Dynamic Profiles of Fermentation Quality and Microbial Community of Kudzu (Pueraria lobata) Ensiled with Sucrose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Fermentation Quality Analysis
2.3. Relative Feed Value Evaluation
2.4. Bacterial Community Analysis
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition of the Kudzu Silages
3.2. Fermentation Dynamics Profile of Kudzu Silage
3.3. Assessment of Relative Feeding Value
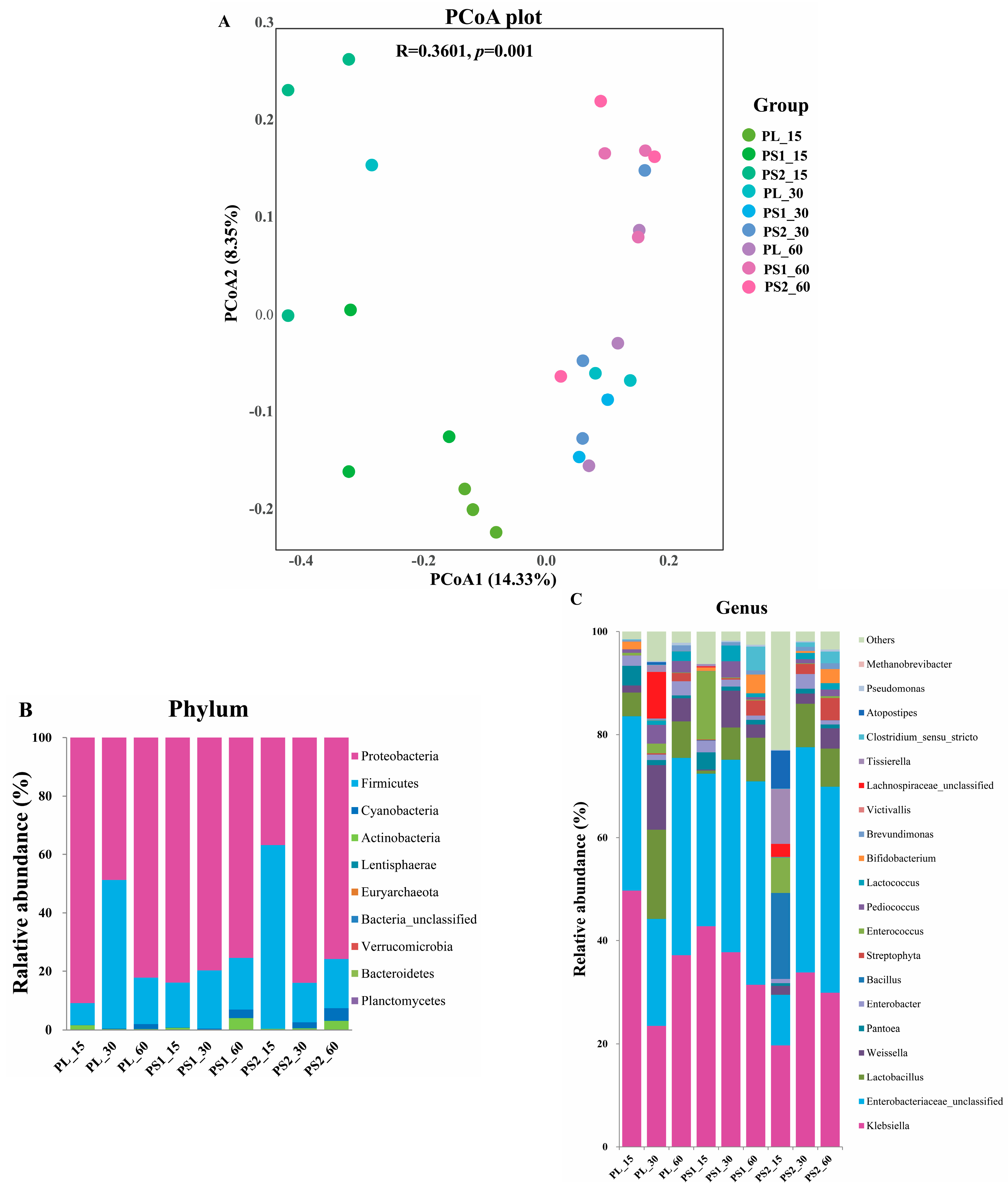
3.4. Microbial Community Diversity in Kudzu Silage
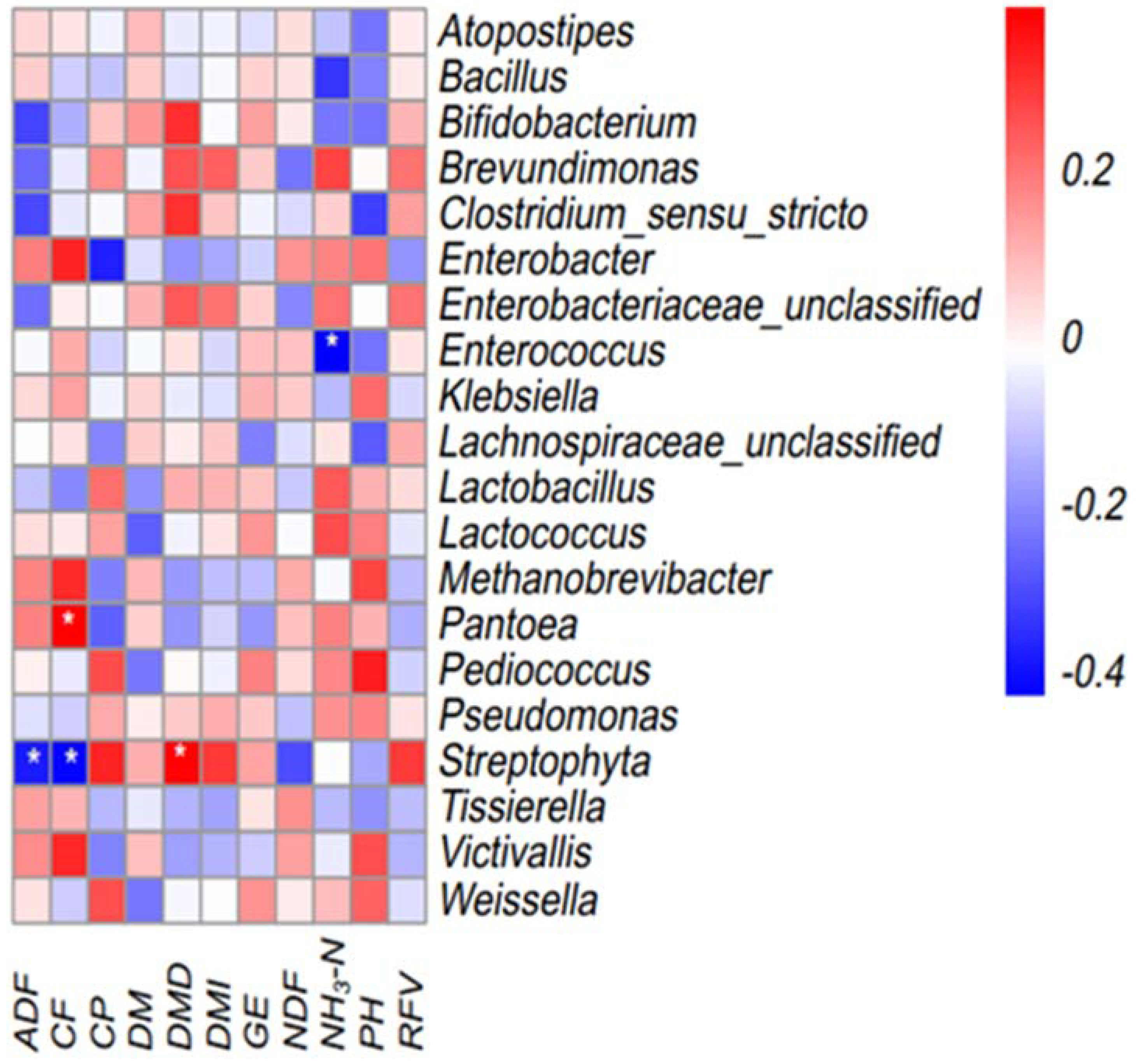
3.5. Relationship between Genera and Silage Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Zhang, J.; Jian, N.Y. Determination of puerarin, daidzein and rutin in Pueraria lobata (Wild.) Ohwi by capillary electrophoresis with electrochemical detection. J. Chromatogr. A 2001, 923, 255–262. [Google Scholar] [CrossRef]
- Jiang, R.W.; Lau, K.M.; Lam, H.M.; Yam, W.S.; Leung, L.K.; Choi, K.L.; Waye, M.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. A comparative study on aqueous root extracts of Pueraria thomsonii and Pueraria lobata by antioxidant assay and HPLC fingerprint analysis. J. Ethnopharmacol. 2005, 96, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wang, D.Y.; Xing, D.M.; Ding, Y.; Wang, R.F.; Lei, F.; Du, L.J. The antidepressant effect of ethanol extract of radix puerariae in mice exposed to cerebral ischaemia reperfusion. Pharmacol. Biochem. Behav. 2004, 78, 319–325. [Google Scholar] [CrossRef]
- Tufarelli, V.; Ragni, M.; Laudadio, V. Feeding forage in poultry: A promising alternative for the future of production systems. Agriculture 2018, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Ustundag, A.O.; Ozdogan, M. Using moringa oleigera in poultry nutrition. 27th International Scientific-Expert Congress of Agriculture and Food Industry, Bursa, Turkey. Agric. Fac. Uludag. Univ. 2016, 30, 195–201. [Google Scholar]
- Ncube, S.; Halimani, T.E.; Mwale, M.; Saidi, P.T. Effect of Acacia angustissma leaf meal on the physiology of broiler intestines. J. Agric. Sci. 2017, 9, 5–62. [Google Scholar]
