Abstract
Studies were undertaken to determine the impact of environmental variables temperature (12.5/9.5, 20/17, 27/24 °C day/night) and soil moisture (100, 50% WHC), and their interaction with Phoma medicaginis infection, on production of the phytoestrogen coumestrol in annual Medicago rugosa cv. Paraponto and M. scutellata cv. Sava. Disease factors measured included leaf disease incidence/severity, petiole/stem disease incidence/severity, and leaf yellowing severity. Coumestrol levels were determined using gas chromatography–mass spectrometry (GC–MS). Increasing temperature from 12.5/9.5 °C to 27/24 °C in inoculated plants significantly (p < 0.05) increased coumestrol from 193 mg kg−1 to 390 mg kg−1, but there were no differences in coumestrol production across all three temperatures in uninoculated plants. Reducing soil moisture from 100% to 50% WHC at the highest temperature (27/24 °C) caused the greatest increase in coumestrol production from 156 to 269 mg kg−1 in inoculated plants. The greatest coumestrol production (600 mg kg−1) was under 27/24 °C/50% WHC for Sava infected with P. medicaginis and least coumestrol (1.6 mg kg−1) was Sava under 20/17 °C/50% WHC in the absence of P. medicaginis. Clearly, situations of higher temperatures in conjunction with lower soil moisture levels cause greatest elevation in coumestrol in the presence of P. medicaginis, levels far exceeding the animal risk threshold of 25 mg kg−1.
1. Introduction
Annual Medicago spp. play a critical role in many dryland farming areas [1,2], and produce high-quality forage legume for grazing animals [3]. One of the most serious threats to annual Medicago spp. productivity is Phoma medicaginis which is among the most serious necrotrophic plant pathogens, causing Phoma black stem and leaf spot disease on perennial and annual Medicago spp. [4]. Phoma black stem and leaf spot disease first appears as blackish-brown lesions on leaves and stems followed by leaf yellowing and leaf and stem collapse [5,6,7]. It can also cause serious root disease [8,9] and result in substantial seed contamination [5]. However, the impact of this disease goes way beyond its influence on herbage and seed yield, even when resulting in complete defoliation and premature death of forage plants [2,6,10], as it stimulates the host plants to produce phytoestrogens [2,6,11]. Phytoestrogens affect the ability of plants to resist biotic attack [12], for example, they can provide plants with some protection against fungal and bacterial pathogens [13] and pests [14]. However, phytoestrogens reduce the feed value of the annual Medicago spp. and can lead to dietary problems [15], particularly in relation to their devastating effects on animal fertility [16] where levels greatly exceed animal requirements, as occurs with both sheep [17,18,19,20] and cattle [6].
Of the phytoestrogens, the coumestan coumestrol is a crucial phytoestrogen [21] that can accumulate at high concentrations in Medicago spp. tissues following fungal infection [12,22] or aphid infestation [22]. In addition, environmental conditions affect the phytoestrogen concentration through diverse and complex enzymatic pathways, particularly leading to their accumulation during times of environmental stress [23]. A range of other factors have also been shown to stimulate increased phytoestrogen in annual Medicago spp. forages [24], including soil type [25], superphosphate level [26], waterlogging, frost and radiation [16], drought [27], and differences in plant growth stage [24]. In other forage legumes, genetic factors such as plant genotype [28] and sward mowing frequency [29] have also been shown to influence phytoestrogen production.
In soybean, there have been quite a few studies in relation to the effects of temperature on consequent levels of coumestrol [30,31,32,33,34,35]. For instance, Tsukamoto [30] and Chennupati [31] indicated that temperature was a significant factor determining soybean isoflavone content among biotic and abiotic factors. However, even for perennial Medicago spp., studies investigating the role of temperature or moisture on phytoestrogen production are relatively few. Examples include Tucak et al. [36], who showed higher phytoestrogen accumulation occurred in alfalfa populations from more crop-favourable air temperatures and with higher precipitation, and Fields et al. [37], who concluded that rainfall was an important driver of coumestrol production in alfalfa, where years with a large amount of rainfall resulting in elevated coumestrol levels. In contrast, Adams [38] noted that concentration of coumestans was not influenced by extreme temperatures in alfalfa.
Despite it being known that higher levels of coumestrol occur in some annual Medicago cultivars during a prolonged dry season [12], in contrast to perennial Medicago spp., studies relating to effects of temperature and/or moisture on coumestrol levels in annual Medicago spp. are lacking. This is even though commercial annual Medicago spp. forage stands are exposed to a range of different temperature and soil moisture conditions during the growing season. Hence, studies were undertaken to determine the impact of the environmental variables temperature (12.5/9.5, 20/17, 27/24 °C day/night) and soil moisture (100, 50% WHC), and their interaction with Phoma medicaginis infection, on production of the phytoestrogen coumestrol in annual Medicago rugosa cv. Paraponto and M. scutellata cv. Sava.
2. Materials and Methods
2.1. Annual Medicago Species and P. medicaginis Isolate
Two different annual Medicago species, M. rugosa cv. Paraponto and M. scutellata cv. Sava, were used, both of which have already been found to produce substantial quantities of phytoestrogens when infected by P. medicaginis [39], which were acquired from the SARDI Genetic Resource Centre, South Australia. Isolate WAC3658 of P. medicaginis, that was found to be highly pathogenic on annual Medicago sp. in an earlier study by Omidvari et al. [7], was again used for the current study.
2.2. Inoculum Preparation and Inoculation Method
Production of inoculum was performed according to Omidvari et al. [39]. Briefly, isolate WAC3658 was subcultured onto Sanderson and Srb medium [40] for 28 days at 25 ± 2 °C under 16/8 h light/dark. After 4 weeks, the conidia were collected by adding 5 mL of sterile water to each Petri plate and the concentration adjusted to 5 × 106 spores mL−1 using a haemocytometer counting chamber and immediately utilised [41]. Seeds of the two annual Medicago species were surface-sterilised with 70% ethanol and germinated according to Mhadhbi et al. [42] and then sown into pots. Pots were maintained at 22 °C (day) and 18 °C (night) in a controlled-environment room with a 12 h photoperiod. Plants were fertilised weekly with Thrive™ (Yates, Australia) at the manufacturer’s recommended rate. At 6 weeks of age, plants were inoculated according to Omidvari et al. [43]. Briefly, plants were spray-inoculated with the conidial suspension, including 0.01% Tween-20® (Sigma-Aldrich Pty Ltd., Darmstadt, Germany), until run off using a hand-held and operated aerosol sprayer. Control plants were also treated with 0.01% Tween-20®, but in sterile distilled water. Plants were then transferred into the clear plastic boxes with lids, measuring 770 mm (L), 570 mm (W), 475 mm (H), used as inoculation/incubation chambers. An internal mist of sterile distilled water was hand-sprayed into the incubator boxes, and the lids were kept closed for 72 h post-inoculation (hpi) in order to maintain high humidity [44].
