Abstract
To discover new acetyl-CoA carboxylase (ACCase) inhibiting-based herbicides, twenty-nine novel quinazolin-4(3H)-one derivatives were designed and synthesized based on the aryloxyphenoxypropionate motif. The bioassay results showed that most of the target compounds showed better pre-emergent herbicidal activity against monocotyledonous weeds in a greenhouse. Especially, when applied at 375 g ha−1 under pre-emergence conditions, compound QPP-7 displayed excellent herbicidal activity against monocotyledonous weeds (i.e., E. crusgalli, D. sanguinalis, P. alopecuroides, S. viridis, E. indica, A. fatua, E. dahuricu, S. alterniflora) with inhibition rate >90%, and displayed excellent crop safety to O. sativa, T. aestivum, G. spp, and A. hypogaea. The study of structure-activity relationship (SAR) revealed that the herbicidal activity of target compounds is strongly influenced by the spatial position of R group and the bulk of R1 group on quinazolin-4(3H)-one, and the (R = 6-F, R1 = Me) pattern is confirmed as the optimal orientation. Furthermore, the molecular docking study and the good inhibitory activity of QPP-7 against E. crusgalli ACCase enzyme (IC50 = 54.65 nM) indicated that it may be a ACCase inhibitor. Taken together, the present work demonstrated that compound QPP-7 could serve as a potential lead structure for further developing novel ACCase inhibiting-based herbicide.
1. Introduction
Herbicides play an important role in weeds control, protecting crops, and increase yields in agriculture. Among the known herbicides, aryloxyphenoxypropionate (APP) are a class of herbicides that inhibit the synthesis of fatty acids and destroy the membrane structure by inhibiting the activity of acetyl-CoA carboxylase in gramineous plants to achieve herbicidal effects [1,2,3,4]. Since the first launch of diclofop-methyl in 1971, many of APP herbicides, such as haloxyfop-P-methyl, fluazifop-P-butyl, fenoxaprop-P-ethyl, quizalofop-P-ethyl, have been reached the marketplace (Figure 1A). However, an inevitable problem associated with long-term irrational use of APP herbicides is the reduced efficacy due to weed resistance [5,6,7,8,9,10]. To overcome this problem, developing APP herbicides with novel structure or improved herbicidal activity is necessary.
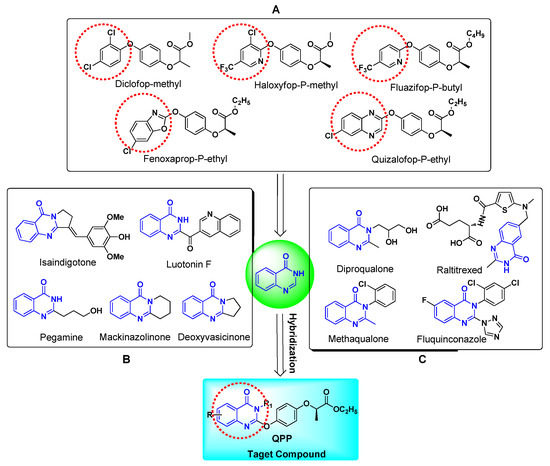
Figure 1.
Design of target compound QPP by molecular hybridization strategy. (A)commercial APP herbicides; (B) the natural quinazolin-4(3H)-ones; (C) commercial drugs containing quinazolin-4(3H)-one motif.
Biologically active natural products (NPs) are often served as the lead structures for novel agrochemical discovery in that their advantages associated with unique mode of action, easy degradation, and good environmental compatibility [11,12,13,14]. In addition, many of previous works have shown that the introduction of natural active groups is also an efficient method for agrichemical discovery, and a variety of NP-derived pesticides have been successfully developed and brought to market [15,16]. Quinazolin-4(3H)-ones, an important class of N-containing heterocyclic compounds based on a benzopyrimidone alkaloid structure, are widely distributed in plants and microorganisms (Figure 1B) [17]. Over the last few decades, natural quinazolin-4(3H)-ones have been found to possess a wide range of biological activities such as antifungal, [18] anticancer, [19] antiviral, [20,21] radical-scavenging, [22] antimicrobial, [23] cytotoxicity, [24] and anti-inflammatory, [25] and anti-malaria activities [26]. As such, the quinazolin-4(3H)-one skeleton have received considerable attention in recent years and is considered to be a privileged structure for developing drugs and pesticides [27,28,29,30,31,32,33]. So far, many of the quinazolin-4(3H)-one derivatives have been introduced into the market as drugs or pesticides, such as diproqualone (an anti-rheumatic drug), methaqualone (anti-convulsant drugs), raltitrexed (an anticancer drug), and fluquinconazole (an agricultural fungicide) have been developed to reach the market. Although there are many therapeutic drugs and pesticides based on the quinazolin-4(3H)-one skeleton, commercial herbicides based on the quinazolin-4(3H)-one skeleton are rarely reported.
Based on the above facts, in order to develop novel APP herbicides containing quinazolin-4(3H)-one skeleton with commercial potential, we intend to replace the aromatic ring part of the APP herbicides with quinazolin-4-one motif to construct quinazolinone-APP hybrids (Figure 1), which is expected to possess good herbicidal activity. Therefore, as part of our continuous efforts to develop novel structures with potential use as herbicides, [34,35,36,37] twenty-nine novel quinazolin-4-one derivatives based on the APP motif were designed, synthesized and tested for herbicidal activity and molecular mode action. To the best of our knowledge, this is the first report on the herbicidal activity of quinazolin-4(3H)-one derivatives with an APP motif.
2. Materials and Methods
2.1. General Information
In most cases, the reagents and solvents, purchased form Energy Chemical or Tokyo Chemical Industry, were analytical grade and used without further purification. Column chromatography purification was carried out using silica gel column chromatography (silica gel 200–300 mesh) (Qingdao Makall Group Co., Ltd., Qingdao, China). 1H and 13C NMR spectrum are obtained at 500 MHz and 125 MHz, respectively, using an AV-500 spectrometer (Bruker, Billerica, MA, USA) in CDCl3 or DMSO-d6 solution with tetramethylsilane (TMS) as the internal standard. The chemical shifts are reported as δ values relative to TMS. High-resolution mass spectra is conducted using an Ionspec 7.0 T spectrometer (Varian, Palo Alto, CA, USA) by the electrospray ionisation fourier transform ion cyclotron resonance (ESI-FTICR) technique. The crystal structure was determined on a Saturn 724 CCD area-detector diffractometer (Rigaku, Tokyo, Japan). High-performance liquid chromatography (HPLC) data is obtained on a SHIMADZU LC-20AT (Japan).
2.2. Chemical Synthesis Procedures
The synthetic pathway used to prepare the target compounds QPP-1 to QPP-29 is outlined in Scheme 1. The yields were not optimized.
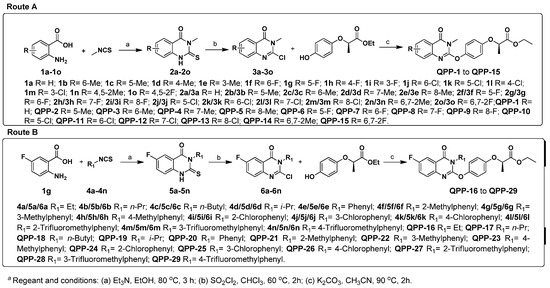
Scheme 1.
Synthetic route of preparing target compounds QPP-1 to QPP-29.
2.2.1. General Procedure for the Synthesis of Intermediates 2a–2o and 5a–5n
Intermediates 2a–2o and 5a–5n were prepared following a reported method [38]. To a 100 mL round-bottom flask was added anthranilic acid 1a (2.74 g, 20.0 mmol), methyl isothiocyanate (1.61 g, 22.0 mmol), Et3N (2.22 g, 22.0 mmol) and EtOH (30 mL). The reaction mixture was stirred at 80 °C for 3 h. After the reaction cooled to room temperature, the resulting precipitates was filtered, and the solid was washed with 20 mL EtOH/20 mL hexane, and dried to acquire the pure product 2a as a white solid (3.56 g, yield: 92.7%).
Intermediates 2b–2o and 5a–5n were prepared by the similar procedure to 2a. For data on 2a–2o and 5a–5n, see the supporting information.
2.2.2. General Procedure for the Synthesis of Intermediates 3a–3o and 6a–6n
Intermediates 3a–3o and 6a–6n were prepared following a reported method [38]. To a suspension of compound 2a (1.92 g, 10.0 mmol) in CHCl3 (25 mL) was added SO2Cl2 (1.46 g, 11.0 mmol). The reaction mixture was stirred at 60 °C for 2 h. After the completion of the reaction, the mixture was cooled to room temperature and diluted with CH2Cl2 (30 mL). The organic mixture was washed with brine, dried with Na2SO4, filtered and concentrated to be purified through chromatograph on silica gel using petroleum ether/ethyl acetate (V:V = 20:1) as eluent to give white solid 3a (1.17 g, yield: 60.3%).
Intermediates 3b–3o and 6a–6n were prepared by the similar procedure to 3a. For data on 3a–3o and 6a–6n, see the supporting information.