- Muck, R.E.; Nadeau, E.M.G.; McAlloster, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Wang, B.; Gao, R.; Wu, Z.; Yu, Z. Functional analysis of sugars in modulating bacterial communities and metabolomics profiles of Medicago sativa silage. Front. Microbiol. 2020, 11, 641. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Wang, X.G.; Tian, J.P. Effects of inoculants and environmental temperature on fermentation quality and bacterial diversity of alfalfa silage. Anim. Sci. J. 2018, 89, 1085–1092. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Martens, S.D.; Avila, P.; Hoedtke, S. The effect of inoculant and sucrose addition on the silage quality of tropical forage legumes with varying ensilability. Anim. Feed Sci. Technol. 2012, 174, 201–210. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Jia, T.T.; Gao, R.; Xu, S.Y.; Wu, Z.; Wang, B.; Yu, Z. Effects of chopping length and additive on the fermentation quality and aerobic stability in silage of Leymus chinensis. Processes 2020, 8, 1283. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Ali, N.; Wang, S.R.; Zhao, J.; Dong, Z.H.; Li, J.F.; Nazar, M.; Shao, T. Microbial diversity and fermentation profile of red clover silage inoculated with reconstituted indigenous and exogenous epiphytic microbiota. Bioresour. Technol. 2020, 314, 123606. [Google Scholar] [CrossRef]
- William, H.; George, W. (Eds.) Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Rohweder, D.A.; Barnes, R.F.; Jorgensen, N. Proposed hay grading standards based on laboratory analyses for evaluating quality. J. Anim. Sci. 1978, 47, 747–759. [Google Scholar] [CrossRef]
- Wang, C.; He, L.W.; Xing, Y.Q.; Zhou, W.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 2019, 284, 240–247. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for 516 metagenomics. Peer J. 2016, 4, e2584. [Google Scholar] [CrossRef]
- Mceniry, J.; O’Kiely, P.; Clipson, N.J.W.; Forristal, P.D.; Doyle, E.M. The relative impacts of wilting, chopping, compaction and air infiltration on the conservation characteristics of ensiled grass. Grass Forage Sci. 2007, 62, 470–484. [Google Scholar] [CrossRef]
- Li, P.; Ji, S.R.; Hou, C.; Tang, H.Y.; Wang, Q.; Shen, Y.X. Effects of chemical additives on the fermentation quality and N distribution of alfalfa silage in south of China. Anim. Sci. J. 2016, 87, 1472–1479. [Google Scholar] [CrossRef]