2.3. Disease Assessment
Disease assessment was conducted according to Omidvari et al. [43]. Two weeks post-inoculation, the presence and development of disease symptoms were evaluated using five different disease assessment parameters as follows: (a) leaf disease incidence (%LDI), by assessing the percentage of leaves per pot showing disease symptoms; (b) leaf disease severity (%LDS), by calculating the percent leaf area diseased per pot as a percentage of the total leaf area; (c) petiole/stem disease incidence (%PDI), by calculating the percentage of petioles/stems per pot showing disease symptoms; (d) petiole/stem disease severity (%PDS), by visually calculating the petiole/stem area diseased per pot as a percentage of the total petiole/stem area; and (e) leaf yellowing severity (%LYS), by calculating the leaf area showing yellow coloration per pot as a percentage of the total leaf area. All disease assessments were conducted by the same individual.
2.4. Temperature and Moisture Regimes
After recording the disease symptoms, the plants were transferred to three growth chambers with temperatures maintained at 12.5/9.5 °C, 20/17 °C, and 27/24 °C day/night with 12 h photoperiod. These temperatures were selected to mimic temperatures commonly seen in annual Medicago spp. fields in Australia [45]. In each temperature, inoculated and control uninoculated plants of both species were treated with two different water regimes: 100% water holding capacity (WHC) (pots were watered to free draining with deionised water (DI) daily) or to 50% water holding capacity (WHC) until the end of the trial [46].
2.5. Phytoestrogen Assessment
2.5.1. Chemicals and Reagents
Coumestrol and derivatisation reagent N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) containing 1% trimethylchlorosilane (TMCS) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Derivatisation reagents were added to the dry extracts before injection into the GC. The stock standard solutions of coumestrol were prepared in methanol at final concentration of 10 μg mL−1 and stored in the dark at 4 °C and allowed to equilibrate at ambient temperature for at least 2 h before use. Pure coumestrol standard was used to prepare a calibration curve from 0.001 to 0.1 mg L−1 with nine different concentrations and the relationship between peak area and concentration was linear with the correlation coefficient (r > 0.9798) for coumestrol.
2.5.2. Quantitation of Phytoestrogens
Plants were harvested four weeks post treatment. Coumestrol content was determined by analysing stem samples from all treatments as these are known to represent a reliable indicator of phytoestrogen levels [47]. To obtain fine-ground samples, samples were oven dried at 70 °C and coarsely ground in a mechanical grinder, then frozen with liquid nitrogen and finely ground in a mortar and pestle. In this study, the methodology by Ferrer et al. [48] with some modifications was used for quantification of coumestrol in plant samples. Because of their polar and non-volatile properties, phytoestrogens such as coumestrol must be derivatised to make them suitable for GC–MS analysis [48], which was done by BSTFA as in the study by Ding and Chiang [49]. The analytes were derivatised to their trimethylsilyl ethers with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) containing 1% trimethylchlorosilane (TMCS). The ground plant samples (0.1 g) were extracted with 1 mL of methanol in glass sample vials (8 mL). The vials were manually mixed for 30 s and then placed on an end-over-end mixer for 16 h at room temperature. Samples were filtered using glass fibre syringe filters. A total of 50 µL of the supernatant was added to 450 µL of methanol, including cholesterol as an internal standard prepared in 0.02 mg mL−1 concentration to confirm effective derivatisation reactions for each sample. Then, 50 µL of this mixture was evaporated to dryness under a stream of nitrogen and 50 µL of 1% TMCS/BSTFA derivatising reagent and 50 µL of pyridine were added to the vials. The vials were then placed on a heating block at 60 °C for 20 min. This temperature was selected as the optimum derivatisation condition after several tests. Then, the vials were removed from the heating block and set aside 20 min to cool. The samples were analysed for coumestrol content using gas chromatography (GC). Coumestrol was identified in the samples on the basis of the relative retention time (RT) of the standard at 28.5 min and mass spectrum, while cholesterol was confirmed not to deviate by more than 2% throughout the experiments. Amounts of coumestrol were quantified as mg kg−1 dry weight of plant material extracted.
2.5.3. GC–MS Conditions
Coumestrol was analyzed using GC–MS on an Agilent 6890 GC connected to an Agilent 5973 mass-selective detector (Agilent Technologies, La Jolla, CA, USA) or a Shimadzu GCMS-QP2010 (Kyoto, Japan). GC columns used included a SGX BPX-5 column (5% phenyl polysilphenylene–siloxane, 30 m × 0.25 mm × 0.25 mm film thickness), with ultra-high purity helium as the carrier gas at a constant flow rate of 1 mL/min. A total of 1 μL of samples was injected in splitless injection mode with the inlet temperature set to 320 °C. The initial oven temperature was set at 50 °C and held for 1 min then ramped at 15 °C/min to 250 °C and then ramped at 3 °C/min to 320 °C and finally held for 10 min. The ion source was set at 200 °C, and the spectrometer was set to record in selective ion monitoring (SIM) mode, measuring ions m/z 329 and 368 for cholesterol and m/z 397 and 412 for coumestrol.
2.6. Experimental Design and Statistical Analyses
The experiment was fully repeated once, and each experiment had 5 replications. There were two Medicago species (M. rugosa cv. Paraponto and M. scutellata cv. Sava), one P. medicaginis isolate (WAC3658), one control (mock inoculation), two moisture levels (100% and 50% WHC), and three temperatures (12.5/9.5 °C, 20/17 °C, and 27/24 °C day/night). The total number of pots in each round was 120.