2.2.3. General Procedure for the Synthesis of Target Compounds QPP-1 to QPP-29
Compound 3a (194 mg, 1.0 mmol) was dissolved in 20 mL acetonitrile followed by addition of (R)-ethyl 2-(4-hydroxyphenoxy)propanoate (210 mg, 1.0 mmol) and potassium carbonate (207 mg, 1.5 mmol). The reaction mixture was stirred at 90 °C for 2 h. After the reaction was completed according to TLC detection, the solvent was removed under reduced pressure. The residue was purified through chromatograph on silica gel using petroleum ether/ethyl acetate (V:V = 10:1) as eluent to give target compound QPP-1 as a white solid (317 mg, yield: 86.2%). Target compounds QPP-2 to QPP-29 were prepared by the similar procedure to QPP-1.
(R)-ethyl 2-(4-((3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-1): white solid, yield 86.2%, m.p. 78–80 °C; 1H NMR (500 MHz, CDCl3) δ: 8.21 (dd, J = 8.0, 1.3 Hz, 1H), 7.62–7.58 (m, 1H), 7.38–7.30 (m, 2H), 7.20–7.12 (m, 2H), 6.98–6.88 (m, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.31–4.20 (m, 2H), 3.70 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.1, 163.1, 155.4, 152.6, 146.6, 145.9, 134.3, 127.1, 126.0, 124.9, 122.7, 118.9, 115.9, 73.2, 61.4, 28.8, 18.6, 14.2; HRMS, m/z calcd. for C20H21N2O5+ [M + H]+ 369.1445, found 369.1452.
(R)-ethyl 2-(4-((3,5-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-2): white solid, yield 85.1%, m.p. 101–104 °C; 1H NMR (500 MHz, CDCl3) δ: 7.45–7.38 (m, 1H), 7.19–7.13 (m, 3H), 7.06 (d, J = 7.3 Hz, 1H), 6.96–6.91 (m, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.30–4.18 (m, 2H), 3.63 (s, 3H), 2.85 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ: 172.1, 163.5, 155.3, 152.2, 148.0, 145.9, 141.2, 133.3, 127.7, 124.2, 122.7, 117.4, 115.9, 73.2, 61.4, 28.6, 22.9, 18.6, 14.2; HRMS, m/z calcd. for C21H23N2O5+ [M + H]+ 383.1601, found 383.1612.
(R)-ethyl 2-(4-((3,6-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-3): white solid, yield 83.3%, m.p. 91–94 °C; 1H NMR (500 MHz, CDCl3) δ: 7.99 (d, J = 0.8 Hz, 1H), 7.41 (dd, J = 8.3, 2.0 Hz, 1H), 7.26 (d, J = 8.6 Hz, 1H), 7.19–7.13 (m, 2H), 6.99–6.90 (m, 2H), 4.75 (q, J = 6.8 Hz, 1H), 4.27–4.23 (m, 2H), 3.68 (s, 3H), 2.42 (s, 3H), 1.64 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.1, 163.1, 155.4, 152.1, 145.9, 144.4, 135.8, 134.9, 126.5, 125.8, 122.7, 118.6, 116.7, 116.1, 115.9, 73.2, 61.4, 28.8, 21.1, 18.6, 14.2; HRMS, m/z calcd. for C21H23N2O5+ [M + H]+ 383.1601, found 383.1607.
(R)-ethyl 2-(4-((3,7-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-4): white solid, yield 81.6%, m.p. 87–90 °C; 1H NMR (500 MHz, CDCl3) δ: 8.09 (d, J = 8.1 Hz, 1H), 7.20–7.09 (m, 4H), 6.98–6.87 (m, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.25 (qd, J = 7.1, 1.6 Hz, 2H), 3.68 (s, 3H), 2.39 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.1, 162.9, 155.4, 152.7, 146.7, 145.9, 145.3, 126.9, 126.5, 125.8, 122.7, 116.5, 115.9, 73.2, 61.4, 28.7, 21.8, 18.6, 14.2; HRMS, m/z calcd. for C21H23N2O5+ [M + H]+ 383.1601, found 383.1612.
(R)-ethyl 2-(4-((3,8-dimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-5): white solid, yield 80.3%, m.p. 70–72 °C; 1H NMR (500 MHz, CDCl3) δ: 8.05 (d, J = 7.9 Hz, 1H), 7.45 (d, J = 7.2 Hz, 1H), 7.24–7.16 (m, 3H), 7.01–6.90 (m, 2H), 4.77 (q, J = 6.8 Hz, 1H), 4.30–4.14 (m, 2H), 3.69 (s, 3H), 2.24 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.1, 163.4, 155.2, 151.6, 146.2, 145.1, 134.8, 134.3, 124.7, 124.5, 122.7, 118.7, 115.7, 73.3, 61.4, 28.7, 18.6, 16.7, 14.1; HRMS, m/z calcd. for C21H23N2O5+ [M + H]+ 383.1601, found 383.1610.
(R)-ethyl 2-(4-((5-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-6): white solid, yield 78.0%, m.p. 82–85 °C; 1H NMR (500 MHz, CDCl3) δ: 7.52–7.48 (m, 1H), 7.17–7.11 (m, 3H), 6.99–6.91 (m, 3H), 4.76 (q, J = 6.8 Hz, 1H), 4.32–4.18 (m, 2H), 3.66 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 161.52 (d, J = 265.1 Hz), 159.9 (d, J = 4.0 Hz), 155.5, 153.2, 148.6, 145.7, 134.5 (d, J = 10.5 Hz), 122.7, 121.9 (d, J = 4.0 Hz), 115.9, 111.6 (d, J = 20.7 Hz), 73.2, 61.4, 28.5, 18.6, 14.2; HRMS, m/z calcd. for C20H20FN2O5+ [M + H]+ 387.1351, found 387.1355.
(R)-ethyl 2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-7): white solid, yield 87.5%, m.p. 88–91 °C; 1H NMR (500 MHz, CDCl3) δ: 7.86–7.80 (m, 1H), 7.38–7.28 (m, 2H), 7.19–7.11 (m, 2H), 6.99–6.89 (m, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.28–4.23 (m, 2H), 3.69 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 162.4 (d, J = 3.6 Hz), 159.7 (d, J = 245.5 Hz), 155.5, 152.2 (d, J = 1.3 Hz), 145.8, 143.1 (d, J = 1.5 Hz), 128.1 (d, J = 8.1 Hz), 122.8 (d, J = 24.1 Hz), 122.7, 119.8 (d, J = 8.5 Hz), 116.7, 116.1, 115.9, 111.9 (d, J = 23.7 Hz), 73.2, 61.4, 28.9, 18.6, 14.2; HRMS, m/z calcd. for C20H20FN2O5+ [M + H]+ 387.1351, found 387.1533.
(R)-ethyl 2-(4-((7-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-8): white solid, yield 88.2%, m.p. 61–64 °C; 1H NMR (500 MHz, CDCl3) δ: 8.19 (dd, J = 8.8, 6.2 Hz, 1H), 7.22–7.11 (m, 2H), 7.05–6.97 (m, 2H), 6.97–6.92 (m, 2H), 4.77 (q, J = 6.8 Hz, 1H), 4.25 (qd, J = 7.1, 3.2 Hz, 2H), 3.67 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 166.6 (d, J = 253.1 Hz), 162.2, 155.5, 153.5, 148.8 (d, J = 13.8 Hz), 145.7, 129.7 (d, J = 10.9 Hz), 122.7, 115.9, 115.6, 113.6 (d, J = 23.4 Hz), 111.4 (d, J = 22.5 Hz), 73.2, 61.4, 28.8, 18.6, 14.2; HRMS, m/z calcd. for C20H20FN2O5+ [M + H]+ 387.1351, found 387.1365.
(R)-ethyl 2-(4-((8-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-9): white solid, yield 80.0%, m.p. 67–70 °C; 1H NMR (500 MHz, CDCl3) δ: 7.99 (d, J = 8.0 Hz, 1H), 7.36–7.30 (m, 1H), 7.25 (dd, J = 8.0, 3.4 Hz, 1H), 7.23–7.19 (m, 2H), 7.02–6.87 (m, 2H), 4.77 (q, J = 6.8 Hz, 1H), 4.30–4.15 (m, 2H), 3.70 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.27 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.1, 162.2, 162.1, 154.9 (d, J = 273.1 Hz), 155.5, 152.8, 145.8, 136.1 (d, J = 12.3 Hz), 124.7 (d, J = 7.2 Hz), 122.5, 120.9 (d, J = 1.6 Hz), 119.8 (d, J = 18.5 Hz), 115.9, 73.3, 61.4, 28.9, 18.6, 14.1; HRMS, m/z calcd. for C20H20FN2O5+ [M + H]+ 387.1351, found 387.1361.
(R)-ethyl 2-(4-((5-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-10): white solid, yield 71.5%, m.p. 90–92 °C; 1H NMR (500 MHz, CDCl3) δ: 8.12–8.10 (m, 1H), 7.68 (dd, J = 7.7, 1.3 Hz, 1H), 7.31–7.26 (m, 2H), 7.25–7.20 (m, 1H), 6.97–6.91 (m, 2H), 4.78 (q, J = 6.8 Hz, 1H), 4.28–4.14 (m, 2H), 3.69 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.1, 162.6, 155.3, 152.7, 145.9, 143.5, 134.5, 130.4, 125.8, 124.9, 122.5, 120.4, 115.8, 73.2, 61.4, 28.9, 18.6, 14.1; HRMS, m/z calcd. for C20H20ClN2O5+ [M + H]+ 403.1055, found 403.1060.