- Li, R.R.; Jiang, D.; Zheng, M.L.; Tian, P.J.; Zheng, M.H.; Xu, C.C. Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci. Rep. 2020, 10, 17782. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tang, S.; Zhong, R.; Tan, Z.; Wu, D. Alfalfa Silage Treated with Sucrose Has an Improved Feed Quality and More Beneficial Bacterial Communities. Front. Microbiol. 2021, 12, 670165. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, O.C.M.; Ogunade, I.M.; Weinberg, Z.; Adesogan, A.T. Silage review: Foodborne pathogens in silage and their mitigation by silage additives. J. Dairy Sci. 2018, 101, 4132–4142. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, J.A.; Franco, R.B.; Palumbo, J.D.; Hnasko, R.; Stanker, L.; Mitloehner, F.M. Bacterial population dynamics during the ensiling of Medicago sativa (alfalfa) and subsequent exposure to air. J. Appl. Microbiol. 2013, 114, 1661–1670. [Google Scholar] [CrossRef]
- Hou, Z.; Zheng, X.; Zhang, X.; Chen, Q.; Wu, D. Effects of urea supplementation on the nutritional quality and microbial community of alfalfa (Medicago sativa L.) silage. Arch. Microbiol. 2022, 204, 414. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.Y.; Wang, C.; He, L.W.; Zhou, W.; Yang, F.Y.; Zhang, Q. The bacterial community and fermentation quality of mulberry (Morus alba) leaf silage with or without Lactobacillus casei and sucrose. Bioresour. Technol. 2019, 293, 122059. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Lin, H.; Lin, S.; Awasthi, M.K.; Wang, Y.; Xu, P. Exploring the bacterial community and fermentation characteristics during silage fermentation of abandoned fresh tea leaves. Chemosphere 2021, 283, 131234. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, H.; Yu, Z. Effects of sucrose, formic acid and lactic acid bacteria inoculant on quality, in vitro rumen digestibility and fermentability of drooping wild ryegrass (Elymus nutans Griseb.) silage. J. Anim. Feed Sci. 2017, 26, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Ni, K.; Zhao, J.; Zhu, B.; Su, R.; Pan, Y.; Ma, J.; Zhou, G.; Tao, Y.; Liu, X.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef]
- Hu, Z.; Chang, J.; Yu, J.; Li, S.; Niu, H. Diversity of bacterial community during ensiling and subsequent exposure to air in whole-plant maize silage. Asian-Australas. J. Anim. Sci. 2018, 31, 1464–1473. [Google Scholar] [CrossRef]
- Wang, C.; Pian, R.; Chen, X.; Lv, H.; Zhou, W.; Zhang, Q. Beneficial effects of tannic acid on the quality of bacterial communities present in high-moisture mulberry leaf and stylo silage. Front. Microbiol. 2020, 11, 586412. [Google Scholar] [CrossRef]