Initially, the duplicate experiments were conducted in controlled environment room maintained at 22 °C (day) and 18 °C (night), arranged as a “one-way design (in randomised blocks)” generated using the “Generate a Standard Design” function of GenStat 18.1 (18th Edition, Lawes Agricultural Trust, Rothamsted Research, UK). Normality of data and homogeneity of the original and repeat experiments were tested before the analyses were conducted. As data from the original and the repeat experiments were not significantly different, the two datasets were combined and re-analysed together in GenStat. Data for leaf disease incidence (%LDI), petiole/stem disease incidence (%PDI), leaf disease severity (%LDS), petiole/stem disease severity (%PDS), and leaf yellowing severity (%LYS) were analysed using the “one-way ANOVA” function of GenStat. Fisher’s least significant differences (LSDs) were used to separate significant differences between species.
Subsequently, pots were transferred to three growth chambers maintained at 12.5/9.5 °C, 20/17 °C, and 27/24 °C day/night with 12/12 h photoperiod. This component of the duplicate experiments was arranged as “split-split plot design” in every temperature using the “Generate a Standard Design” function of GenStat 18.1. For quantification of targeted phytoestrogen in the plants harvested from controlled environment growth chambers, four replicates from each treatment were used and the data for the treatments were analysed according to this ”split-split plot design” with the randomisation determined using GenStat release 18.1 for every temperature separately. In every temperature, species were arranged as whole plots, and inoculation was arranged as subplots; within each subplot, moisture level was arranged as a sub-sub plot, and four replications were considered as blocks. All three temperature regimes were run concurrently for the original and for the repeat experiments. Fisher’s protected least significant differences (LSDs) at p ≤ 0.05 were used to separate significant differences between treatments. The three temperature regimes were compared using a t-test for mean of coumestrol production.
3. Results
3.1. Disease Expression
The main effects of annual Medicago species for %LDI, %PDI, %LDS, %PDS, and %LYS disease parameters were significant (all p ≤ 0.001). Sava was more sensitive than Paraponto in terms of %LDI (92.2 and 59.5, respectively), %PDI (81.3 and 57.4, respectively), %LDS (82.2 and 58.3, respectively), %PDS (71.3 and 40.5, respectively), and %LYS (7.7 and 2.8, respectively) (Table 1). Symptom expression across the three different temperature regimes and two different moisture levels on Phoma black stem and leaf disease severity in inoculated plants compared to disease-free plants in the two species is as illustrated in Figure 1a–f.

Table 1.
Statistical analysis of the annual Medicago species main effects on %LDI †, %PDI, %LDS, %PDS, and %LYS.

Figure 1.
Effect of three different temperature regimes (12.5/9.5 °C, 20/17 °C, and 27/24 °C, day/night) and two different moisture levels (100% and 50% water holding capacity) on Phoma black stem and leaf disease severity in inoculated plants compared to disease-free plants in annual Medicago rugosa cv. Paraponto and M. scutellata cv. Sava. All photos, from left to right, show 50% WHC control plant, 100% WHC control plant, 50% WHC inoculated plant, 100% WHC inoculated plant: (a), (b), (c) = M. rugosa cv. Paraponto under 12.5/9.5 °C, 20/17 °C, and 27/24 °C, respectively; (d), (e), (f) = M. scutellata cv. Sava under 12.5/9.5 °C, 20/17 °C, and 27/24 °C, respectively.
3.2. Phytoestrogen Expression
Statistical main effects and interactions (p-values and LSDs) for mean of coumestrol induced by P. medicaginis in two annual Medicago species M. rugosa cv. Paraponto and M. scutellata cv. Sava under two moisture levels (100% and 50% WHC) at three separate temperature regimes (12.5/9.5 °C, 20/17 °C, and 27/24 °C) are provided in Table 2. There was a significant effect of moisture level overall and at both 20/17 °C and 27/24 °C but not at 12.5/9.5 °C. There was significant two-way interaction of species with moisture, two-way interaction of inoculation with moisture, and three-way interactions at both 20/17 °C and 27/24 °C but not at 12.5/9.5 °C.

Table 2.
Statistical main effects and interactions (p-value and LSD) from mean percentage coumestrol production in two species of annual Medicago spp. inoculated with Phoma medicaginis at three different temperatures and two different moisture levels.
3.2.1. Main Treatment Effects
Temperature: The results of the t-test between tested temperatures showed that while there was not a significant difference for coumestrol production in control plants across tested temperatures, the concentrations of coumestrol increased significantly under high-temperature stress in inoculated plants. In fact, there was a significant difference between 12.5/9.5 °C with 20/17 °C and 27/24 °C (p ≤ 0.05 and p ≤ 0.001, respectively), while 20/17 °C and 27/24 °C were not significantly different. The greatest and lowest level of coumestrol in inoculated plants was produced at 27/24 °C and 12.5/9.5 °C, respectively (391 and 193 mg kg−1, respectively), and 20/17 °C was intermediate (313 mg kg−1).