(R)-ethyl 2-(4-((6-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-11): white solid, yield 91.7%, m.p. 100–102 °C; 1H NMR (500 MHz, CDCl3) δ: 8.16 (d, J = 2.4 Hz, 1H), 7.52 (dd, J = 8.7, 2.5 Hz, 1H), 7.28 (d, J = 8.7 Hz, 1H), 7.17–7.12 (m, 2H), 6.97–6.92 (m, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.25 (qd, J = 7.1, 2.6 Hz, 2H), 3.69 (s, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ:172.0, 162.0, 155.5, 152.8, 145.7, 145.2, 134.6, 130.5, 127.6, 126.4, 122.7, 119.9, 115.9, 73.2, 61.4, 28.9, 18.6, 14.2; HRMS, m/z calcd. for C20H20ClN2O5+ [M + H]+ 403.1055, found 403.1066.
(R)-ethyl 2-(4-((7-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-12): white solid, yield 80.6%, m.p. 77–80 °C; 1H NMR (500 MHz, CDCl3) δ: 7.27 (t, J = 8.0 Hz, 1H), 7.17–7.13 (m, 1H), 7.10–7.02 (m, 3H), 6.83 (dd, J = 9.8, 2.9 Hz, 2H), 4.65 (q, J = 6.8 Hz, 1H), 4.16–4.10 (m, 2H), 3.52 (s, 3H), 1.53 (d, J = 6.8 Hz, 3H), 1.16 (t, J = 7.2 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ:170.9, 159.8, 154.4, 151.7, 148.1, 144.6, 133.1, 132.4, 126.6, 124.2, 121.7, 114.9, 114.8, 72.1, 60.3, 27.8, 17.5, 13.1; HRMS, m/z calcd. for C20H20ClN2O5+ [M + H]+ 403.1055, found 403.1064.
(R)-ethyl 2-(4-((8-chloro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-13): white solid, yield 85.8%, m.p. 97–98 °C; 1H NMR (500 MHz, CDCl3) δ: 7.27 (t, J = 8.0 Hz, 1H), 7.17–7.12 (m, 1H), 7.10–7.02 (m, 3H), 6.83 (dd, J = 9.8, 2.9 Hz, 2H), 4.65 (q, J = 6.8 Hz, 1H), 4.16–4.10 (m, 2H), 3.52 (s, 3H), 1.53 (d, J = 6.8 Hz, 3H), 1.16 (t, J = 7.2 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 170.9, 159.8, 154.4, 151.7, 148.1, 144.6, 133.1, 132.4, 126.6, 124.2, 121.7, 114.9, 114.8, 72.1, 60.3, 27.8, 17.5, 13.1; HRMS, m/z calcd. for C20H20ClN2O5+ [M + H]+ 403.1055, found 403.1067.
(R)-ethyl 2-(4-((3,6,7-trimethyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-14): white solid, yield 89.8%, m.p. 124–125 °C; 1H NMR (500 MHz, CDCl3) δ: 7.93 (s, 1H), 7.19–7.09 (m, 3H), 6.94 (d, J = 8.6 Hz, 2H), 4.75 (q, J = 6.7 Hz, 1H), 4.31–4.17 (m, 2H), 3.67 (dd, J = 3.5, 2.5 Hz, 3H), 2.31 (d, J = 17.4 Hz, 3H), 1.65 (d, J = 6.8 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.1, 162.9, 155.3, 152.3, 146.0, 144.8, 144.5, 134.2, 134.2, 126.8, 126.3, 122.7, 116.6, 115.9, 73.2, 61.4, 28.7, 20.2, 19.5, 18.6, 14.2; HRMS, m/z calcd. for C22H25N2O5+ [M + H]+ 397.1758, found 397.1765.
(R)-ethyl 2-(4-((6,7-difluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)- propanoate (QPP-15): white solid, yield 84.6%, m.p. 76–79 °C; 1H NMR (500 MHz, CDCl3) δ: 7.81 (dd, J = 9.8, 8.9 Hz, 1H), 7.07–7.02 (m, 2H), 7.02–6.97 (m, 1H), 6.88–6.80 (m, 2H), 4.67 (q, J = 6.8 Hz, 1H), 4.19–4.12 (m, 2H), 3.57 (s, 3H), 1.55 (d, J = 6.8 Hz, 3H), 1.19 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.9, 161.5 (d, J = 2.7 Hz), 155.6, 154.84 (dd, J = 256.25 Hz,14.5 Hz), 153.8 (dd, J = 246.25 Hz, 14.6 Hz), 153.2, 145.6, 144.29 (dd, J = 11.8, 1.6 Hz), 122.6, 115.9, 115.43 (d, J = 4.7 Hz), 114.26 (dd, J = 19.2, 1.5 Hz), 113.72 (d, J = 18.3 Hz), 73.1, 61.4, 28.9, 18.5, 14.1; HRMS, m/z calcd. for C20H19F2N2O5+ [M + H]+ 405.1257, found 405.1266.
(R)-ethyl 2-(4-((3-ethyl-6-fluoro-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-16): white solid, yield 91.4%, m.p. 124–127 °C; 1H NMR (500 MHz, CDCl3) δ: 7.90–7.77 (m, 1H), 7.35–7.29 (m, 2H), 7.17–7.12 (m, 2H), 6.97–6.90 (m, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.33 (q, J = 7.1 Hz, 2H), 4.29–4.21 (m, 2H), 1.65 (d, J = 6.8 Hz, 3H), 1.42 (t, J = 7.1 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 161.9 (d, J = 3.4 Hz), 159.7 (d, J = 245.3 Hz), 155.4, 152.1, 145.8, 143.2, 128.1 (d, J = 8.0 Hz), 122.8, 122.6, 120.2 (d, J = 8.5 Hz), 115.9, 111.9 (d, J = 23.5 Hz), 73.2, 61.4, 37.6, 18.6, 14.2, 13.8; HRMS, m/z calcd. for C21H22FN2O5+ [M + H]+ 401.1507, found 405.1514.
(R)-ethyl 2-(4-((6-fluoro-4-oxo-3-propyl-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-17): white solid, yield 79.7%, m.p. 122–123 °C; 1H NMR (500 MHz, CDCl3) δ: 7.83 (d, J = 8.5 Hz, 1H), 7.32 (d, J = 5.5 Hz, 2H), 7.14 (d, J = 8.9 Hz, 2H), 6.94 (d, J = 8.8 Hz, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.28–4.21 (m, 4H), 1.97–1.78 (m, 2H), 1.65 (d, J = 6.8 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H), 1.03 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 162.1 (d, J = 3.1 Hz), 159.7 (d, J = 245.5 Hz), 155.4, 152.2, 145.9, 143.2, 128.1 (d, J = 8.0 Hz), 122.8, 122.6, 120.1 (d, J = 8.4 Hz), 115.9, 111.9 (d, J = 23.7 Hz), 73.2, 61.4, 43.9, 21.9, 18.6, 14.2, 11.4; HRMS, m/z calcd. for C22H24FN2O5+ [M + H]+ 415.1664, found 415.1675.
(R)-ethyl 2-(4-((3-butyl-6-fluoro-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-18): white solid, yield 75.1%, m.p. 108–109 °C; 1H NMR (500 MHz, CDCl3) δ: 7.89–7.71 (m, 1H), 7.35–7.31 (m, 2H), 7.16–7.11 (m, 2H), 6.97–6.92 (m, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.34–4.18 (m, 4H), 1.89–1.74 (m, 2H), 1.65 (d, J = 6.8 Hz, 3H), 1.55–1.38 (m, 2H), 1.29 (t, J = 7.1 Hz, 3H), 0.99 (t, J = 7.4 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 162.1 (d, J = 3.3 Hz), 159.7 (d, J = 245.3 Hz), 155.4, 152.2, 145.9, 143.2, 128.1 (d, J = 7.8 Hz), 122.8, 122.6, 120.1 (d, J = 8.4 Hz), 115.9, 111.9 (d, J = 23.5 Hz), 73.2, 61.4, 42.3, 30.6, 20.2, 18.6, 14.2, 13.8; HRMS, m/z calcd. for C23H26FN2O5+ [M + H]+ 429.1820, found 429.1830.
(R)-ethyl 2-(4-((6-fluoro-3-isobutyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-19): white solid, yield 85.8%, m.p. 100–103 °C; 1H NMR (500 MHz, CDCl3) δ: 7.85–7.80 (m, 1H), 7.37–7.30 (m, 2H), 7.17–7.10 (m, 2H), 6.98–6.92 (m, 2H), 4.76 (q, J = 6.8 Hz, 1H), 4.31–4.20 (m, 2H), 4.10 (d, J = 7.5 Hz, 2H), 2.29 (dp, J = 14.0, 7.0 Hz, 1H), 1.65 (d, J = 6.8 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H), 1.02 (s, 3H), 1.01 (s, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 162.3 (d, J = 3.2 Hz), 159.7 (d, J = 245.4 Hz), 155.4, 152.4, 145.8, 143.2, 128.1 (d, J = 7.7 Hz), 122.8, 122.6 (d, J = 2.6 Hz), 120.1 (d, J = 8.4 Hz), 115.9, 112.0 (d, J = 23.8 Hz), 73.2, 61.4, 49.2, 29.7, 27.8, 20.2, 18.6, 14.2; HRMS, m/z calcd. for C23H26FN2O5+ [M + H]+ 429.1820, found 429.1830.