- Santos, A.O.; Ávila, C.L.; Pinto, J.C.; Carvalho, B.F.; Dias, D.R.; Schwan, R.F. Fermentative profile and bacterial diversity of corn silages inoculated with new tropical lactic acid bacteria. J. Appl. Microbiol. 2016, 120, 266–279. [Google Scholar] [CrossRef]
- Da Silva, E.B.; Smith, M.L.; Savage, R.M.; Polukis, S.A.; Drouin, P.; Kung, L., Jr. Effects of Lactobacillus hilgardii 4785 and Lactobacillus buchneri 40788 on the bacterial community, fermentation and aerobic stability of high-moisture corn silage. J. Appl. Microbiol. 2021, 130, 1481–1493. [Google Scholar] [CrossRef]
| Items | Treatment | Days Ensiled | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | T | D | I | |||
| DM | PL | 238.03 ab | 236.13 b | 246.10 a | 5.35 | ns | * | * |
| PS1 | 243.00 b | 234.23 b | 275.73 a | |||||
| PS2 | 252.33 | 242.93 | 241.80 | |||||
| CP | PL | 145.83 b | 142.75 b | 151.65 a | 3.86 | ns | *** | ns |
| PS1 | 144.50 b | 138.13 b | 157.58 a | |||||
| PS2 | 146.17 ab | 138.22 b | 160.73 a | |||||
| NDF | PL | 523.70 | 501.43 | 487.93 | 10.81 | ns | ns | ns |
| PS1 | 489.77 | 500.50 | 476.23 | |||||
| PS2 | 475.30 | 473.97 | 477.30 | |||||
| ADF | PL | 338.90 b | 361.33 αa | 335.33 b | 5.24 | * | ** | ns |
| PS1 | 350.30 b | 369.90 αa | 328.87 c | |||||
| PS2 | 333.97 | 334.43 β | 324.83 | |||||
| CF | PL | 284.47 b | 295.73 a | 250.10 c | 10.97 | ns | ** | ns |
| PS1 | 294.47 b | 314.10 a | 247.27 c | |||||
| PS2 | 263.40 b | 292.20 a | 256.93 b | |||||
| GE | PL | 17.07 | 16.45 | 17.31 | 0.18 | ns | ns | ns |
| PS1 | 16.88 | 16.74 | 17.06 | |||||
| PS2 | 16.61 | 16.40 | 16.78 | |||||
| Items | Treatment | Days Ensiled | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | T | D | I | |||
| pH | PL | 5.15 | 5.34 α | 5.24 α | 0.12 | *** | ns | ns |
| PS1 | 4.90 | 5.09 αβ | 4.59 β | |||||
| PS2 | 4.68 | 4.78 β | 4.54 β | |||||
| NH3-N (g/kg TN) | PL | 11.58 b | 14.13 a | 11.85 b | 0.45 | ns | *** | ns |
| PS1 | 12.33 b | 14.74 a | 12.69 b | |||||
| PS2 | 12.09 b | 15.42 a | 10.81 b | |||||
| Items | Treatment | Days Ensiled | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | T | D | I | |||
| DMI% | PL | 2.30 | 2.39 | 2.47 | 0.05 | ns | ns | ns |
| PS1 | 2.45 | 2.40 | 2.52 | |||||
| PS2 | 2.53 | 2.53 | 2.52 | |||||
| DDM% | PL | 62.50 | 60.75 | 62.78 | 0.41 | ns | ** | ns |
