, 23, 593–603. [Google Scholar] [CrossRef]
- Lauerer, M.; Saftic, D.; Quick, W.P.; Labate, C.; Fichtner, K.; Schulze, E.D.; Rodermel, S.; Bogorad, L.; Stitt, M. Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with “antisense” rbcS. VI. Effect on photosynthesis in plants grown at different irradiance. Planta 1993, 190, 332–345. [Google Scholar] [CrossRef]
- Makino, A. Rubisco and nitrogen relationships in rice: Leaf photosynthesis and plant growth. J. Soil Sci. Plant Nutr. 2003, 49, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R. Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol. 1983, 72, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Ohkubo, M.; Hatakeyama, H.; Ohashi, K.; Yoshizawa, R.; Kojima, S.; Makino, A. Increased Rubisco content in transgenic rice transformed with the ‘sense’ rbcS gene. Plant Cell Physiol. 2007, 48, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.B.; Ogren, W.L. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Planta 1984, 161, 308–313. [Google Scholar] [CrossRef]
- Lin, M.T.; Occhialini, A.; Andralojc, P.J.; Parry, M.A.J.; Hanson, M.R. A faster Rubisco with potential to increase photosynthesis in crops. Nature 2014, 513, 547–550. [Google Scholar] [CrossRef]
- Taylaran, R.D.; Adachi, S.; Ookawa, T.; Usuda, H.; Hirasawa, T. Hydraulic conductance as well as nitrogen accumulation plays a role in the higher rate of leaf photosynthesis of the most productive variety of rice in Japan. J. Exp. Bot. 2011, 62, 4067–4077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polesskaya, O.G.; Kashirina, E.I.; Andreeva, S.E.; Goryaeva, O.V.; Glazunova, M.A.; Alekhina, N.D. Morphophysiological indices of the source leaf in wheat plants acclimated to conditions of nitrogen nutrition. Russ. J. Plant Physiol. 2001, 48, 716–722. [Google Scholar] [CrossRef]
- Li, Y.; Ren, B.; Ding, L.; Shen, Q.; Peng, S.; Guo, S. Does chloroplast size influence photosynthetic nitrogen use efficiency? PLoS ONE 2013, 8, e62036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, D.; Liu, X.; Liu, L.; Douthe, C.; Li, Y.; Peng, S.; Huang, J. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplies in rice. Plant Cell Environ. 2015, 38, 2541–2550. [Google Scholar] [CrossRef]
- Peng, S.; Garcia, F.V.; Laza, R.C.; Sanico, A.L.; Visperas, R.M.; Cassman, K.G. Increased N-use efficiency using a chlorophyll meter on high-yielding irrigated rice. Field Crops Res. 1996, 47, 243–252. [Google Scholar] [CrossRef]
- Ye, M.; Peng, S.; Li, Y. Intraspecific variation in photosynthetic nitrogen-use efficiency is positively related to photosynthetic rate in rice (Oryza sativa L.) plants. Photosynthetica 2019, 57, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Ocheltree, T.W.; Nippert, J.B.; Prasad, P.V.V. Changes in stomatal conductance along grass blades reflect changes in leaf structure. Plant Cell Environ. 2012, 35, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef]