(R)-ethyl 2-(4-((6-fluoro-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-20): white solid, yield 69.7%, m.p. 135–136 °C; 1H NMR (500 MHz, CDCl3) δ: 7.86 (dd, J = 8.4, 2.8 Hz, 1H), 7.54 (t, J = 7.5 Hz, 2H), 7.51–7.46 (m, 1H), 7.43–7.36 (m, 4H), 7.05 (d, J = 9.0 Hz, 2H), 6.87 (d, J = 9.0 Hz, 2H), 4.72 (q, J = 6.8 Hz, 1H), 4.27–4.17 (m, 2H), 1.62 (d, J = 6.8 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 162.2 (d, J = 3.5 Hz), 159.9 (d, J = 246.2 Hz), 155.4, 151.6, 145.8, 143.3, 134.9, 129.5, 129.1, 128.3 (d, J = 7.8 Hz), 128.1, 123.1 (d, J = 24.0 Hz), 122.5, 120.5 (d, J = 8.8 Hz), 115.8, 112.3 (d, J = 23.7 Hz), 73.1, 61.4, 18.6, 14.1; HRMS, m/z calcd. for C25H22FN2O5+ [M + H]+ 449.1507, found 449.1516.
(R)-ethyl 2-(4-((6-fluoro-4-oxo-3-(o-tolyl)-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-21): white solid, yield 69.5%, m.p. 47–49 °C; 1H NMR (500 MHz, CDCl3) δ: 7.88 (dd, J = 8.4, 2.8 Hz, 1H), 7.47–7.33 (m, 5H), 7.25 (t, J = 3.6 Hz, 1H), 7.06–7.01 (m, 2H), 6.90–6.84 (m, 2H), 4.72 (q, J = 6.8 Hz, 1H), 4.22 (q, J = 7.1 Hz, 2H), 2.23 (s, 3H), 1.62 (d, J = 6.8 Hz, 3H), 1.25 (t, J = 7.1, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.9, 161.8 (d, J = 3.5 Hz), 159.9 (d, J = 246.2 Hz), 155.4, 151.7, 145.8, 143.6, 135.5, 134.2, 131.2, 129.5, 127.3, 123.1 (d, J = 24.0 Hz), 122.5, 120.5 (d, J = 8.6 Hz), 115.8, 112.4 (d, J = 23.8 Hz), 73.1, 61.4, 18.6, 17.6, 14.2; HRMS, m/z calcd. for C26H24FN2O5+ [M + H]+ 463.1664, found 463.1672.
(R)-ethyl 2-(4-((6-fluoro-4-oxo-3-(m-tolyl)-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-22): white solid, yield 84.3%, m.p. 119–122 °C; 1H NMR (500 MHz, CDCl3) δ: 7.86 (dd, J = 8.4, 2.8 Hz, 1H), 7.39 (ddt, J = 11.8, 8.9, 5.4 Hz, 3H), 7.29 (d, J = 7.7 Hz, 1H), 7.16 (d, J = 8.5 Hz, 2H), 7.08–7.02 (m, 2H), 6.91–6.85 (m, 2H), 4.72 (q, J = 6.8 Hz, 1H), 4.27–4.13 (m, 2H), 2.43 (s, 3H), 1.62 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 162.3 (d, J = 3.5 Hz), 159.9 (d, J = 246.0 Hz), 155.4, 151.7, 145.9, 143.4, 139.6, 134.9, 129.9, 129.3, 128.6, 128.3 (d, J = 8.0 Hz), 124.9, 123.1 (d, J = 23.9 Hz), 122.6, 120.6 (d, J = 8.8 Hz), 115.8, 112.3 (d, J = 23.7 Hz), 73.2, 61.4, 29.7, 21.4, 18.6, 14.2; HRMS, m/z calcd. for C26H24FN2O5+ [M + H]+ 463.1664, found 463.1669.
(R)-ethyl 2-(4-((6-fluoro-4-oxo-3-(p-tolyl)-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-23): white solid, yield 68.0%, m.p. 151–152 °C; 1H NMR (500 MHz, CDCl3) δ: 7.86 (dd, J = 8.4, 2.8 Hz, 1H), 7.44–7.32 (m, 4H), 7.24 (d, J = 8.3 Hz, 2H), 7.07–7.02 (m, 2H), 6.90–6.85 (m, 2H), 4.72 (q, J = 6.8 Hz, 1H), 4.25–4.19 (m, 2H), 2.43 (s, 3H), 1.62 (d, J = 6.8 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 172.0, 162.3 (d, J = 3.5 Hz), 159.9 (d, J = 245.7 Hz), 155.3, 151.8, 145.9, 143.3, 139.1, 132.3, 130.2, 128.3 (d, J = 8.0 Hz), 127.7, 123.1 (d, J = 24.0 Hz), 122.5, 120.6 (d, J = 8.6 Hz), 115.8, 112.4 (d, J = 23.8 Hz), 73.2, 61.4, 21.3, 18.6, 14.2; HRMS, m/z calcd. for C26H24FN2O5+ [M + H]+ 463.1664, found 463.1670.
(R)-ethyl 2-(4-((3-(2-chlorophenyl)-6-fluoro-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-24): white solid, yield 74.3%, m.p. 51–54 °C; 1H NMR (500 MHz, CDCl3) δ: 7.88 (dd, J = 8.3, 2.8 Hz, 1H), 7.64–7.57 (m, 1H), 7.50–7.36 (m, 5H), 7.10–7.05 (m, 2H), 6.93–6.84 (m, 2H), 4.72 (q, J = 6.8 Hz, 1H), 4.22 (q, J = 7.1 Hz, 2H), 1.62 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.9, 161.5 (d, J = 3.0 Hz), 159.9 (d, J = 246.3 Hz), 155.5, 151.2, 145.7, 143.5, 132.9, 132.4, 130.5 (d, J = 29.2 Hz), 130.1, 128.4 (d, J = 8.0 Hz), 128.0, 123.3 (d, J = 23.9 Hz), 122.6, 120.3 (d, J = 8.8 Hz), 115.8, 112.5 (d, J = 23.7 Hz), 73.1, 61.4, 18.6, 14.2; HRMS, m/z calcd. for C25H21ClFN2O5+ [M + H]+ 483.1118, found 483.1122.
(R)-ethyl 2-(4-((3-(3-chlorophenyl)-6-fluoro-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-25): white solid, yield 82.0%, m.p. 113–116 °C; 1H NMR (500 MHz, CDCl3) δ: 7.85 (dd, J = 8.3, 2.6 Hz, 1H), 7.51–7.45 (m, 2H), 7.42–7.38 (m, 3H), 7.29–7.26 (m, 1H), 7.08–7.03 (m, 2H), 6.92–6.85 (m, 2H), 4.73 (q, J = 6.8 Hz, 1H), 4.22 (qd, J = 7.1, 0.7 Hz, 2H), 1.63 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.9, 161.9 (d, J = 3.5 Hz), 160.0 (d, J = 246.5 Hz), 155.5, 151.1, 145.7, 143.2, 135.9, 135.1, 130.5, 129.5, 128.6, 128.4 (d, J = 8.1 Hz), 126.6, 123.3 (d, J = 24.0 Hz), 122.5, 120.4 (d, J = 8.7 Hz), 115.8, 112.4 (d, J = 23.9 Hz), 73.1, 61.4, 18.6, 14.2; HRMS, m/z calcd. for C25H21ClFN2O5+ [M + H]+ 483.1118, found 483.1124.
(R)-ethyl 2-(4-((3-(4-chlorophenyl)-6-fluoro-4-oxo-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-26): white solid, yield 78.1%, m.p. 146–148 °C; 1H NMR (500 MHz, CDCl3) δ: 7.85 (dd, J = 8.3, 2.5 Hz, 1H), 7.52 (d, J = 8.4 Hz, 2H), 7.45–7.36 (m, 2H), 7.31 (d, J = 8.5 Hz, 2H), 7.05 (d, J = 8.5 Hz, 2H), 6.88 (d, J = 8.3 Hz, 2H), 4.72 (q, J = 6.7 Hz, 1H), 4.22 (q, J = 7.0 Hz, 2H), 1.63 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.9, 162.0 (d, J = 3.1 Hz), 160.0 (d, J = 246.2 Hz), 155.5, 151.2, 145.7, 143.2, 135.2, 133.4, 128.4 (d, J = 8.0 Hz), 123.3 (d, J = 24.0 Hz), 122.4, 115.9, 112.4 (d, J = 23.9 Hz), 73.2, 61.4, 18.6, 14.2; HRMS, m/z calcd. for C25H21ClFN2O5+ [M + H]+ 483.1118, found 483.1123.