| PS1 | 61.61 ab | 60.08 b | 63.60 a | |||||
| PS2 | 62.89 | 62.85 | 63.28 | |||||
| RFV | PL | 111.55 | 112.74 | 120.23 | 2.98 | ns | ns | ns |
| PS1 | 117.03 | 111.73 | 123.78 | |||||
| PS2 | 123.26 | 123.46 | 124.23 | |||||
| Items | Treatment | Days Ensiled | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | T | D | I | |||
| OTUs | PL | 692.67 | 498.33 | 633.67 | 71.87 | ns | ns | ns |
| PS1 | 643.00 | 521.33 | 467.67 | |||||
| PS2 | 575.67 | 646.00 | 494.67 | |||||
| Observed species | PL | 468.33 | 363.67 | 471.00 | 38.86 | ns | ns | ns |
| PS1 | 462.67 | 432.67 | 471.00 | |||||
| PS2 | 390.00 | 545.33 | 427.67 | |||||
| Chao1 | PL | 841.92 | 648.36 | 797.85 | 53.44 | ns | ns | ns |
| PS1 | 852.67 | 810.56 | 729.29 | |||||
| PS2 | 651.68 | 884.47 | 744.11 | |||||
| Shannon | PL | 3.47 | 3.79 | 3.86 | 0.28 | ns | ns | ns |
| PS1 | 3.70 | 3.79 | 3.94 | |||||
| PS2 | 4.35 | 4.28 | 4.11 | |||||
| Simpson | PL | 0.72 | 0.83 | 0.80 | 0.02 | ns | ns | ns |
| PS1 | 0.76 | 0.80 | 0.82 | |||||
| PS2 | 0.83 | 0.83 | 0.84 | |||||
| Coverage | PL | 0.97 | 0.98 | 0.98 | 0.00 | ns | ns | ns |
| PS1 | 0.97 | 0.98 | 0.98 | |||||
| PS2 | 0.98 | 0.97 | 0.98 | |||||
| Items | Treatment | Days Ensiled | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | T | D | I | |||
| Proteobacteria | PL | 90.89 αa | 48.70 βb | 82.21 ab | 5.24 | ns | ns | *** |
| PS1 | 83.88 α | 79.75 αβ | 75.36 | |||||
| PS2 | 36.72 βb | 75.71 αab | 84.00 a | |||||
| Firmicutes | PL | 7.54 c | 50.75 αa | 15.89 b | 4.79 | ns | ns | *** |
| PS1 | 15.24 | 19.78 β | 17.74 | |||||
| PS2 | 62.86 a | 16.93 βb | 13.45 b | |||||
| Cyanobacteria | PL | 0.04 b | 0.24 b | 1.59 a | 0.70 | ns | *** | ns |
| PS1 | 0.13 b | 0.27 b | 2.92 a | |||||
| PS2 | 0.02 c | 1.94 b | 4.30 a | |||||
| Actinobacteria | PL | 1.51 a | 0.22 b | 0.17 βb | 0.58 | ns | * | ** |
| PS1 | 0.70 b | 0.11 b | 2.90 αβa | |||||
| PS2 | 0.33 b | 0.53 b | 3.55 αa | |||||
| Items | Treatment | Days Ensiled | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 60 | T | D | I | |||
| Klebsiella | PL | 49.73 α | 23.44 | 37.17 | 4.15 | ns | ns | ** |
| PS1 | 42.79 α | 37.74 | 31.44 | |||||
| PS2 | 19.70 β | 33.84 | 29.91 | |||||
| Enterobacteriaceae | PL | 38.34 a | 33.84 a | 20.80 αb | 2.59 | ns | *** | *** |
| PS1 | 39.49 a | 37.39 a | 29.62 αb | |||||
| PS2 | 43.73 a | 39.96 a | 9.78 βb | |||||