- Sarwar, A.G.; Karim, M.A.; Rana, S.M. Influence of stomatal characteristics on yield and yield attributes of rice. J. Bangladesh Agric. Univ. 2013, 11, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Boer, H.J.; Zhang, Z.; Chen, X.; Shi, Y.; Peng, S.; Wang, F. The coordinated increase in stomatal density and vein dimensions during genetic improvement in rice. Agron. J. 2020, 112, 2791–2804. [Google Scholar] [CrossRef]
- Li, S.; Hamani, A.K.M.; Zhang, Y.; Liang, Y.; Gao, Y.; Duan, A. Coordination of leaf hydraulic, anatomical, and economical traits in tomato seedlings acclimation to long-term drought. BMC Plant Biol. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Feild, T.S.; Jordan, G.J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.; Nadal, M. Linking water relations and hydraulics with photosynthesis. Plant J. 2020, 101, 800–815. [Google Scholar] [CrossRef]
- Tabassum, M.A.; Ye, Y.; Yu, T.; Zhu, G.; Rizwan, M.S.; Wahid, M.A.; Li, Y. Rice (Oryza sativa L.) hydraulic conductivity links to leaf venation architecture under well-watered condition rather than PEG-induced water deficit. Acta Physiol. Plant. 2016, 38, 92. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, T.; Tsuchida, M.; Ishihara, K. Relationship between resistance to water transport and exudation rate and the effect of the resistance on the midday depression of stomatal aperture in rice plants. Jpn. J. Crop Sci. 1992, 61, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Wu, M.; Zhang, H.; Zhang, Z.; Zhang, Z. High leaf vein density promotes leaf gas exchange by enhancing leaf hydraulic conductance in Oryza sativa L. plants. Front. Plant Sci. 2021, 12, 693815. [Google Scholar] [CrossRef]
- Rockwell, F.E.; Holbrook, N.M.; Stroock, A.D. The competition between liquid and vapor transport in transpiring leaves. Plant Physiol. 2014, 164, 1741–1758. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.; Flexas, J.; Yu, T.; Peng, S.; Huang, J. Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in Oryza. New Phytol. 2017, 213, 572–583. [Google Scholar] [CrossRef]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef]
- Simonneau, T.; Inra, R.H. The use of tree root suckers to estimate root water potential. Plant Cell Environ. 1991, 14, 585–591. [Google Scholar] [CrossRef]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Miyamoto, N.; Steudle, E.; Hirasawa, T.; Lafitte, R. Hydraulic conductivity of rice roots. J. Exp. Bot. 2001, 52, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Ren, B.; Ding, L.; Gao, C.; Shen, Q.; Guo, S. Drought-induced root aerenchyma formation restrains water uptake in nitrate-supplied rice seedlings. Plant Cell Physiol. 2012, 53, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Postaire, O.; Tournaire-Roux, C.; Grondin, A.; Boursiac, Y.; Morillon, R.; Schäffner, A.R.; Maurel, C. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol. 2010, 152, 1418–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, P.J. Water Relations of Plants; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Steudle, E.; Peterson, C.A. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- Lu, Z.; Neumann, P.M. Water stress inhibits hydraulic conductance and leaf growth in rice seedlings but not the transport of water via mercury-sensitive water channels in the root. Plant Physiol. 1999, 120, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Grondin, A.; Rodrigues, O.; Verdoucq, L.; Merlot, S.; Leonhardt, N.; Maurel, C. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 2015, 27, 1945–1954. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Ribas-Carbó, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliani, R.; Koteyeva, N.; Voznesenskaya, E.; Evans, M.A.; Cousins, A.B.; Edwards, G.E. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiol. 2013, 162, 1632–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carriquí, M.; Cabrera, H.M.; Conesa, M.À.; Coopman, R.E.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Ribas-Carbo, M.; Tomás, M.; et al. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ. 2015, 38, 448–460. [Google Scholar] [CrossRef]
- Scafaro, A.P.; von Caemmerer, S.; Evans, J.R.; Atwell, B.J. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant Cell Environ. 2011, 34, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Peguero-Pina, J.J.; Flexas, J.; Galmes, J.; Niinemets, U.; Sancho-Knapik, D.; Barredo, G.; Villarroya, D.; Gil-Pelegrin, E. Leaf anatomical properties in relation to differences in mesophyll conductance to CO2 and photosynthesis in two related Mediterranean Abies species. Plant Cell Environ. 2012, 35, 2121–2129. [Google Scholar] [CrossRef]
- Tosens, T.; Niinemets, Ü.; Westoby, M.; Wright, I.J. Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: News of a long and winding path. J. Exp. Bot. 2012, 63, 5105–5119. [Google Scholar] [CrossRef] [Green Version]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmés, J.; Hallik, L.; Medrano, H.; Niinemets, Ü. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef]
- Muir, C.D.; Hangarter, R.P.; Moyle, L.C.; Davis, P.A. Morphological and anatomical determinants of mesophyll conductance in wild relatives of tomato (Solanum sect. Lycopersicon, sect. Lycopersicoides; Solanaceae). Plant Cell Environ. 2014, 37, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Renton, M.; Ludwig, M.; Evans, J.R.; Veneklaas, E.J. Photosynthesis at an extreme end of the leaf trait spectrum: How does it relate to high leaf dry mass per area and associated structural parameters? J. Exp. Bot. 2010, 61, 3015–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Zhang, Z.C.; Huang, G.J.; Xiong, Z.; Peng, S.B.; Li, Y. High leaf mass per area Oryza genotypes invest more leaf mass to cell wall and show a low mesophyll conductance. AoB Plants 2020, 12, plaa028. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, Z.; Huang, G.; Li, Y. Leaf photosynthesis and its temperature response are different between growth stages and N supplies in rice plants. Int. J. Mol. Sci. 2022, 23, 3885. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Ludwig, M.; Renton, M.; Veneklaas, E.J.; Evans, J.R. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J. Exp. Bot. 2009, 60, 2303–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanba, Y.T.; Kogami, H.; Terashima, I. The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ. 2002, 25, 1021–1030. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sisó, S.; Flexas, J.; Galmés, J.; García-Nogales, A.; Niinemets, Ü.; Gil-Pelegrín, E. Cell-level anatomical characteristics explain high mesophyll conductance and photosynthetic capacity in sclerophyllous Mediterranean oaks. New Phytol. 2017, 214, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Fini, A.; Loreto, F.; Tattini, M.; Giordano, C.; Ferrini, F.; Brunetti, C.; Centritto, M. Mesophyll conductance plays a central role in leaf functioning of Oleaceae species exposed to contrasting sunlight irradiance. Physiol. Plant. 2016, 157, 54–68. [Google Scholar] [CrossRef]
- Ren, T.; Weraduwage, S.M.; Sharkey, T.D. Prospects for enhancing leaf photosynthetic capacity by manipulating mesophyll cell morphology. J. Exp. Bot. 2019, 70, 1153–1165. [Google Scholar] [CrossRef]
- Nakhoul, N.L.; Davis, B.A.; Romero, M.F.; Boron, W.F. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am. J. Physiol. Cell Physiol. 1998, 274, C543–C548. [Google Scholar] [CrossRef]
- Uehlein, N.; Lovisolo, C.; Siefritz, F.; Kaldenhoff, R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 2003, 425, 734–737. [Google Scholar] [CrossRef]
- Fukuzawa, H.; Suzuki, E.; Komukai, Y.; Miyachi, S. A gene homologous to chloroplast carbonic anhydrase (icfA) is essential to photosynthetic carbon dioxide fixation by Synechococcus PCC7942. Proc. Natl. Acad. Sci. USA 1992, 89, 4437–4441. [Google Scholar] [CrossRef] [Green Version]
- Badger, M. The roles of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth. Res. 2003, 77, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Shatil-Cohen, A.; Attia, Z.; Maurel, C.; Boursiac, Y.; Kelly, G.; Granot, D.; Yaaran, A.; Lerner, S.; Moshelion, M. The role of plasma membrane aquaporins in regulating the bundle sheath-mesophyll continuum and leaf hydraulics. Plant Physiol. 2014, 166, 1609–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secchi, F.; Zwieniecki, M.A. Down-regulation of plasma intrinsic protein1 aquaporin in poplar trees is detrimental to recovery from embolism. Plant Physiol. 2014, 164, 1789–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Wang, Z.; Huang, J.; Peng, S.; Xiong, D. Mesophyll conductance variability of rice aquaporin knockout lines at different growth stages and growing environments. Plant J. 2021, 107, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Heckwolf, M.; Pater, D.; Hanson, D.T.; Kaldenhoff, R. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J. 2011, 67, 795–804. [Google Scholar] [CrossRef]
- Uehlein, N.; Sperling, H.; Heckwolf, M.; Kaldenhoff, R. The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant Cell Environ. 2012, 35, 1077–1083. [Google Scholar] [CrossRef]