(R)-ethyl 2-(4-((6-fluoro-4-oxo-3-(2-(trifluoromethyl)phenyl)-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-27): white solid, yield 96.9%, m.p. 45–48 °C; 1H NMR (500 MHz, CDCl3) δ: 7.77 (d, J = 8.1 Hz, 2H), 7.66 (t, J = 7.7 Hz, 1H), 7.54 (t, J = 7.7 Hz, 1H), 7.38 (d, J = 7.9 Hz, 1H), 7.35–7.26 (m, 2H), 6.93 (d, J = 8.8 Hz, 2H), 6.78 (d, J = 8.8 Hz, 2H), 4.62 (q, J = 6.8 Hz, 1H), 4.12 (q, J = 7.1 Hz, 2H), 1.52 (d, J = 6.8 Hz, 3H), 1.16 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.9, 161.9 (d, J = 3.3 Hz), 159.9 (d, J = 246.2 Hz), 155.5, 151.3, 145.6, 143.5, 133.4, 133.2 (d, J = 1.5 Hz), 130.8, 129.9, 128.5 (d, J = 7.9 Hz), 127.9 (q, J = 31.2 Hz), 127.7 (q, J = 4.2 Hz), 123.3 (d, J = 23.9 Hz), 123.1 (q, J = 216.7 Hz), 122.5, 120.1 (d, J = 8.8 Hz), 115.8, 112.4 (d, J = 23.9 Hz), 73.1, 61.4, 18.6, 14.1; HRMS, m/z calcd. for C26H21F4N2O5+ [M + H]+ 517.1381, found 517.1386.
(R)-ethyl 2-(4-((6-fluoro-4-oxo-3-(3-(trifluoromethyl)phenyl)-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-28): white solid, yield 85.0%, m.p. 39–42 °C; 1H NMR (500 MHz, CDCl3) δ: 7.86 (dd, J = 8.2, 2.6 Hz, 1H), 7.76 (d, J = 7.9 Hz, 1H), 7.72–7.66 (m, 2H), 7.58 (d, J = 7.9 Hz, 1H), 7.45–7.38 (m, 2H), 7.08–7.03 (m, 2H), 6.91–6.86 (m, 2H), 4.72 (q, J = 6.8 Hz, 1H), 4.22 (q, J = 7.1 Hz, 2H), 1.63 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.9, 161.9 (d, J = 3.2 Hz), 160.1 (d, J = 246.6 Hz), 155.5, 150.9, 145.6, 143.2, 135.5, 132.2 (q, J = 33.1 Hz), 131.8, 130.2, 128.5 (d, J = 7.8 Hz), 126.1 (q, J = 3.2 Hz), 125.5 (q, J = 3.6 Hz), 123.5 (d, J = 23.9 Hz), 122.4, 120.3 (d, J = 8.4 Hz), 115.8, 112.4 (d, J = 23.9 Hz), 73.1, 61.4, 18.6, 14.2; HRMS, m/z calcd. for C26H21F4N2O5+ [M + H]+ 517.1381, found 517.1387.
(R)-ethyl 2-(4-((6-fluoro-4-oxo-3-(4-(trifluoromethyl)phenyl)-3,4-dihydroquinazolin-2-yl)oxy)phenoxy)propanoate (QPP-29): white solid, yield 77.0%, m.p. 146–147 °C; 1H NMR (500 MHz, CDCl3) δ: 7.86 (dd, J = 8.2, 2.6 Hz, 1H), 7.82 (d, J = 8.4 Hz, 2H), 7.52 (d, J = 8.2 Hz, 2H), 7.46–7.37 (m, 2H), 7.07–7.02 (m, 2H), 6.91–6.85 (m, 2H), 4.72 (q, J = 6.8 Hz, 1H), 4.22 (qd, J = 7.1, 0.9 Hz, 2H), 1.63 (d, J = 6.8 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ: 171.9, 161.9 (d, J = 3.4 Hz), 160.1 (d, J = 246.8 Hz), 155.5, 150.9, 145.6, 143.2, 138.1, 131.4 (q, J = 32.9 Hz), 128.9, 128.5 (d, J = 7.9 Hz), 126.7 (q, J = 7.3 Hz), 123.5 (d, J = 23.9 Hz), 122.4, 120.3 (d, J = 8.3 Hz), 112.4 (d, J = 23.8 Hz), 73.1, 61.4, 18.6, 14.2; HRMS, m/z calcd. for C26H21F4N2O5+ [M + H]+ 517.1381, found 517.1387.
2.3. X-ray Diffraction Analysis of Target Compound QPP-7
The single crystal of QPP-7 was slowly cultivated from a mixture of dichloromethane and ethanol (1/1 by volume). Crystallographic data of compound QPP-7 had been deposited with the Cambridge Crystallographic Data Centre as supplementary publications with the deposition number 2183936. The detail data can be acquired free of charge from http://www.ccdc.cam.ac.uk/ (15 July 2022).
2.4. Evaluation of Herbicidal Activity
Herbicidal activity was evaluated based on the reported methods [34]. Commercial herbicide quizalofop-P-ethyl (QZ) was selected as the positive control. The preliminary in vitro herbicidal activity of target compounds QPP-1 to QPP-15 was determined with Brassica campestris root test and Echinochloa crusgalli cup test at a dosage of 10 μg/mL. Further herbicidal activity of target compounds QPP-1 to QPP-29 against two dicotyledonous species Brassica campestris and Amaranthus retroflexus, and two monocotyledonous species Echinochloa crusgalli and Digitaria sanguinalis was tested in a greenhouse. Briefly, the target compounds were dissolved in 100 μL of N, N-dimethylformamide with the addition of a little Tween 80 and then were sprayed using a laboratory belt sprayer delivering a 750 L/ha spray volume. The dosage (activity ingredient) for each compound corresponded to 1500 g/ha. Compounds were sprayed immediately after seed planting (preemergence treatment) or after the expansion of the first true leaf (postemergence treatment). The mixture of same amount of water, N, N-dimethylformamide, and Tween 80 was sprayed as the control. The fresh weight of the above ground tissues was measured 14 days after treatment. The inhibition percent was used to describe the control efficiency of the compounds. The data represented the percent displaying herbicidal damage as compared to the control, where complete control of the target is 100 and no control is 0. Compounds QPP-3, QPP-7, QPP-11 were selected to study the herbicidal activity at 750 g/ha, 375 g/ha and 187.5 g/ha, respectively. Compound QPP-7 was selected to study the herbicidal spectrum. All of the bioassays were tested for three parallel experiments. Details of the experimental procedure is given in the Supplementary Materials section.
2.5. Crop Selectivity
Based on the reported methods, [34] the crop selectivity of target compound QPP-7 was evaluated with three replicates per treatment. Six representative crops, namely, Oryza sativa, Zea mays, Triticum aestivum, Gossypium spp, Glycine max and Arachis hypogaea, were selected for crop selectivity studies in the greenhouse. The procedure is given in the Supplementary Materials section.
2.6. Molecular Docking Study
As we known, the APP herbicides as predrug, played major role in plants through hydrolysis into acids [39]. Therefore, the free acid of compound QPP-7 and QZ were dock into the binding site of ACCase by using the molecular docking module (CDOCKER) in the DS software (Discovery Studio 2020, Dassault Systemes, France). The crystal structure of ACCase enzyme was extracted from crystal structure PDB code 1UYR, and H2O and all binding ligands were deleted. The structures of small molecules were optimized with the ligand minimization protocol. The free acid of compound QPP-7 and QZ were set near the original diclofop-methyl binding site (amino acid residues from 1596 to 2025) in ACCase, respectively. The combined spherical area were (x = 26.6004, y = 40.6731, z = 77.6454, Radius = 10). After calculations with the parameters set as default values, the generated conformations were clustered together and ranked by the lowest docking energy, and a cluster analysis was performed.
2.7. ACCase Extraction and Inhibition Activity Assay
When E. crusgalli was grown to the 3-leaf stage in a greenhouse, shoots were cut at the base and stored at −80 °C. ACCase was extracted and partially purified using the method described by Cocker et al. [40]. The ACCase enzyme inhibition assay was performed by Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China). The ACCase enzyme was treated with the inhibitors QPP-7 and QZ, and the enzyme activity was measured using the method of enzyme-linked immune-sorbent assay (ELISA) according to the manufacturer’s instructions (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). The inhibitor concentrations ranged from 6.25 to 100 nM, and each experiment was repeated at least three times. The absorbance [optical density (OD) value] was determined at 450 nm and used to calculate the half maximal inhibitory concentration (IC50) values.
3. Results and Discussion
3.1. Synthetic Chemistry
As depicted in Scheme 1, the target compounds QPP-1 to QPP-15 were prepared via a three-step synthetic route using methyl isothiocyanate and several anthranilic acids as the starting material (Route A); the target compounds QPP-16 to QPP-29 were prepared via a three-step synthetic route using 2-amino-5-fluorobenzoic acid and several isothiocyanates as the starting material (Route B). Briefly, anthranilic acids 1a–1o reacted with isothiocyanates in ethanol by using triethylamine as a base to provide intermediates 2a–2o and 5a–5n, which was then reacted with sulfuryl chloride in trichloromethane to provide intermediates 3a–3o and 6a–6n. Finally, intermediates 3a–3o and 6a–6n reacted with commercial (R)-2-(4-hydroxyphenoxy)propionic acid in acetonitrile using potassium carbonate as a base to provide target compounds QPP-1 to QPP-29 in 68.0% to 96.9% yields. The structures of all the target compounds were confirmed by 1H NMR, 13C NMR, and HRMS.