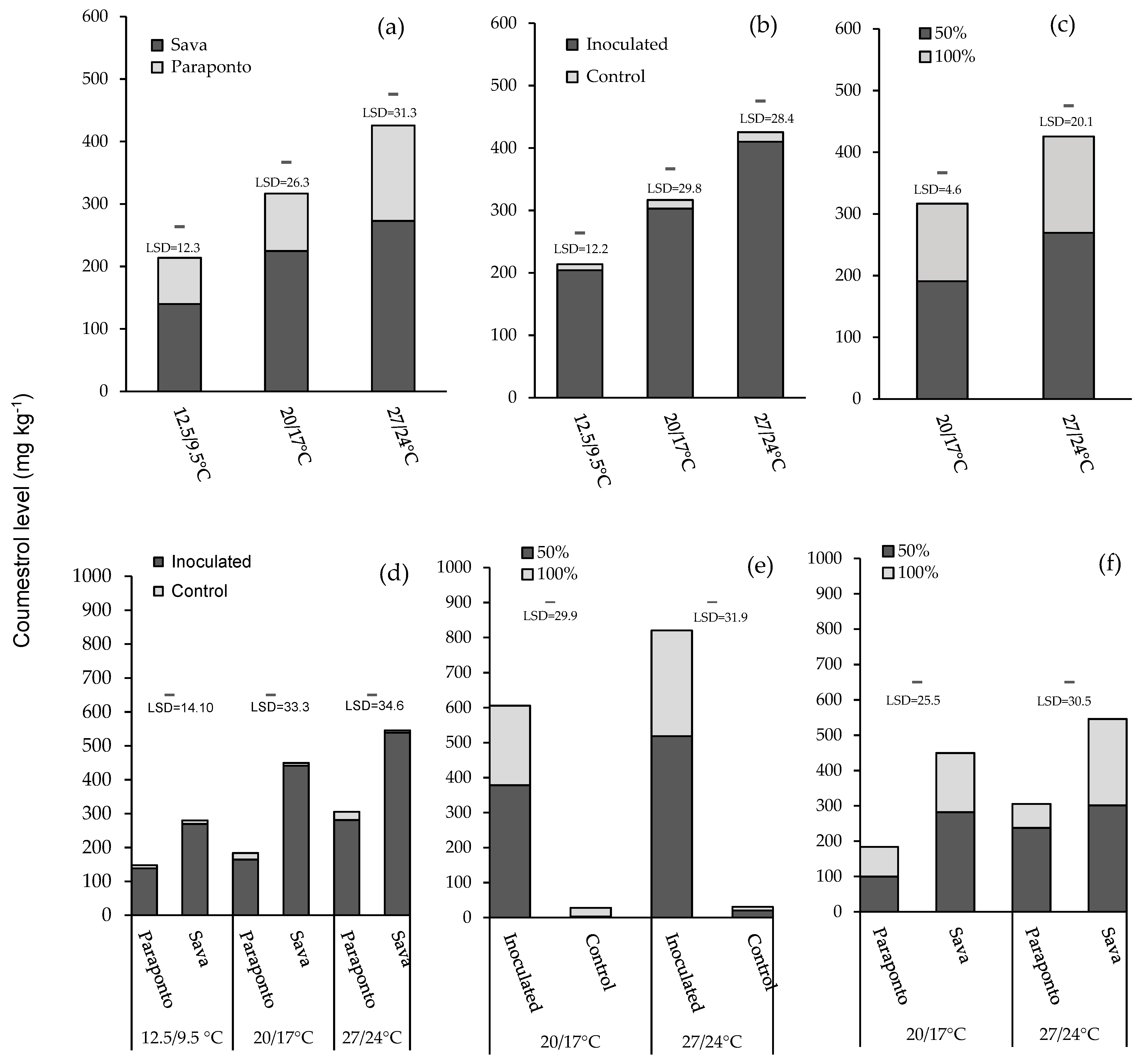
Species: There was a significant main effect of species in terms of coumestrol production in all three temperatures (p < 0.001; Table 2). Across both species, Sava showed greater amounts of coumestrol than Paraponto at 12.5/9.5 °C (140 and 74.0 mg kg−1, respectively), 20/17 °C (225 and 92.0 mg kg−1, respectively), and 27/24 °C (273 and 153 mg kg−1, respectively) (Figure 2a).
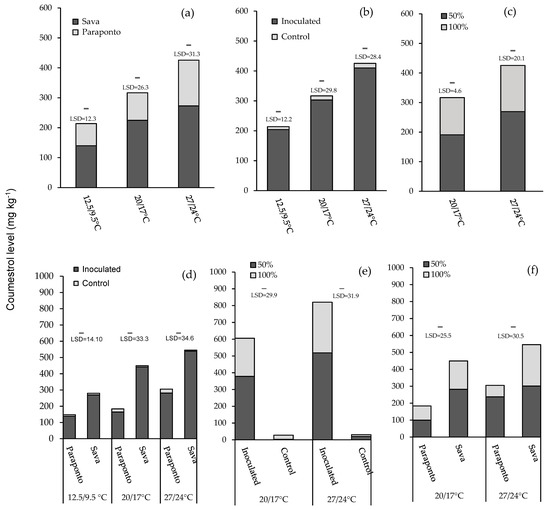
Figure 2.
The bar charts show the effect of three different temperature regimes (12.5/9.5 °C, 20/17 °C, and 27/24 °C, day/night) and two different moisture levels (100% and 50% water holding capacity) across control and inoculated plants of annual Medicago rugosa cv. Paraponto and M. scutellata cv. Sava on the content of coumestrol. Data shown represent mean coumestrol content values in relation to (a) main effect of species at three different temperatures; (b) main effect of inoculation at three different temperatures; (c) main effect of moisture at 20/17 °C and 27/24 °C; (d) two-way interactions of species and inoculation at 20/17 °C and 27/24 °C; (e) two-way interactions of inoculation and moisture level at 20/17 °C and 27/24 °C; (f) two-way interactions of species and moisture level at 20/17 °C and 27/24 °C.
Inoculation: Presence of Phoma black stem and leaf spot significantly increased the coumestrol production in all three temperatures (p ≤ 0.001; Table 2). The level of the coumestrol in inoculated plants was much higher than control plants at 12.5/9.5 °C (204 and 9.7 mg kg−1, respectively), 20/17 °C (303 and 13.9 mg kg−1, respectively), and 27/24 °C (410 and 15.4 mg kg−1, respectively) (Figure 2b).
Moisture level: The main effect of moisture level on coumestrol was significant at 20/17 °C and 27/24 °C (p ≤ 0.001; Table 2). At both 20/17 °C and 27/24 °C, the level of coumestrol was greater in low moisture (191 and 269 mg kg−1, respectively) compared to the plants with high moisture (126 and 156 mg kg−1, respectively) (Figure 2c).
3.2.2. Two-Way Interaction Effects
Species with inoculation: There was a significant two-way interaction of Medicago species with inoculation at all three temperatures (all p < 0.001). At 12.5/9.5 °C, the greatest overall mean was for inoculated plants of M. scutellata cv. Sava (269 mg kg−1), and the lowest was for control plants of M. rugosa cv.Paraponto (9.1 mg kg−1). The highest and lowest level of coumestrol at 20/17 °C was recorded for inoculated and control plants in Sava (441 and 8.1 mg kg−1, respectively). Inoculated and control plants of Sava exhibited the greatest and lowest values (539 and 6.6 mg kg−1, respectively) for coumestrol production at 27/24 °C (Figure 2d).
Inoculation with moisture level: Two-way interaction of inoculation and moisture level only occurred at 20/17 °C and 27/24 °C (both p < 0.01). At 20/17 °C, the greatest and lowest mean of coumestrol production was for inoculated and control plants treated at low moisture (378 and 3.31 mg kg−1, respectively). At 27/24 °C, inoculated plants with low moisture recorded the highest coumestrol production (518 mg kg−1), while the least coumestrol was produced in control plants under high moisture (10.4 mg kg−1) (Figure 2e).
