Assessing the Genetic Improvement in Inbred Late Rice against Chilling Stress: Consequences for Spikelet Fertility, Pollen Viability and Anther Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Observations
2.3.1. Spikelet Fertility and Yield Components
2.3.2. Pollen Viability
2.3.3. Anther Characteristics
2.3.4. SPAD, Chlorophyll Assay
2.4. Statistical Analysis
3. Results
3.1. Yield Components
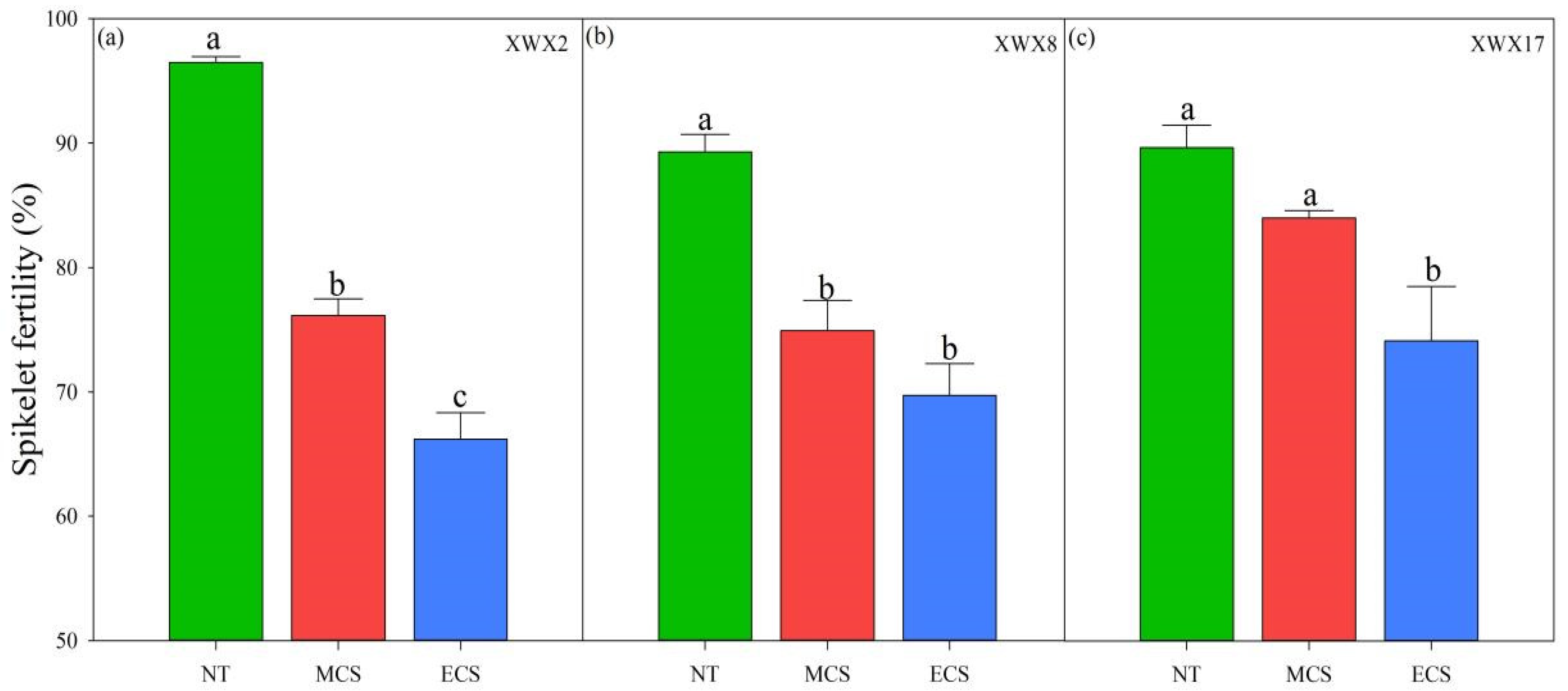
3.2. Spikelet Fertility
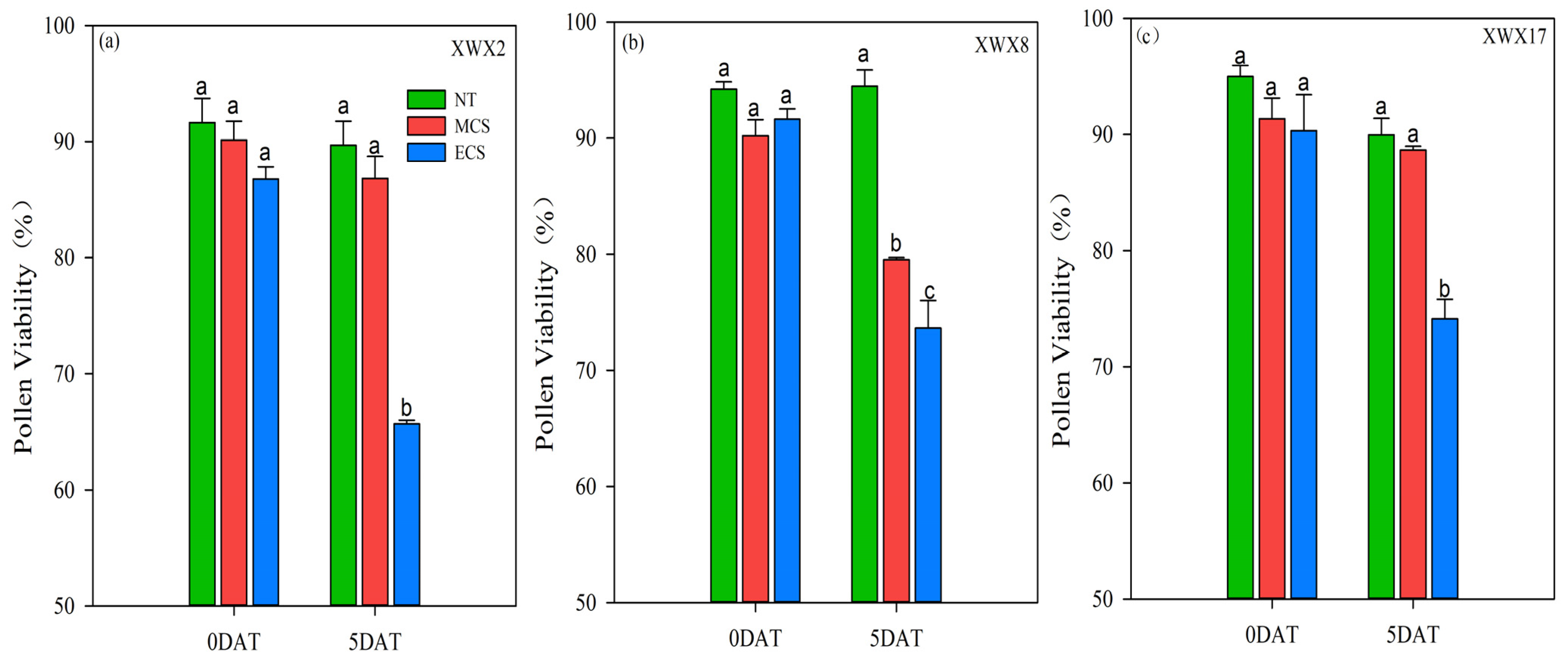
3.3. Pollen Viability
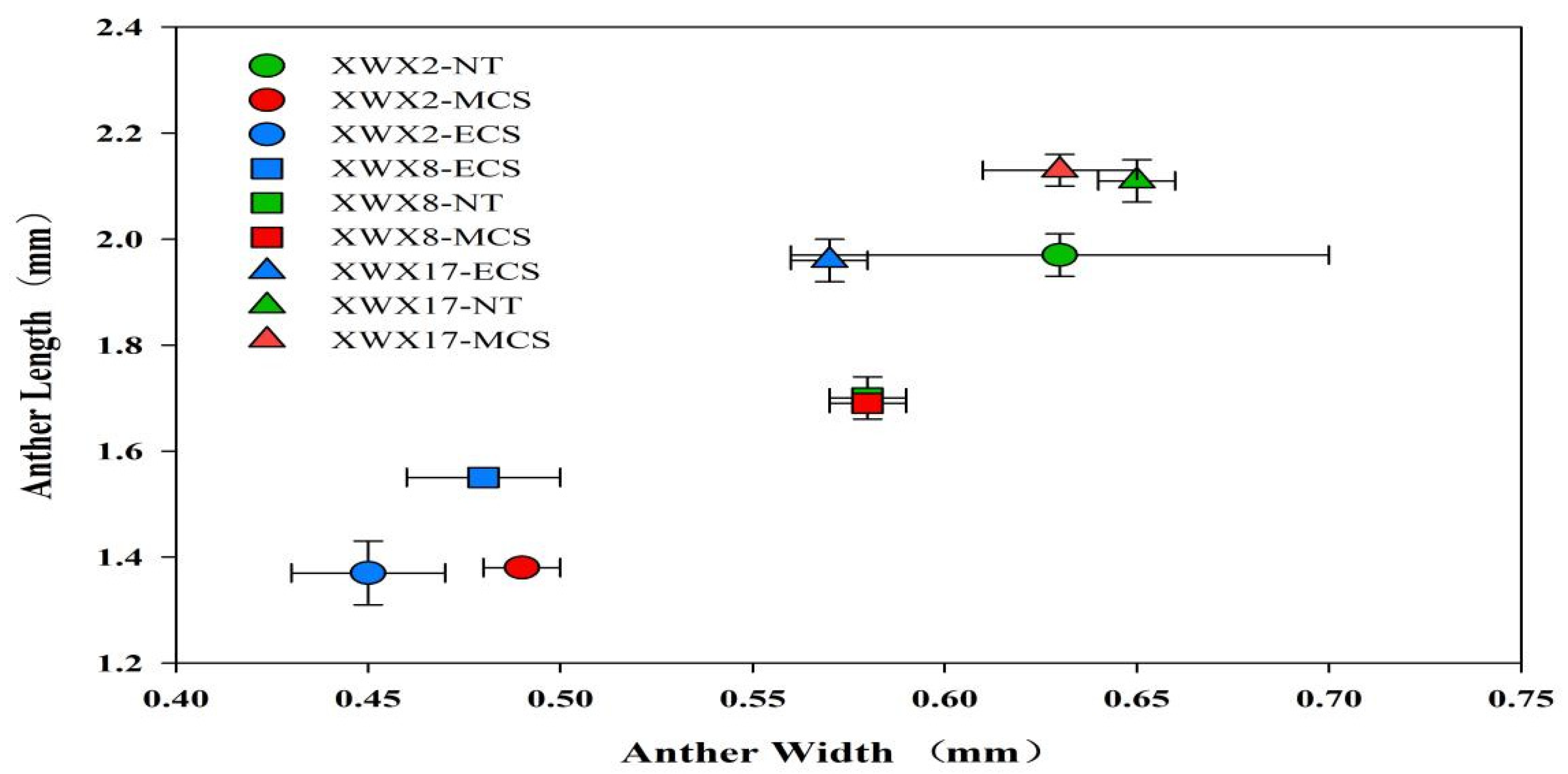
3.4. Anther Characters
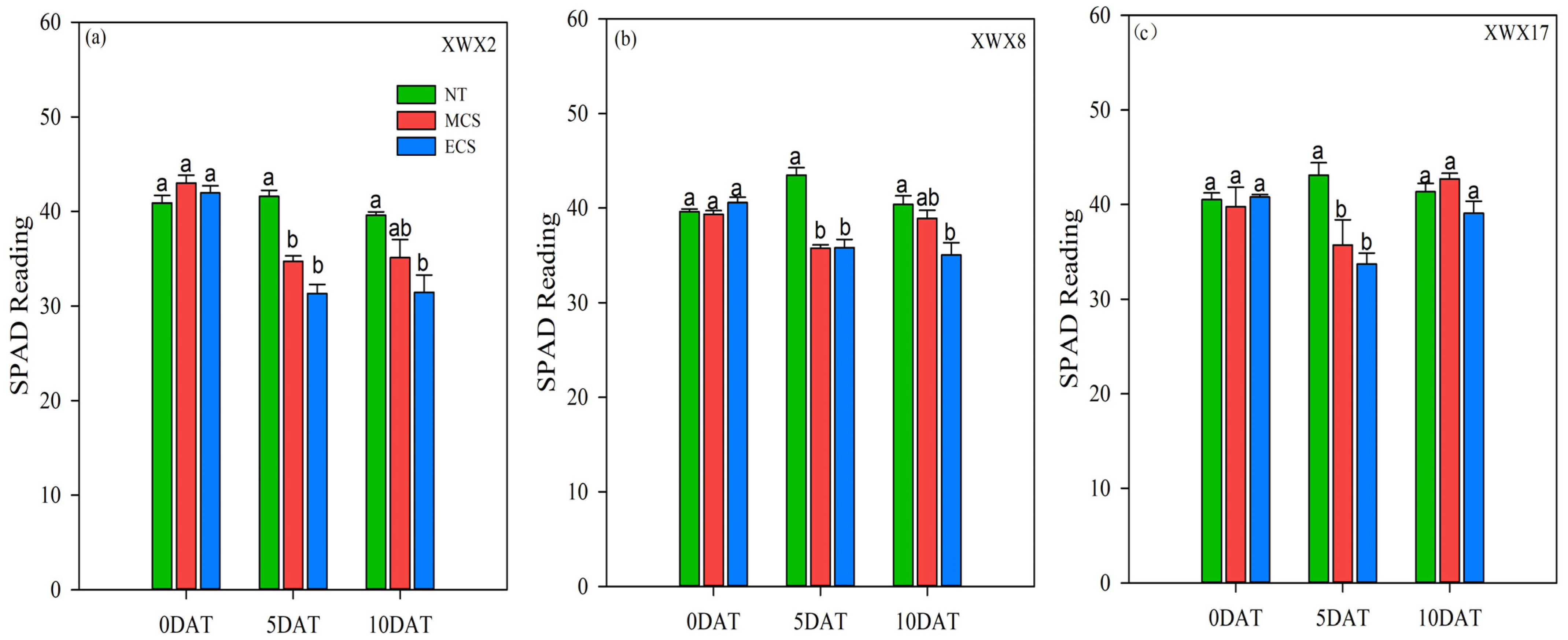
3.5. SPAD of Different Late Rice
3.6. Chlorophyll of Different Late Rices
4. Discussion
4.1. Chilling Stress at Heading Decreased the Grain Filling Percentage via Reducing Spikelet Fertility
4.2. The Variances in Chilling Tolerance among Three Inbred Late Varieties