Furthermore, to demonstrate that the reaction conditions have little effect on the chiral carbon configuration of the target compounds, the enantiomeric excesses (ee) values of the target compound QPP-7 and intermediate (R)-2-(4-hydroxyphenoxy)propionic acid were tested. The enantiomeric excesses were determined by HPLC analysis over a chiral column (Daicel Chiralcel OD-H, eluted with hexane-isopropyl alcohol; monitored by UV detector). The results showed that the ee values of (R)-2-(4-hydroxyphenoxy)propionic acid and QPP-7 are 100% and 95.8%, respectively. In addition, the configuration of compound QPP-7 was confirmed using the X-ray diffraction analysis (CCDC 2183936, Figure 2).
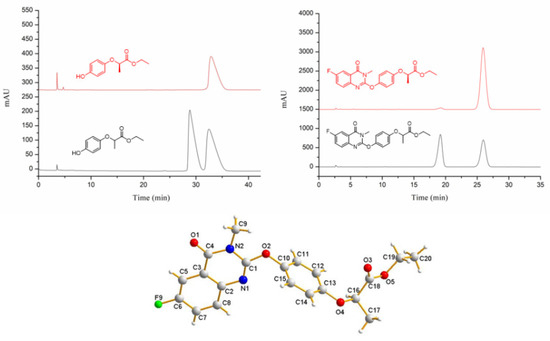
Figure 2.
ee value testing and X-ray crystal structure of target compound QPP-7.
3.2. In Vitro Herbicidal Activity of Target Compounds QPP-1 to QPP-15
The in vitro herbicidal activities of the target compounds QPP-1 to QPP-15 were preliminarily determined by the B. campestris root test and E. crusgalli cup test at a dosage of 10 μg/mL. Commercial herbicide quizalofop-P-ethyl was selected as the positive control sample. As shown in Figure 3, some of the target compounds, such as QPP-1 to QPP-3, QPP-7 to QPP-9, QPP-11, QPP-12, and QPP-15, exhibited good herbicidal activity against the monocotyledonous plant E. crusgalli with >50% inhibition. Among them, compound QPP-7 exhibited excellent herbicidal activity against E. crusgalli with 100% inhibition, which is equal to commercial herbicide quizalofop-P-ethyl. However, the target compounds have no inhibition against the dicotyledonous plant B. campestris. This finding indicated that the target compounds have stronger herbicidal activity against monocotyledonous plant.
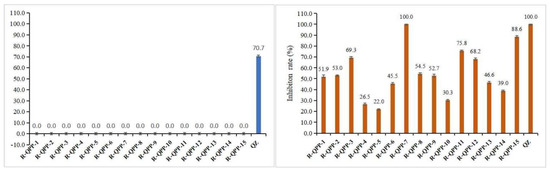
Figure 3.
In vitro herbicidal activity of target compounds QPP-1 to QPP-15 at a dosage of 10 μg/mL; B. campestris root test (Left), E. crusgalli cup test (Right).
3.3. Herbicidal Activity of Target Compounds QPP-1 to QPP-29 in Greenhouse Tests and SAR Study
Based on the above preliminary bioassay results, the herbicidal activity of target compounds QPP-1 to QPP-15 was further tested on four species that were representative of monocotyledonous and dicotyledonous plants at a dosage of 1500 g ha−1 located in a greenhouse. As shown in Figure 4, in most cases, the target compounds displayed stronger herbicidal activity against monocotyledonous plants than dicotyledonous plants. Moreover, it was found that most of the target compounds have stronger pre-emergent herbicidal activity than post-emergent herbicidal activity. For example, compounds QPP-1, QPP-3, QPP-7, and QPP-11 exhibited good herbicidal activity against all the weeds tested with sum inhibition 167.7%, 215.7%, 259.4%, and 225.8% under pre-emergence conditions, respectively, while these compounds have lower sum inhibition (75.9%, 60.0%, 208.4%, 107.2%, respectively) under post-emergence conditions.
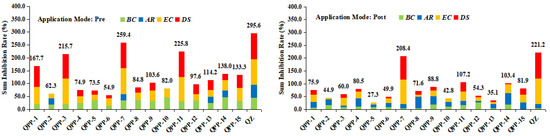
Figure 4.
Effects (% inhibition) of compounds QPP-1 to QPP-15 on the loss of plant weight at a dosage of 1500 g ha−1 in greenhouse testing; pre: pre-emergence; post: post-emergence; BC: B. campestris; AR: A. retroflexus; EC: E. crusgalli; DS: D. sanguinalis.
Analyzing the herbicidal activity of QPP-1 to QPP-15 under pre-emergence conditions, it was found that R group on the benzene ring of quinazolin-4-one has significant influence on the herbicidal activity. Generally, when a single substituent was introduced at the 6-position on benzene ring of quinazolin-4-one, the herbicidal activity of target compounds was improved. For example, compounds QPP-3 (R = 6-Me, sum inhibition = 215.7%), QPP-7 (R = 6-F, sum inhibition = 259.4%), and QPP-11 (R = 6-Cl, sum inhibition = 225.8%) exhibited stronger herbicidal activity than that of compound QPP-1 (R = H, sum inhibition = 167.7%). Simultaneously, the herbicidal activity was enhanced with increasing the electron-withdrawing ability of the substituent at the 6-position, i.e., R = 6-F (QPP-7) > 6-Cl (QPP-11) > 6-Me (QPP-3). When a single substituent (regardless of whether electron-withdrawing group or electron-donating group) was introduced at the 5-position (i.e., QPP-2, QPP-6, QPP-10), or 7-position (i.e., QPP-4, QPP-8, QPP-12), or 8-position (i.e., QPP-5, QPP-9, QPP-13) on the benzene ring of quinazolin-4-one, the herbicidal activity of target compound was decreased sharply. In addition, when a substituent was introduced at the 7-position of QPP-3 and QPP-7, the corresponding disubstituted compounds QPP-14 and QPP-15 showed lower herbicidal activity than that of QPP-3 and QPP-7. These results suggested that the spatial position of R group on the benzene ring of quinazolin-4-one has more important influence on the herbicidal activity than that of electronic effect and introducing a single electron-withdrawing group at the 6-position on benzene ring of quinazolin-4-one would be essential for improving herbicidal activity.
As a result of the higher sum inhibition against all the weeds tested, compounds QPP-3, QPP-7, and QPP-11 were chosen for further testing at lower doses. As shown in Table 1, these compounds displayed stronger herbicidal activity against monocotyledonous plants than dicotyledonous plants. Moreover, upon decreasing the dosage, the herbicidal activity of these compounds under post-emergence conditions decreased faster than that observed under pre-emergence conditions. These findings confirmed that the target compounds have better selective to monocotyledonous plants and exhibit stronger herbicidal activity under pre-emergence conditions than under post-emergence conditions. It was found that compounds QPP-3, QPP-7, and QPP-11 exhibit good herbicidal activity against monocotyledonous plants under pre-emergence conditions at a dosage of 750 g ha−1. Unfortunately, these compounds have lower herbicidal activity against monocotyledonous plants than that of QZ when the dosage reduced to 187.5 g ha−1. Nevertheless, to our relief, compound QPP-7 still exhibited excellent pre-emergent herbicidal activities against E. crusgalli and D. sanguinalis with inhibition 96.7% and 100% at the dosage of 375 g ha−1, respectively, which are almost equal to QZ (Figure 5). This promising result indicate that compound QPP-7 may serve as a potential lead compound for further optimization.

Table 1.
Effects (inhibition/%) of compounds QPP-3, QPP-7, and QPP-11 on loss of plant weight at lower dosage in greenhouse testing a.

Figure 5.
Photographs illustrating the herbicidal activity of QPP-7 under pre-emergence conditions at a dosage of 375 g ha−1; From left to right: B. campestris, A. retroflexus, E. crusgalli, D. sanguinalis.
In order to explore the effect of R1 group on herbicidal activity, subsequently, the target compounds QPP-16 to QPP-29 were synthesized by using QPP-7 as lead compound, and their herbicidal activity was evaluated under pre-emergence conditions. Lead compound QPP-7 and QZ were selected as the positive control samples. As shown in Figure 6, the target compounds QPP-16 to QPP-29 showed lower sum inhibition against all the weeds tested at a dosage of 1500 g ha−1 than that of lead compound QPP-7 and QZ. Meanwhile, it was found that the decreased sum inhibition was mainly attributed to the reduced inhibitory effect of the compound on monocotyledonous plants. Analyzing the herbicidal activity of QPP-7 and QPP-16 to QPP-18, it was found that, with the extension of the carbon chain, compounds with longer carbon chain progressively lost herbicidal activity. When a branched-chain alkyl groups was introduced on the nitrogen atom, QPP-19 showed lower herbicidal activity than that of the corresponding straight-chain compound QPP-18. With the alkyl group at the nitrogen atom of quinazolin-4(H)-one replaced by a benzene ring, the herbicidal activity of QPP-20 did not improve. Although the introduction of substituent on the benzene ring at the nitrogen atom increased the herbicidal activity compared to QPP-20, the herbicidal activity of QPP-21 to QPP-29 was still lower than that of lead compound QPP-7. These results suggested that the introduction of a substituent with bulker than the methyl at the nitrogen atom did not conducive to improving the herbicidal activity.