Species with moisture level: The two-way interaction effects of Medicago species and moisture level for coumestrol production was significant for 20/17 °C and 27/24 °C (both p < 0.01) but not at 12.5/9.5 °C. The greatest coumestrol production was for M. scutellata cv. Sava under low moisture at both 20/17 °C and 27/24 °C (282 and 301 mg kg−1) and the least coumestrol was for M. rugosa cv. Paraponto under high moisture at both 20/17 °C and 27/24 °C (84.4 and 67.7 mg kg−1) (Figure 2f).
3.2.3. Three-Way Interaction Effects
There was a significant three-way interaction of Medicago species with isolate and with moisture level at 20/17 °C and 27/24 °C (p < 0.001 and p < 0.01, respectively). At 20/17 °C, the content of coumestrol in M. rugosa cv. Paraponto and M. scutellata cv. Sava treated with low moisture level (5.0 and 1.6 mg kg−1, respectively) increased as the moisture level increased (34.4 and 14.6 mg kg−1, respectively). However, this trend was reversed at 20/17 °C for inoculated plants, where higher moisture level reduced the production of coumestrol. For example, inoculated plants of Paraponto and Sava produced higher amounts of coumestrol in low moisture level (194 and 562 mg kg−1, respectively) than high moisture level (134 and 320 mg kg−1, respectively). A similar trend in coumestrol production was observed in both control and inoculated plants of Paraponto at 27/24 °C. For example, the control and inoculated plants at low moisture level produced higher coumestrol (39 and 436 mg kg−1, respectively) than at high moisture level (9.4 and 126 mg kg−1, respectively). At this same temperature regime, for Sava control plants, the content of coumestrol at low moisture level (1.8 mg kg−1) increased when the moisture level increased (11.5 mg kg−1), but the reverse was the case for Sava inoculated plants, decreasing from 600 to 478 mg kg−1 as the moisture level increased (Table 3).

Table 3.
Interaction of species with inoculation and with moisture level at 20/17 °C and 27/24 °C in relation to coumestrol production (mg kg−1).
4. Discussion
This is the first study determining how environmental factors such as temperature and moisture level can significantly affect phytoestrogen production in annual Medicago spp. in the presence of P. medicaginis. Although fungal diseases, such as Phoma black stem and leaf spot disease, are generally considered as the major cause of elevated phytoestrogens in annual Medicago spp. [4,5,6,7,10,11,39,47,50,51,52,53,54], several other studies suggested that the level of these compounds can also be elevated due to one or more particular environmental parameters [55], such as temperature [30,33] and water stress [27]. The current study highlighted those situations of higher temperatures in conjunction with lower soil moisture, such as may arise from climate change [56], that will result in greatest elevation in coumestrol in presence of P. medicaginis and to levels far exceeding the animal risk threshold of 25 mg kg−1 [22].
In the current study, the effect of temperature on coumestrol production was different across uninoculated versus inoculated plants. In inoculated plants, coumestrol increased significantly with high-temperature stress, but not so for uninoculated plants. Likewise, effects of temperature have been reported in relation to accumulation of various phenolic compounds by Sharma et al. [33]. In particular, a strong effect of temperature on isoflavone concentration has also been demonstrated for some other plants such as soybean. For instance, Ohta et al. [34] found that coumestrol content was higher in sprouts cultivated at 24 °C than in sprouts cultivated at 4 °C. In contrast, Chennupati et al. [57] reported that isoflavone content was reduced in soybeans at the late reproductive stages under high temperature stress. The latter study suggests that the developmental stage of the plant may also play an important role in plant responses towards increased phytoestrogen and this warrants further investigation. However, Adams [38] noted that alfalfa appeared unaffected by temperature extremes, so the effect of temperature phytoestrogen is clearly not universal.
In the current study, coumestrol concentration was influenced by Medicago species, being much higher in M. scutellata cv. Sava than M. rugosa cv. Paraponto in all tested temperatures. Such species/cultivar differences are evident even at a single temperature, such as in a greenhouse study at 18 ± 4 °C by Omidvari et al. [39]. Moreover, in the current study, Paraponto was more resistant than Sava against P. medicaginis in terms of all disease parameters including %LDI, %PDI, %LDS, %PDS, and %LYS. Similarly, Barbetti [58] also reported that Sava was among the most susceptible cultivars. In addition, Omidvari et al. [7] showed that some cultivars, such as M. murex cv. Zodiac, had lower rate of acceleration of phytoestrogen production from disease than M. truncatula cv. Cyprus and M. polymorpha var. brevispina cv. Serena, primarily due to Zodiac producing higher levels of coumestrol than the other two other cultivars in disease-free plants. That Paraponto demonstrated significant resistance against P. medicaginis in the current study suggests it could provide an important source of resistance [5,50,59] and potentially be used both in breeding programs [6,60], and more directly, in minimising Phoma black stem and leaf spot damage.
The current study demonstrated that the production of coumestrol was significantly associated with Phoma black stem and leaf spot disease, with little coumestrol produced across all three temperatures in the absence of disease. Similarly, Fields et al. [22] showed that alfalfa inoculated with Stemphylium vesicarium contained 169 ± 25.1 mg kg−1 of coumestrol on a dry matter basis as compared with 3.4 ± 0.8 mg kg−1 in control plants. The current study confirms that, in the absence of disease, species such as M. rugosa and M. scutellata can safely be used in sheep farming systems during mating since the coumestrol content in dry stems of uninoculated plants across all tested temperatures remained lower than animal risk threshold of 25 mg kg−1 [22,61].
Production of coumestrol in the current study was not only driven by temperature, species and inoculation, but also by moisture level. For example, the level of coumestrol was higher in the plants with lower moisture in both 20/17 °C and 27/24 °C. Coumestrol concentration increased by 51.5 and 72.5% when moisture was reduced at 20/17 °C and 27/24 °C, respectively. Similar effects of water stress on isoflavones production have been highlighted for soybean [62,63] and in relation to various phenolic compounds in Brassicaceae [64]. These above results are in contrast with Fields et al. [27], who reported a little-to-no coumestrol response following water stress, and even where severe drought conditions resulted in foliage death, there was only slight elevation of coumestrol content, from 1.3 to 3.0 mg kg−1 dry material.