- Groszmann, M.; Osborn, H.L.; Evans, J.R. Carbon dioxide and water transport through plant aquaporins. Plant Cell Environ. 2017, 40, 938–961. [Google Scholar] [CrossRef] [PubMed]
- Roig-Oliver, M.; Fullana-Pericàs, M.; Bota, J.; Flexas, J. Adjustments in photosynthesis and leaf water relations are related to changes in cell wall composition in Hordeum vulgare and Triticum aestivum subjected to water deficit stress. Plant Sci. 2021, 311, 111015. [Google Scholar] [CrossRef]
- Flexas, J.; Clemente-Moreno, M.J.; Bota, J.; Brodribb, T.J.; Gago, J.; Mizokami, Y.; Nadal, M.; Perera-Castro, A.V.; Roig-Oliver, M.; Sugiura, D.; et al. Cell wall thickness and composition are involved in photosynthetic limitation. J. Exp. Bot. 2021, 72, 3971–3986. [Google Scholar] [CrossRef] [PubMed]
- Canny, M.; Wong, S.C.; Huang, C.; Miller, C. Differential shrinkage of mesophyll cells in transpiring cotton leaves: Implications for static and dynamic pools of water, and for water transport pathways. Funct. Plant Biol. 2012, 39, 91–102. [Google Scholar] [CrossRef]
- Lunn, J.E.; MacRae, E. New complexities in the synthesis of sucrose. Curr. Opin. Plant Biol. 2003, 6, 208–214. [Google Scholar] [CrossRef]
- Sauer, N. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 2007, 581, 2309–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, D.M. SWEET! The pathway is complete. Science 2012, 335, 173–174. [Google Scholar] [PubMed]
- Patrick, J.W.; Botha, F.C.; Birch, R.G. Metabolic engineering of sugars and simple sugar derivations in plant. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Timm, H.C.; Stegemann, J.; Küppers, M. Photosynthetic induction strongly affects the light compensation point of net photosynthesis and coincidentally the apparent quantum yield. Trees 2002, 16, 47–62. [Google Scholar] [CrossRef]
- Lawson, T.; Kramer, D.M.; Raines, C.A. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr. Opin. Biotechnol. 2012, 23, 215–220. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, S.; Li, Y. Increase rate of light-induced stomatal conductance is related to stomatal size in the genus Oryza. J. Exp. Bot. 2019, 70, 5259–5269. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Huang, G.; Peng, S.; Li, Y. Temperature responses of photosynthesis and leaf hydraulic conductance in rice and wheat. Plant Cell Environ. 2020, 43, 1437–1451. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; von Caemmerer, S.; Long, S.P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in Vivo. Plant Physiol. 2002, 130, 1992–1998. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, X.; Li, S.; Salter, W.T.; Barbour, M.M. The role of leaf water potential in the temperature response of mesophyll conductance. New Phytol. 2020, 225, 1193–1205. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Evans, J.R. Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 2015, 38, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R. The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply. J. Exp. Bot. 2004, 55, 2313–2321. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Asensio, J.S.; Rachmilevitch, S.; Bloom, A.J. Responses of Arabidopsis and wheat to rising CO2 depend on nitrogen source and nighttime CO2 levels. Plant Physiol. 2015, 168, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J. Photorespiration and nitrate assimilation: A major intersection between plant carbon and nitrogen. Photosynth. Res. 2015, 123, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Lovelock, C.; Winter, K. Oxygendependent electron transport and protection from photoinhibition in leaves of tropical tree species. Planta 1996, 198, 580–587. [Google Scholar] [CrossRef]
- Heber, U.; Walker, D. Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol. 1992, 100, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Kozaki, A.; Takeba, G. Photorespiration protects C3 crops from photooxidation. Nature 1996, 384, 557–560. [Google Scholar] [CrossRef]
- Zelitch, I.; Schultes, N.P.; Peterson, R.B.; Brown, P.; Brutnell, T.P. High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiol. 2009, 149, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Whitney, S.M.; von Caemmerer, S.; Hudson, G.S.; Andrews, T.J. Directed mutation of the Rubisco large subunit of tobacco influences photorespiration and growth. Plant Physiol. 1999, 121, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Whitney, S.M.; Sharwood, R.E.; Orr, D.; White, S.J.; Alonso, H.; Galmés, J. Isoleucine 309 acts as a C4 catalytic switch that increases ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) carboxylation rate in Flaveria. Proc. Natl. Acad. Sci. USA 2011, 108, 14688–14693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurek, I.; Chang, T.K.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, X.G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 3230–3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Cishan, L.; Portis, A.R., Jr. Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth. Res. 2009, 100, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; von Caemmerer, S. Effect of Rubisco activase deficiency on the temperature response of CO2 assimilation rate and Rubisco activation state: Insights from transgenic tobacco with reduced amounts of Rubisco activase. Plant Physiol. 2009, 151, 2073–2082. [Google Scholar] [CrossRef] [Green Version]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco activase is a key regulator of nonsteady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 2012, 71, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, Y.; Tamoi, M.; Shigeoka, S. Overexpression of a yanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat. Biotechnol. 2001, 19, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Lawson, T.; Zakhleniuk, O.V.; Lloyd, J.C.; Raines, C.A.; Fryer, M. Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 2005, 138, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, D.; Locke, A.; Khozaei, M.; Raines, C.; Long, S.; Ort, D. Overexpressing the C3 photosynthesis cycle enzyme sedoheptulose-1–7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biol. 2011, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Köhler, I.H.; Ruiz-Vera, U.M.; VanLoocke, A.; Thomey, M.L.; Clemente, T.; Long, S.P.; Ort, D.R.; Bernacchi, C.J. Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. J. Exp. Bot. 2017, 68, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Zhong, M.; Wang, Y.; Hou, K.; Shu, S.; Sun, J.; Guo, S. TGase positively regulates photosynthesis via activation of Calvin cycle enzymes in tomato. Hortic. Res. 2019, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, N.; Huang, R.; Chen, C.; Guo, J.; Yang, X.; Zhang, X.; Sun, C.; Deng, X.; Wang, P. A single nucleotide substitution at the 3′-end of SBPase gene involved in Calvin cycle severely affects plant growth and grain yield in rice. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hajirezaei, M.R.; Peisker, M.; Tschiersch, H.; Palatnik, J.F.; Valle, E.M.; Carrillo, N.; Sonnewald, U. Small changes in the activity of chloroplastic NADP+- dependent ferredoxin oxidoreductase lead to impaired plant growth and restrict photosynthetic activity of transgenic tobacco plants. Plant J. 2002, 29, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, P.; Scharfenberg, M.; Weigel, M.; Granlund, I.; Schroder, W.P.; Finazzi, G.; Leister, D. Mutants, overexpressors, and interactors of Arabidopsis plastocyanin isoforms: Revised roles of plastocyanin in photosynthetic electron flow and thylakoid redox state. Mol. Plant 2009, 2, 236–248. [Google Scholar] [CrossRef] [Green Version]
- Takahara, K.; Kasajima, I.; Takahashi, H.; Hashida, S.N.; Itami, T.; Uchimiya, H. Metabolome and photochemical analysis of rice plants overexpressing Arabidopsis NAD kinase gene. Plant Physiol. 2010, 152, 1863–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, G.D.; Badger, M.R.; von Caemmerer, S. The prospect of using cyanobacterial bicarbonate transporters to improve leaf photosynthesis in C3 crop plants. Plant Physiol. 2011, 155, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Price, G.D.; Pengelly, J.J.L.; Forster, B.; Du, J.; Whitney, S.M.; von Caemmerer, S.; Evans, J.R. The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J. Exp. Bot. 2013, 64, 753–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covshoff, S.; Hibberd, J.M. Integrating C4 photosynthesis into C3 crops to increase yield potential. Curr. Opin. Biotechnol. 2012, 23, 209–214. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Quick, W.P.; Furbank, R.T. The development of C4 rice: Current progress and future challenges. Science 2012, 336, 1671–1672. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Hanba, Y.T.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K.; Terashima, I.; Katsuhara, M. Overexpression of the barley aquaporin HvPIP2; 1increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 2004, 45, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Ribas-Carbó, M.; Hanson, D.T.; Bota, J.; Otto, B.; Cifre, J.; Kaldenhoff, R. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006, 48, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leakey, A.D.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Markelz, R.J.; Strellner, R.S.; Leakey, A.D. Impairment of C4 photosynthesis by drought is exacerbated by limiting nitrogen and ameliorated by elevated [CO2] in maize. J. Exp. Bot. 2011, 62, 3235–3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehy, J.E.; Ferrer, A.B.; Mitchell, P.L.; Elmido-Mabilangen, A.; Pablico, P.; Dionora, M.J.A. How the rice crop works and why it needs a new engine. In Charting New Pathways to C4 Rice; Sheehy, J.E., Mitchell, P.L., Hardy, B., Eds.; International Rice Research Institute: Los Banos, Philippines, 2007; pp. 3–26. [Google Scholar]
- Taniguchi, Y.; Ohkawa, H.; Masumoto, C.; Fukuda, T.; Tamai, T.; Lee, K.; Miyao, M. Overproduction of C4 photosynthetic enzymes in transgenic rice plants: An approach to introduce the C4-like photosynthetic pathway into rice. J. Exp. Bot. 2008, 59, 1799–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.G.; Shan, L.; Wang, Y.; Quick, W.P. C4 rice—An ideal arena for systems biology research. J. Integr. Plant Biol. 2010, 52, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Arrivault, S.; Coe, R.A.; Karki, S.; Covshoff, S.; Bagunu, E.; Lunn, J.E.; Stitt, M.; Furbank, R.T.; Hibberd, J.M.; et al. A partial C4 photosynthetic biochemical pathway in rice. Front. Plant Sci. 2020, 11, 564463. [Google Scholar] [CrossRef]
- Ermakova, M.; Arrivault, S.; Giuliani, R.; Danila, F.; AlonsoCantabrana, H.; Vlad, D.; Ishihara, H.; Feil, R.; Guenther, M.; Borghi, G.L.; et al. Installation of C4 photosynthetic pathway enzymes in rice using a single construct. Plant Biotechnol. J. 2021, 19, 575–588. [Google Scholar] [CrossRef] [PubMed]
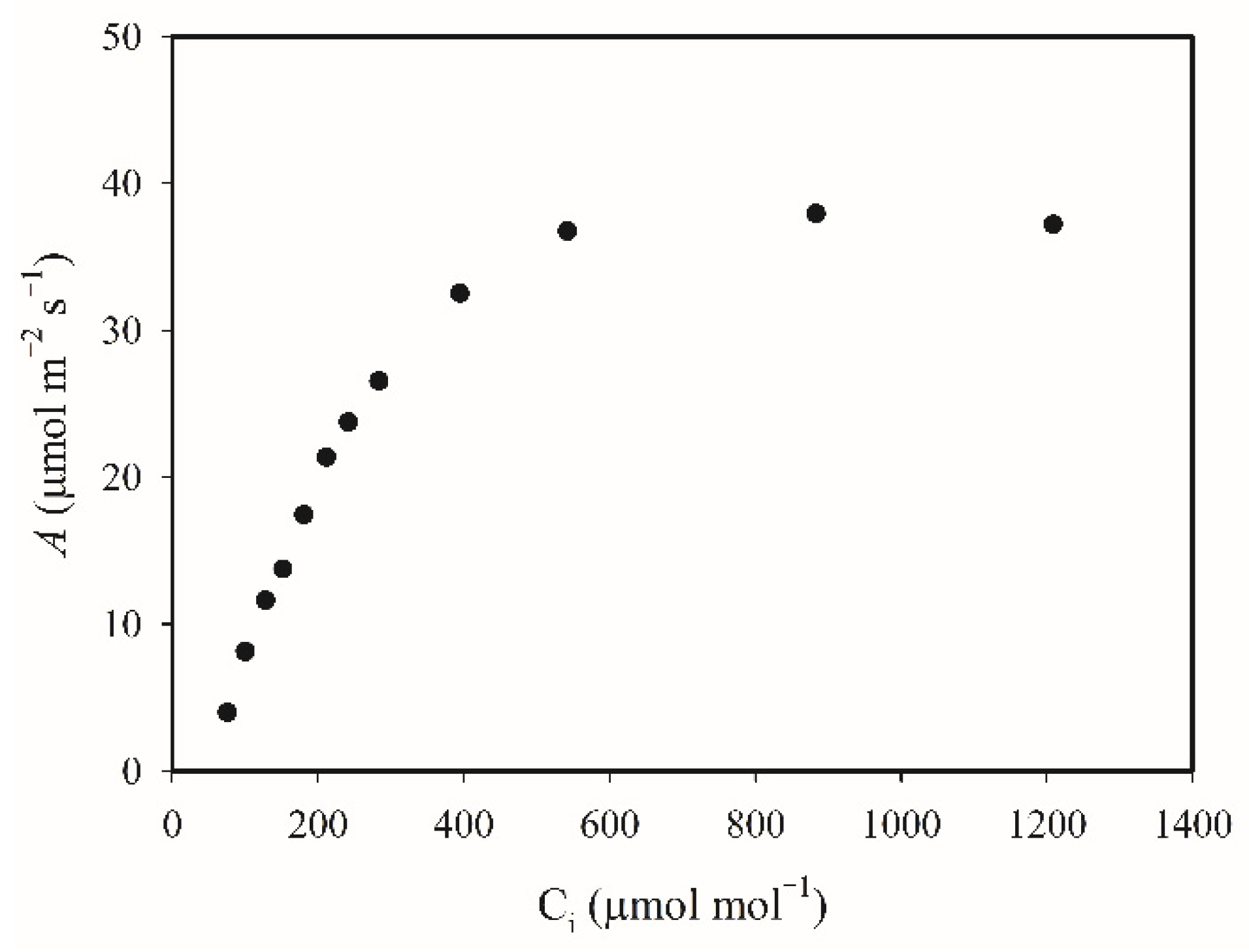

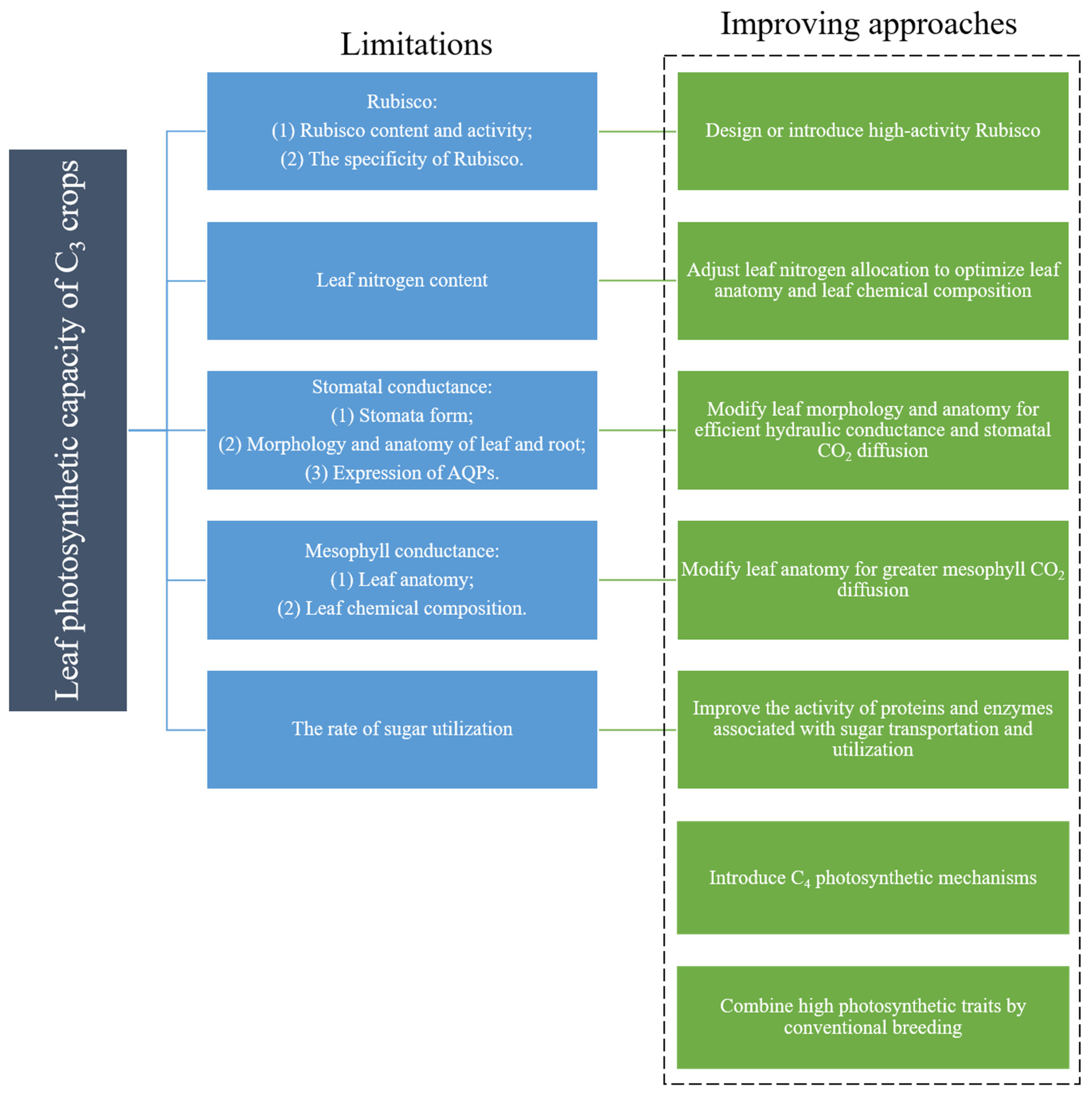
| Section | A (μmol m−2 s−1) | Narea (g m−2) | Number of Rice Lines |
|---|---|---|---|
| A ≥ 30 | 31.5 ± 0.9 a | 1.67 ± 0.17 a | 5 |
| 25 ≤ A < 30 | 27.0 ± 1.5 b | 1.57 ± 0.16 a | 34 |
| 20 ≤ A < 25 | 22.4 ± 1.4 c | 1.53 ± 0.18 a | 60 |
| A < 20 | 18.4 ± 1.2 d | 1.35 ± 0.22 b | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Wu, M.; Zhang, Y.; Wang, Z.; Zhang, H.; Zhang, Z. Physiological Factors Limiting Leaf Net Photosynthetic Rate in C3 Crops like Rice and Approaches for Improving It. Agronomy 2022, 12, 1830. https://doi.org/10.3390/agronomy12081830
Ye M, Wu M, Zhang Y, Wang Z, Zhang H, Zhang Z. Physiological Factors Limiting Leaf Net Photosynthetic Rate in C3 Crops like Rice and Approaches for Improving It. Agronomy. 2022; 12(8):1830. https://doi.org/10.3390/agronomy12081830
Chicago/Turabian StyleYe, Miao, Meng Wu, Yu Zhang, Zeyu Wang, Hao Zhang, and Zujian Zhang. 2022. "Physiological Factors Limiting Leaf Net Photosynthetic Rate in C3 Crops like Rice and Approaches for Improving It" Agronomy 12, no. 8: 1830. https://doi.org/10.3390/agronomy12081830