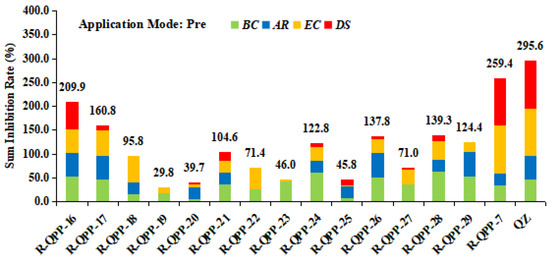
Figure 6.
Effects (% inhibition) of compounds QPP-16 to QPP-29 on the loss of plant weight at a dosage of 1500 g ha−1 in greenhouse testing; pre: pre-emergence; BC: B. campestris; AR: A. retroflexus; EC: E. crusgalli; DS: D. sanguinalis.
The aforementioned results for structure-activity relationship revealed that the herbicidal activity of target compounds is strongly influenced by the spatial position of R group and the bulk of R1 group on quinazolin-4(H)-one, and the (R = 6-F, R1 = Me) pattern was confirmed as the optimal orientation.
3.4. Herbicidal Spectrum and Crop Safety of Compound QPP-7
To further evaluate whether compound QPP-7 has the potential to be developed as a herbicide, its herbicidal spectrum against monocotyledonous plants and crop safety were investigated at a dosage of 375 g ha−1 under pre-emergence conditions. The monocotyledonous plants Echinochloa crusgalli (EC), Digitaria sanguinalis (DS), Pennisetum alopecuroides (PA), Setaria viridis (SV), Eleusine indica (EI), Avena fatua (AF), Elymus dahuricu (ED), Spartina alterniflora (SA) were chosen as the target weeds to evaluate the herbicidal spectrum of compound QPP-7 in a greenhouse. As shown in Figure 7, QPP-7 displays strong control with inhibition >90% against all the weeds tested, which is almost equal to QZ. This finding indicated that compound QPP-7 has a broad herbicidal spectrum for monocotyledonous weeds control. Subsequently, six representative crops, Oryza sativa, Zea mays, Triticum aestivum, Gossypium spp, Glycine max and Arachis hypogaea, were selected for further crop selectivity study (Table 2). The results showed that O. sativa, T. aestivum, and A. hypogaea displayed a high tolerance toward compound QPP-7, while QZ was not selective for O. sativa and T. aestivum (98.7% and 59.3% injury, respectively). These promising results indicated that compound QPP-7 has the potential to be developed as a pre-emergence herbicide lead compound for weed control in O. sativa, T. aestivum, and A. hypogaea Fields.
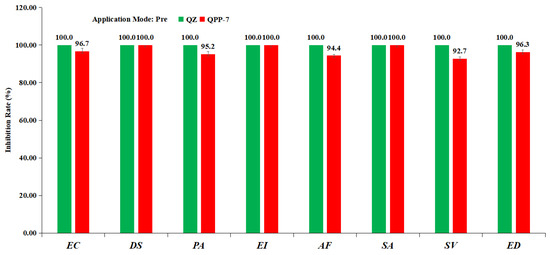
Figure 7.
Herbicidal spectrum testing of compound QPP-7 under pre-emergence conditions at a dosage of 375 g ha−1; Echinochloa crusgalli (EC), Digitaria sanguinalis (DS), Pennisetum alopecuroides (PA), Setaria viridis (SV), Eleusine indica (EI), Avena fatua (AF), Elymus dahuricu (ED), Spartina alterniflora (SA).

Table 2.
Pre-emergence crop selectivity of compound QPP-7 at the dosage of 375 g ha−1 (Injury Inhibition) a.
3.5. Molecular Mode of Action of the Target Compound QPP-7
In order to explore the molecular mode of action of target compounds, compound QPP-7 was selected to study the herbicidal mechanism. Since the target compounds were designed based on the APP motif, we speculated that QPP-7 could be a ACCase inhibitor. Thus, the molecular docking simulations were first carried out. As shown in Figure 8, it was easy to find that the relevant interactions between compound QPP-7 and the target enzyme is different to that of quizalofop-P-ethyl. In the docking complex of compound QPP-7, the carbonyl oxygen atom on the carboxyl group formed two non-classical hydrogen bonds with the amino acid residues of GLY1971; the carbonyl oxygen atom on the quinazolin-4(3H)-one formed a non-classical hydrogen bonds with the amino acid residues of GLY1997. Meanwhile, the benzene ring on the phenoxypropionic acid inserted into the active site and generated a π–π interaction with PHE1956, and the hydroxyl group generated a p–π interaction with TRP1924. In the docking complex of QZ, the hydroxyl group formed a classical hydrogen bond and two non-classical hydrogen bonds with the amino acid residues of ALA1627, GLY1626, GLY1734, respectively. Meanwhile, the benzene ring of quinoxaline formed two π–π interaction with TYR1738, and the nitrogen atom formed two non-classical hydrogen bonds with GLY1998. From the above docking results alone, it is difficult to conclude if it is QPP-7 or QZ has a more prominent inhibitory activity against ACCase. Therefore, ACCase activity test is necessary to carry out to help us make a accurate judgment.
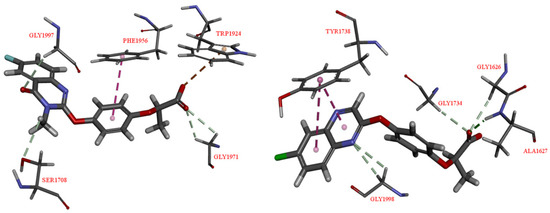
Figure 8.
The docking binding mode of the free acid of QPP-7 (Left) and quizalofop-P-ethyl (Right) to acetyl-CoA carboxylase (ACCase) (PDB code: 1UYR).
To further verify whether compound QPP-7 is a ACCase inhibitor, the tests of E. crusgalli ACCase inhibition activity in vitro was performed. As shown in Table 3, compound QPP-7 displayed good inhibitory activity against E. crusgalli ACCase with an IC50 value of 54.65 nM, which is comparable to commercial herbicide quizalofop-P-ethyl (IC50= 41.19 nM). This result indicate that QPP-7 may be an ACCase inhibitor and has an herbicidal mechanism similar to that of QZ.

Table 3.
In vitro inhibitory activity of compound QPP-7 against E. crusgalli ACCase a.
It was noteworthy that although QPP-7 has a herbicidal mechanism similar to that of QZ, however, the study of molecular docking showed that their interactions with the ACCase is different, indicating that QPP-7 has the potential to control weeds that are resistant to APP herbicides. Furthermore, in our present work, compound QPP-7 has the comparable ACCase inhibitory activity to that of QZ, but its herbicidal activity at a lower dosage is worse than that of QZ. The reason may be attributed to the compound QPP-7 with natural structure fragment being easily metabolized in plants when compared to QZ.
4. Conclusions
In summary, a series of quinazolin-4(3H)-one derivatives based on the aryloxyphenoxypropionate motif have been designed by using molecular hybridization strategy. twenty-nine novel quinazolin-4(3H)-one derivatives were prepared in moderate to good yields. The bioassay results showed that compound QPP-7 displayed good pre-emergent herbicidal activity at a dosage of 375 g ha−1. The herbicidal spectrum and crop selectivity study revealed that compound QPP-7 had a broad spectrum of monocotyledonous weed control and displayed excellent crop safety to O. sativa, T. aestivum, and A. hypogaea, which indicated its great potential as a herbicide lead compound. The study of structure-activity relationship showed that the spatial position of R group and the bulk of R1 group on quinazolin-4-one have strongly influenced on the herbicidal activity of target compounds, and the (R = 6-F, R1 = Me) pattern was confirmed as the optimal orientation. Furthermore, the inhibitory activity against E. crusgalli ACCase enzyme and the molecular docking simulation of the free acid of compound QPP-7 were performed, and the results indicated that compound QPP-7 may be a ACCase inhibitor. For developing improved herbicidal activity of APP herbicide containing quinazolin-4(3H)-one skeleton, further studies on the structural optimization of compound QPP-7 are ongoing in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12081840/s1, Details on the inhibition of the root growth of B. campestris, inhibition of the seedling growth of E. crusgalli, greenhouse tests, and crop selectivity, 1H NMR, 13C NMR and HRMS spectrum of target compounds.