Importantly, in the current study, there were interactions between the different environmental and disease parameters, particularly for Medicago species with inoculation across all tested temperatures. Additionally, there were significant interactions of moisture with Medicago species, of moisture with inoculation, and a three-way interaction of moisture with Medicago species and with inoculation at 20/17 °C and 27/24 °C. Following inoculation, the content of coumestrol increased nearly 26-, 54- and 82-fold in Sava while only 15-, 8- and 12-fold in Paraponto at 12.5/9.5 °C, 20/17 °C, and 27/24 °C, respectively. An interaction of Medicago species and moisture level only occurred at 20/17 °C and 27/24 °C, a possible explanation being that at lower temperatures there was relatively little loss of moisture. Similarly, an interaction of inoculation and moisture level only occurred at 20/17 °C and 27/24 °C, where inoculated plants exposed to lower moisture levels produced greater quantities of coumestrol, up to 66.3 and 71.7% more at 20/17 °C and 27/24 °C, respectively. Other studies on the effect of fungal disease [27] and water stress [62,65] on production of secondary metabolites also showed that both disease in alfalfa and moisture levels in soybean and Scutellaria were significant drivers of coumestrol production. The three-way interaction of Medicago species with inoculation and with moisture level also only occurred at 20/17 °C and 27/24 °C. In this instance, only on diseased plants were there significant differences in terms of coumestrol production between the two Medicago species with different moisture levels, with Sava producing the highest levels of coumestrol on diseased plants at low moisture level. Further studies to better define and understand the exact mechanisms of the above interactions are warranted.
5. Conclusions
Clearly, situations of higher temperatures in conjunction with lower soil moisture levels cause greatest elevation in coumestrol in the presence of P. medicaginis, levels far exceeding the animal risk threshold of 25 mg kg−1. Having a better understanding of the effects of environmental signals such as temperature and moisture levels on phytoestrogen production in diseased annual Medicago spp. will not only assist in identifying optimal locations for mating grazing animals but should promote improved management decisions towards maximising quality and yield of annual Medicago spp. swards. Further, there is potential to select and/or breed new species/cultivars that are not only more resistant to P. medicaginis, but that are the least responsive towards coumestrol production, under the tested environmental conditions of the current study. In addition, further gene expression studies would be useful to unveil the mechanisms by which these abiotic factors affect annual Medicago spp. isoflavone production in the presence of Phoma black stem and leaf spot.
Author Contributions
M.O., M.J.B., M.P.Y., G.R.F. and P.A.-D. conceived the ideas and designed methodology; M.O. and P.A.-D. collected the data; M.O. and M.P.Y. analysed the data; M.O. and M.J.B. led the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the School of Agriculture and Environment and the School of Molecular Sciences at the University of Western Australia.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to sensitivities relating to animal welfare aspects.
Acknowledgments
The authors thank Robert Creasy and Bill Piasini in the UWA Plant Growth Facilities for their technical assistance in plant growth facilities. The first author gratefully acknowledges an Australian Government Postgraduate Scholarship funding her PhD studies.
Conflicts of Interest
All authors state that they have no conflict of interest to declare with regard to the conduct or publication of these studies.
References
- Walsh, M.J.; Delaney, R.H.; Groose, R.W.; Krall, J.M. Performance of annual medic species (Medicago spp.) in southeastern Wyoming. Agron. J. 2001, 93, 1249–1256. [Google Scholar] [CrossRef]
- Barbetti, M.J.; Riley, I.T.; You, M.P.; Li, H.; Sivasithamparam, K. The association of necrotrophic fungal pathogens and plant parasitic nematodes with the loss of productivity of annual medic-based pastures in Australia and options for their management. Australas. Plant Pathol. 2006, 35, 691–706. [Google Scholar] [CrossRef]
- Puschner, B. Anti-nutritional factors in alfalfa hay. In Proceedings of the 29th National Alfalfa Symposium, Las Vegas, NV, USA, 11–12 December 2000; The California Alfalfa Workgroup and The Alfalfa Council: Las Vegas, NV, USA, 2000; pp. 157–162. [Google Scholar]
- Barbetti, M.J. Subterranean clover foliage fungi as root pathogens. Australas. Plant Pathol. 1984, 13, 38–40. [Google Scholar] [CrossRef]
- Barbetti, M.J. Effects of temperature and humidity on disease caused by Phoma medicaginis, resistance in some Medicago cultivars and the incidence of seed-borne inoculum. Aust. J. Exp. Agric. 1987, 27, 851–856. [Google Scholar] [CrossRef]
- Barbetti, M.J.; You, M.; Jones, R.A.C. Medicago truncatula and other annual Medicago spp.—Interactions with root and foliar fungal, oomycete, and viral pathogens. In The Model Legume Medicago truncatula; De Bruijn, F.J., Liu, D.Y., Eds.; Wiley: Chichester, UK, 2020; pp. 293–306. [Google Scholar]
- Omidvari, M.; Flematti, G.; You, M.P.; Abbaszadeh-Dahaji, P.; Barbetti, M.J. Phoma medicaginis isolate differences determine disease severity and phytoestrogen production in annual Medicago spp. Plant Dis. 2021, 105, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, M.J. Response of Medicago cultivars to fungal root pathogens associated with Trifolium subterraneum. Plant Prot. Q. 1989, 4, 1–3. [Google Scholar]
- You, M.P.; Sivasithamparam, K.; Riley, I.T.; Barbetti, M.J. The occurrence of root-infecting fungi and parasitic nematodes in annual Medicago spp. in Western Australian pastures. Aust. J. Agric. Res. 2000, 51, 435–444. [Google Scholar] [CrossRef]
- Barbetti, M.J. Resistance in annual Medicago spp. to Phoma medicaginis and Leptosphaerulina trifolii and its relationship to induced production of a phytoestrogen. Plant Dis. 2007, 91, 239–244. [Google Scholar] [CrossRef]
- Barbetti, M.J. Relative resistance, associated yield losses and phytoestrogen production from fungal foliar diseases in new and old annual Medicago cultivars. Aust. J. Agric. Res. 1995, 46, 441–450. [Google Scholar] [CrossRef]
- Adams, N.R. Detection of the effects of phytoestrogens on sheep and cattle. J. Anim. Sci. 1995, 73, 1509–1515. [Google Scholar] [CrossRef]
- Wasserman, M.D.; Milton, K.; Chapman, C.A. The roles of phytoestrogens in primate ecology and evolution. Int. J. Primatol. 2013, 34, 861–878. [Google Scholar] [CrossRef]
- Deavours, B.E.; Dixon, R.A. Metabolic engineering of isoflavonoid biosynthesis in alfalfa. Plant Physiol. 2005, 138, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Hloucalová, P.; Skládanka, J.; Horký, P.; Klejdus, B.; Pelikán, J.; Knotová, D. Determination of phytoestrogen content in fresh-cut legume forage. Animals 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.F.M. Fertility of Herbivores Consuming Phytoestrogen-containing Medicago and Trifolium Species. Agriculture 2016, 6, 35. [Google Scholar] [CrossRef]
- Croker, K.P.; Barbetti, M.J.; Nichols, P.G.H. Incidence of coumestrol in medic pastures in Western Australia. Proc. Aust. Soc. Anim. Prod. 1994, 20, 416. [Google Scholar]
- Croker, K.P.; Nichols, P.G.H.; Barbetti, M.J.; Adams, N. Sheep Infertility from Pasture Legumes; Farmnote No. 6/94; Department of Agriculture Western Australia: Perth, WA, Australia, 1994.