| Lactobacillus | PL | 4.58 b | 7.31 a | 7.07 ab | 3.20 | ns | * | ns |
| PS1 | 0.59 b | 6.25 a | 8.46 a | |||||
| PS2 | 0.04 b | 8.43 a | 7.46 a | |||||
| Weissella | PL | 1.41 | 7.57 | 4.46 | 2.55 | ns | ns | ns |
| PS1 | 0.21 | 7.15 | 2.59 | |||||
| PS2 | 1.71 | 1.94 | 3.92 | |||||
| Pantoea | PL | 3.81 αa | 0.98 b | 0.56 b | 0.64 | * | * | ** |
| PS1 | 3.35 αa | 0.84 b | 0.88 b | |||||
| PS2 | 0.52 β | 1.00 | 0.75 | |||||
| Enterobacter | PL | 1.99 | 1.05 | 2.77 | 0.53 | ns | ns | ns |
| PS1 | 2.28 | 1.35 | 0.84 | |||||
| PS2 | 0.83 | 2.86 | 0.80 | |||||
| Streptophyta | PL | 0.04 b | 0.24 ab | 1.59 a | 0.70 | ns | ** | ns |
| PS1 | 0.13 b | 0.27 b | 2.92 a | |||||
| PS2 | 0.02 b | 1.94 ab | 4.30 a | |||||
| Enterococcus | PL | 0.48 | 1.86 | 0.10 | 2.99 | ns | * | ns |
| PS1 | 13.30 a | 0.11 b | 0.17 b | |||||
| PS2 | 6.84 a | 0.11 b | 0.36 b | |||||
| Pediococcus | PL | 0.69 b | 3.64 a | 2.23 a | 0.89 | ns | * | ns |
| PS1 | 0.03 b | 3.17 a | 0.46 b | |||||
| PS2 | 0.03 | 0.81 | 1.27 | |||||
| Lactococcus | PL | 0.02 b | 0.89 βab | 1.90 a | 0.26 | ns | *** | ** |
| PS1 | 0.02 b | 3.05 αa | 0.76 ab | |||||
| PS2 | 0.04 b | 1.22 αβab | 1.32 a | |||||
| Brevundimonas | PL | 0.21 | 0.29 | 1.12 | 0.28 | ns | * | ns |
| PS1 | 0.02 | 0.63 | 0.79 | |||||
| PS2 | 0.05 b | 0.81 ab | 1.13 a | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Zheng, X.; Zhang, X.; Yan, L.; Chen, Q.; Wu, D. Dynamic Profiles of Fermentation Quality and Microbial Community of Kudzu (Pueraria lobata) Ensiled with Sucrose. Agronomy 2022, 12, 1853. https://doi.org/10.3390/agronomy12081853
Hou Z, Zheng X, Zhang X, Yan L, Chen Q, Wu D. Dynamic Profiles of Fermentation Quality and Microbial Community of Kudzu (Pueraria lobata) Ensiled with Sucrose. Agronomy. 2022; 12(8):1853. https://doi.org/10.3390/agronomy12081853
Chicago/Turabian StyleHou, Zhenping, Xia Zheng, Xuelei Zhang, Li Yan, Qing Chen, and Duanqin Wu. 2022. "Dynamic Profiles of Fermentation Quality and Microbial Community of Kudzu (Pueraria lobata) Ensiled with Sucrose" Agronomy 12, no. 8: 1853. https://doi.org/10.3390/agronomy12081853
APA StyleHou, Z., Zheng, X., Zhang, X., Yan, L., Chen, Q., & Wu, D. (2022). Dynamic Profiles of Fermentation Quality and Microbial Community of Kudzu (Pueraria lobata) Ensiled with Sucrose. Agronomy, 12(8), 1853. https://doi.org/10.3390/agronomy12081853