Author Contributions
Conceptualization, K.L.; methodology, K.L.; validation, C.W., K.C. and N.L.; formal analysis, S.F. and P.L.; investigation, C.W., K.C. and N.L.; data curation, C.W. and K.C.; writing—original draft preparation, K.L.; writing—review and editing, K.L. and L.J.; project administration, K.L.; funding acquisition, K.L., X.W., G.L. and L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financially supported by the China Postdoctoral Science Foundation (No. 2020M671984); the National Natural Science Foundation of China (No. 31701827); the National Innovation and Entrepreneurship Training Program for College Students (No. 202110447022); Guangyue Young Scholar Innovation Team of Liaocheng University (No. LCUGYTD2022-04); the National Key Research and Development Program of China (No. SQ2020YFF0422322); the Natural Science Foundation of Shandong Province (No. ZR202102180037) and the Research Fund of Liaocheng University (No. 318012106).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef] [PubMed]
- Zagnitko, O.; Jelenska, J.; Tevzadze, G.; Haselkorn, R.; Gornicki, P. An isoleuciney leucine residue in the carboxyltransferase domain of acetyl-CoA carboxylase is critical for interaction with aryloxyphenoxypropionate and cyclohexanedione inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 6617–6622. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.L.; Yang, Z.R.; Shen, Y.; Tong, L. Crystal Structure of the Carboxyltransferase Domain of Acetyl-Coenzyme A Carboxylase. Science 2003, 299, 2064–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Délye, C. Weed resistance to acetyl coenzyme A carboxylase inhibitors: An update. Weed Sci. 2005, 53, 728–746. [Google Scholar] [CrossRef]
- Leach, G.E.; Devine, M.D.; Kirkwood, R.C.; Marshall, G. Target enzyme-based resistance to acetyl-coenzyme A carboxylase inhibitors in Eleusine indica. Pestic. Biochem. Physiol. 1995, 51, 129–136. [Google Scholar] [CrossRef]
- Stoltenberg, D.E.; Wiederholt, R.J. Giant foxtail (Setaria faberi) resistance to aryloxyphenoxypropionate and cyclohexanedione herbicides. Weed Res. 1995, 43, 527–535. [Google Scholar] [CrossRef]
- Heap, I.M.; Morrison, I.N. Resistance to aryloxyphenoxypropionate and cyclohexanedione herbicides in green foxtail (Setaria viridis). Weed Sci. 1996, 44, 25–30. [Google Scholar] [CrossRef]
- Preston, C.; Tardif, F.J.; Christoffer, J.T.; Powles, S.B. Multiple resistance to dissimilar herbicide chemistries in a biotype of Lolium rigidum due to enhanced activity of several herbicide degrading enzymes. Pestic. Biochem. Physiol. 1996, 54, 123–134. [Google Scholar] [CrossRef]
- Liu, W.; Harrison, D.K.; Chalupska, D.; Gornicki, P.; O’Donnell, C.C.; Adkins, S.W.; Haselkorn, R.; Williams, R.R. Single-site mutations in the carboxyltransferase domain of plastid acetyl-CoA carboxylase confer resistance to grass-specific herbicides. Proc. Natl. Acad. Sci. USA 2007, 104, 3627–3632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Q.; Powles, S.B. Six amino acid substitutions in the carboxyltransferase domain of the plastidic acetyl-CoA carboxylase gene are linked with resistance to herbicides in a Lolium rigidum population. New Phytol. 2006, 172, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Sparks, T.C.; Duke, S.O. Structure simplification of natural products as a lead generation approach in agrochemical discovery. J. Agric. Food Chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef]
- Ahrens, H.; Lange, G.; Müller, T.; Rosinger, C.; Willms, L.; van Almsick, V. 4-Hydroxyphenylpyruvate dioxygenase inhibitors in combination with safeners: Solutions for modern and sustainable agriculture. Angew. Chem. Int. Ed. 2013, 52, 9388–9398. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products-status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- He, D.; Wang, M.L.; Zhao, S.Y.; Shu, Y.S.; Zeng, H.L.; Xiao, C.; Lu, C.; Liu, Y.Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Fu, H.; Zhong, H.; Hong, K.; Zhu, W. Antifungal quinazolinones from marine-derived Bacillus cereus and their preparation. Bioorg. Med. Chem. Lett. 2011, 21, 4005–4007. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Z.L.; Li, D.H.; Sun, Y.T.; Shan, D.T.; Bai, J.; Pei, Y.H.; Jing, Y.K.; Hua, H.M. Quinolone and indole alkaloids from the fruits of Euodia rutaecarpa and their cytotoxicity against two human cancer cell lines. Phytochemistry 2015, 109, 133–139. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.; Che, Y. N-Hydroxypyridones, phenylhydrazones, and a quinazolinone from Isaria farinosa. J. Nat. Prod. 2011, 74, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Lin, T.; Wang, W.; Xin, Z.; Zhu, T.; Gu, Q.; Li, D. Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. J. Nat. Prod. 2013, 76, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.M.; Li, X.M.; Li, C.S.; Sun, H.F.; Gao, S.S.; Wang, B.G. Benzodiazepine alkaloids from marine-derived endophytic fungus Aspergillus ochraceus. Helv. Chim. Acta 2009, 92, 1366–1370. [Google Scholar] [CrossRef]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Li, G.; Zhu, W. New quinazolinone alkaloids within rare amino acid residue from coralassociated fungus, Aspergillus versicolor LCJ-5-4. Org. Lett. 2011, 13, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Cagir, A.; Jones, S.H.; Gao, R.; Eisenhauer, B.M.; Hecht, S.M. Luotonin A. A naturally occurring human DNA topoisomerase I poison. J. Am. Chem. Soc. 2003, 125, 13628–13629. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Tárraga, A.; Gonzalez-Tejero, A.; Rioja, I.; Ubeda, A.; Terencio, M.C.; Alcaraz, M.J. Inhibition of leukocyte functions by the alkaloid isaindigotone from Isatis indigotica and some new synthetic derivatives. J. Nat. Prod. 2001, 64, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Chatterjee, T.K. Pharmacophore modeling and 3D quantitative structureactivity relationship analysis of febrifugine analogues as potent antimalarial agent. J. Adv. Pharm. Technol. Res. 2013, 4, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Kshirsagar, U.A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4(3H)-Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef]
- Amrutkar, R.D.; Amrutkar, S.V.; Ranawat, M.S. Quinazolin-4-one: A varsatile molecule. Curr. Bioact. Compd. 2020, 16, 370–382. [Google Scholar] [CrossRef]
- Xing, Z.M.; Wu, W.H.; Miao, Y.X.; Tang, Y.Q.; Zhou, Y.K.; Zheng, L.F.; Fu, Y.; Song, Z.B.; Peng, Y.Y. Recent advances in quinazolinones as an emerging molecular platform for luminescent materials and bioimaging. Org. Chem. Front. 2021, 8, 1867–1889. [Google Scholar] [CrossRef]
- Fan, Z.J.; Shi, J.; Luo, N.; Ding, M.H.; Bao, X.P. Synthesis, crystal structure, and agricultural antimicrobial evaluation of novel quinazoline thioether derivatives incorporating the 1,2,4-triazolo[4,3-a]pyridine moiety. J. Agric. Food Chem. 2019, 67, 11598–11606. [Google Scholar] [CrossRef]
- Peng, J.W.; Yin, X.D.; Li, H.; Ma, K.Y.; Zhang, Z.J.; Zhou, R.; Wang, Y.L.; Hu, G.F.; Liu, Y.Q. Design, synthesis, and structure-activity relationship of quinazolinone derivatives as potential fungicides. J. Agric. Food Chem. 2021, 69, 4604–4614. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, P.; Li, Z.N.; Yin, J.; He, M.; Xue, W.; Chen, Z.W.; Song, B.A. Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)anilino]-4(3H)-quinazolinone moiety. J. Agric. Food Chem. 2013, 61, 9575–9582. [Google Scholar] [CrossRef]
- Lei, K.; Li, P.; Yang, X.F.; Wang, S.B.; Wang, X.K.; Hua, X.W.; Sun, B.; Ji, L.S.; Xu, X.H. Design and synthesis of novel 4-hydroxyl-3-(2-phenoxyacetyl)-pyran-2-one derivatives for use as herbicides and evaluation of their mode of action. J. Agric. Food Chem. 2019, 67, 10489–10497. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Liu, Y.; Wang, S.B.; Sun, B.; Hua, X.W.; Xu, X.H. Synthesis and herbicidal activity of 3-Acetyl-4-hydroxy-2,1-benzothiazine derivatives. Chem. Res. Chin. Univ. 2019, 35, 609–615. [Google Scholar] [CrossRef]
- Lei, K.; Li, P.; Zhou, X.Y.; Wang, S.B.; Wang, X.K.; Ji, L.S.; Liu, R.M.; Xu, X.H. Design, synthesis and herbicidal activity of 5-acylbarbituric acid derivatives and study of molecular mode of action. Chin. J. Org. Chem. 2020, 40, 2788–2797. [Google Scholar] [CrossRef]
- Lei, K.; Hua, X.W.; Tao, Y.-Y.; Liu, Y.; Liu, N.; Ma, Y.; Li, Y.H.; Xu, X.H.; Kong, C.H. Discovery of (2-benzoylethen-1-ol)-containing 1,2-benzothiazine derivatives as novel 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibiting-based herbicide lead compounds. Bioorg. Med. Chem. 2016, 24, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zekarias, B.L.; Li, X.Y.; Jaffett, V.A.; Guzei, I.A.; Golden, J.E. Divergent 2-chloroquinazolin-4(3H)-one rearrangement: Twistedcyclic guanidine formation or ring-fused N-acylguanidines via a domino process. Chem. Eur. J. 2020, 26, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tweel, B.; Tong, L. Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop. Proc. Natl. Acad. Sci. USA 2004, 101, 5910–5915. [Google Scholar] [CrossRef] [Green Version]
- Cocker, K.M.; Moss, S.R.; Coleman, J.O.D. Multiple mechanisms of resistance to fenoxaprop-P-ethyl in United Kingdom and other European populations of herbicideresistant Alopecurus myosuroides (black-grass). Pestic. Biochem. Physiol. 1999, 65, 169–180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).