- Croker, K.P.; Nichols, P.G.H.; Barbetti, M.J.; Adams, N. Sheep Infertility from Pasture Legumes; Farmnote No. 79/99; Department of Agriculture Western Australia: Perth, WA, Australia, 1999.
- Croker, K.P.; Nichols, P.G.H.; Barbetti, M.J.; Adams, N. Sheep Infertility from Pasture Legumes; Farmnote No. 41/2005; Department of Agriculture Western Australia: Perth, WA, Australia, 2005.
- Sirtori, C.R.; Arnoldi, A.; Johnson, S.K. Phyto-oestrogens: End of a tale? Ann. Med. 2005, 37, 423–438. [Google Scholar] [CrossRef]
- Fields, R.L.; Graham, K.B.; Alan, G.; Jenny, Z.; Derrick, J.M. Alfalfa coumestrol content in response to development stage, fungi, aphids, and cultivar. Ecol. Crop Physiol. 2018, 110, 910–921. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.M.; Millington, A.J. Wether bioassay of annual pasture legumes. IV. The oestrogenic activity of annual medic pastures. Aust. J. Agric. Res. 1965, 16, 927–935. [Google Scholar] [CrossRef]
- Francis, C.M.; Millington, A.J. The presence of methylated coumestans in annual Medicago species: Response to a fungal pathogen. Aust. J. Agric. Res. 1971, 22, 75–80. [Google Scholar] [CrossRef]
- Marshall, T.; Parkin, R.J. Phosphate applications affect the coumestrol level of medics. J. Agric. West. Aust. 1970, 11, 8. [Google Scholar]
- Fields, R.L.; Moot, D.J.; Barrell, G.K. Coumestrol content of lucerne under drought stress. In Proceedings of the 18th Australian Agronomy Conference, Ballarat, Australia, 24–28 September 2017. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Saviranta, N.M.M.; Anttonen, M.J.; von Wright, A.; Karjalainen, R.O. Red clover (Trifolium pratense L.) isoflavones: Determination of concentrations by plant stage, flower colour, plant part and cultivar. J. Sci. Food Agric. 2008, 88, 125–132. [Google Scholar] [CrossRef]
- Tsukamoto, C.; Shimada, S.; Igita, K.; Kudou, S.; Kokubun, M.; Okubo, K.; Kitamura, K. Factors affecting isoflavones content in soybean seeds: Changes in isoflavones, saponins, and composition of fatty acids at different temperatures during seed development. J. Agric. Food Chem. 1995, 43, 1184–1192. [Google Scholar] [CrossRef]
- Chennupati, P.; Seguin, P.; Liu, W. Effects of high temperature stress at different development stages on soybean isoflavone and tocopherol concentrations. J. Agric. Food Chem. 2011, 59, 13081–13088. [Google Scholar] [CrossRef]
- Lozovaya, V.V.; Lygin, A.V.; Ulanov, A.V.; Nelson, R.L.; Dayde, J.; Widholm, J.M. Effect of temperature and soil moisture status during seed development on soybean seed isoflavone concentration and composition. Crop Sci. 2005, 45, 1934–1940. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Ohta, T.; Uto, T.; Tanaka, H. Effective methods for increasing coumestrol in soybean sprouts. PLoS ONE 2021, 16, e0260147. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Lee, E.A.; Woodrow, L.; Seguin, P.; Kumar, J.; Rajcan, J.; Ablett, G.R. Association of seed and agronomic traits with isoflavone levels in soybean. Can. J. Plant Sci. 2009, 89, 477–484. [Google Scholar] [CrossRef]
- Tucak, M.; Cupi’c, T.; Horvat, D.; Popovi’c, S.; Krizmani´c, G.; Ravli´c, M. Variation of phytoestrogen content and major agronomic traits in alfalfa (Medicago sativa L.) populations. Agronomy 2020, 10, 87. [Google Scholar] [CrossRef]
- Fields, R.L.; Sedcole, J.R.; Barrell, G.K.; Moot, D.J. Prediction of coumestrol content in unirrigated lucerne crops using weather variables. N. Z. J. Agric. Res. 2018, 62, 528–542. [Google Scholar] [CrossRef]
- Adams, N.R. Phytoestrogens. In Toxicants of Plant Origin; Cheeke, P.R., Ed.; CRC Press: Boca Raton, FL, USA, 1989; Volume 4, pp. 25–32. [Google Scholar]
- Omidvari, M.; Flematti, G.; You, M.P.; Abbaszadeh-Dahaji, P.; Barbetti, M.J. Phoma black stem severity and phytoestrogen production in annual Medicago spp. is primarily determined by interaction of cultivar and pathogen isolate. Plant Pathol. 2022, 105, 2851–2860. [Google Scholar] [CrossRef]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Chihaoui, S.A.; Djébali, N.; Mrabet, M.; Barhoumi, F.; Mhamdi, R.; Mhadhbi, H. Phoma medicaginis colonizes Medicago truncatula root nodules and affects nitrogen fixation capacity. Eur. J. Plant Pathol. 2014, 141, 375–383. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Jebara, M.; Limam, F.; Huguet, T.; Elarbi Aouani, M. Interaction between Medicago truncatula lines and Sinorhizobium meliloti strains for symbiotic efficiency and nodule antioxidant activities. Physiol. Plant 2005, 124, 4–11. [Google Scholar] [CrossRef]
- Omidvari, M.; Flematti, G.; You, M.P.; Abbaszadeh-Dahaji, P.; Barbetti, M.J. Sequential infections by 32 isolates of Phoma medicaginis increase production of phytoestrogens in Medicago polymorpha var. brevispina. Crop Pasture Sci. 2022; in press. [Google Scholar] [CrossRef]
- Ellwood, S.; Kamphuis, L.G.; Pfaff, T.; Oliver, R.P.; Samac, D.A.; Foster-Hartnett, D.; Tivoli, B.; Onfroy, C.; Moussart, A. Inoculation and growth with foliar pathogenic fungi. In The Medicago truncatula Handbook; Mathesius, U., Journet, E.P., Sumner, L.W., Eds.; The Samuel Roberts Noble Foundation: Ardmore, PA, USA, 2007; pp. 1–14. [Google Scholar]
- Barbetti, M.J. Effects of temperature on development and progression in rape of crown canker caused by Leptosphaeria maculans. Aust. J. Exp. Agric. 1975, 15, 705–708. [Google Scholar] [CrossRef]
- You, M.P.; Barbetti, M.J. Severity of phytophthora root rot and pre-emergence damping-off in subterranean clover influenced by moisture, temperature, nutrition, soil type, cultivar and their interactions. Plant Pathol. 2017, 66, 1162–1181. [Google Scholar] [CrossRef]
- Barbetti, M.J.; Fang, C.S. Relationship between Phoma black stem severity and herbage and seed yield and coumestrol content in three Medicago polymorpha var. brevispina cultivars. Aust. J. Agric. Res. 1991, 42, 409–415. [Google Scholar] [CrossRef]
- Ferrer, I.; Barber, L.B.; Thurman, E.M. Gas chromatographic-mass spectrometric fragmentation study of phytoestrogens as their trimethylsilyl derivatives: Identification in soy milk and wastewater samples. J. Chromatogr. 2009, 1216, 6024–6032. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.H.; Chiang, C.C. Derivatization procedures for the detection of estrogenic chemicals by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, M.J. Resistance in annual Medicago species to Phoma medicaginis and Leptosphaerulina trifolii under field conditions. Aust. J. Exp. Agric. 1995, 35, 209–214. [Google Scholar] [CrossRef]
- Barbetti, M.J.; Nichols, P.G.H. Effect of Phoma medicaginis and Leptosphaerulina trifolii on herbage and seed yield and coumestrol content of annual Medicago species. Phytophylactica 1991, 23, 223–228. [Google Scholar]
- Castell-Miller, C.V.; Zeyen, R.J.; Samac, D.A. Infection and development of Phoma medicaginis on moderately resistant and susceptible alfalfa genotypes. Can. J. Plant Pathol. 2007, 29, 290–298. [Google Scholar] [CrossRef]
- Djebali, N. Aggressiveness and host range of Phoma medicaginis isolated from Medicago species growing in Tunisia. Phytopathol. Mediterr. 2013, 51, 3–15. [Google Scholar]
- Rodriguez, R.D.P.; Leath, K.T. Pathogenicity of Phoma medicaginis var. medicaginis to crowns of alfalfa. Plant Dis. 1992, 76, 1237–1240. [Google Scholar] [CrossRef]
- Wyse, J.M.; Latif, S.; Gurusinghe, S.; Berntsen, E.D.; Weston, L.A.; Stephen, C.P. Characterization of phytoestrogens in Medicago sativa L. and grazing beef cattle. Metabolites 2021, 11, 550. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Barbetti, M.J. Influence of Climate Change on Plant Disease Infections and Epidemics Caused by Viruses and Bacteria; CAB Reviews: Perspectives Agric., Veterinary Sci., Nutrition and Natural Resources; CABI: Wallingford, UK, 2012; Volume 7, pp. 1–31. [Google Scholar]
- Chennupati, P.; Seguin, P.; Chamoun, R.; Jabaji, S. Effects of high-temperature stress on soybean isoflavone concentration and expression of key genes involved in isoflavone synthesis. J. Agric. Food Chem. 2012, 60, 12421–12427. [Google Scholar] [CrossRef]
- Barbetti, M.J. Resistance in annual Medicago species to Phoma medicaginis under controlled environment and field conditions. Aust. J. Exp. Agric. 1990, 30, 209–214. [Google Scholar] [CrossRef]
- Barbetti, M.J. Strategies for control of Phoma black stem in annual Medicago species. Aust. J. Exp. Agric. 1989, 29, 635–640. [Google Scholar] [CrossRef]
- Barbetti, M.J.; Nicholas, D.A. Effect of Phoma black stem and pepper spot diseases on yield, regeneration, sward composition and phyto-oestrogen levels in grazed annual medic pastures. In Proceedings of the Australasian Plant Pathology Society: 11th Biennial Conference Proceedingsel, Perth, Australia, 29 September–2 October 1997; p. 65. [Google Scholar]
- Ramòn, J.P.; Valderràbano, J.; Folch, J. Reproductive performance of Rasa Aragonesa ewes mated on lucerne (Medicago sativa ‘Aragon’) pastures. Small Rumin. Res. 1993, 11, 323–329. [Google Scholar] [CrossRef]
- Tripathi, P.; Rabara, R.C.; Reese, R.N.; Miller, M.A.; Rohila, J.S.; Subramanian, S.; Shen, Q.J.; Morandi, D.; Bücking, H.; Shulaev, V.; et al. A toolbox of genes, proteins, metabolites and promoters for improving drought tolerance in soybean includes the metabolite coumestrol and stomatal development genes. BMC Genom. 2016, 9, 102. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Guttikonda, S.K.; Tran, L.-S.P.; Aldrich, D.L.; Zhong, R.; Yu, O.; Nguyen, H.T.; Sleper, D.A. Differential expression of isoflavone biosynthetic genes in soybean during water deficits. Plant Cell Physiol. 2010, 51, 936–948. [Google Scholar] [CrossRef]
- Linić, I.; Šamec, D.; Grúz, J.; Vujčić Bok, V.; Strnad, M.; Salopek Sondi, B. Involvement of phenolic acids in short-term adaptation to salinity stress is species-specific among brassicaceae. Plants 2019, 8, 155. [Google Scholar] [CrossRef]
- Qin, S.S.; Chen, S.Q.; Huang, L.Q. Effect of water stress on relationship of endogenous phytohormone and active compound content in roots of Scutellaria baicalensis Georgi. Chin. J. Exp. Tradit. Med. Formulae 2010, 16, 99–101. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).