4.3. Morphological and Physiological Characteristics Underlying the Varietal Differences in Chilling Tolerance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shinada, H.; Iwata, N.; Sato, T.; Fujino, K. Genetical and morphological characterization of cold tolerance at fertilization stage in rice. Breed. Sci. 2013, 63, 197–204. [Google Scholar] [CrossRef]
- Jia, Y.; Zou, D.; Wang, J.; Liu, H.; Inayat, M.A.; Sha, H.; Zheng, H.; Sun, J.; Zhao, H. Effect of low water temperature at reproductive stage on yield and glutamate metabolism of rice (Oryza sativa L.) in China. Field Crop. Res. 2015, 175, 16–25. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Prod Sci. 2001, 4, 90–93. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H., Jr.; Sheehy, J.E.; Thomas, J.M.G. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crop. Res. 2006, 95, 98–411. [Google Scholar] [CrossRef]
- Rang, Z.W.; Jagadish, S.V.K.; Zhou, Q.M.; Craufurd, P.Q.; Heuer, S. Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environ. Exp. Bot. 2011, 70, 58–65. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc Oxide Nanoparticles Alleviate Chilling Stress in Rice (Oryza sativa L.) by Regulating Antioxidative System and Chilling Response Transcription Factors. Molecules 2021, 26, 2196. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, H.; Sonoike, K. Irreversible damage to photosystemi by chilling in the light: Cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 2002, 215, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of Low Temperature on Chlorophyll Biosynthesis and Chloroplast Biogenesis of Rice Seedlings during Greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, Y.; Xiang, J.; Uphoff, N.T.; Pan, X.; Zhu, D. Effects of Low Temperature Stress on Spikelet-Related Parameters during Anthesis in Indica–Japonica Hybrid Rice. Front. Plant Sci. 2017, 8, 1350. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.C.; Fox, K.M.; Williams, R.L.; Fukai, S. Genotypic variation for cold tolerance during reproductive development in rice: Screening with cold air and cold water. Field Crop. Res. 2006, 98, 178–194. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Kesawat, M.S.; Kumar, M.; Kim, S.; Mani, V.; Subramanian, P.; Hahn, B. Lack of the α1,3-fucosyltransferase gene (osfuct) affects anther development and pollen viability in rice. Int. J. Mol. Sci. 2018, 19, 1225. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, X.; Jiang, L.; Li, X.; Yang, S.; Li, Y. Hyperspectral Reflectance Characteristics of Rice Canopies under Changes in Diffuse Radiation Fraction. Remote Sens. 2022, 14, 285. [Google Scholar] [CrossRef]
- Wintermans, J.; De Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochim. Biophys. Acta (BBA)-Biophys. Incl. Photosynth 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, E.; Wang, L.; Li, T.; Jiang, S.; Xiang, H.; Yang, X. Effects of chilling at the booting and flowering stages on rice phenology and yield: A case study in Northeast China. J. Agron. Crop Sci. 2022, 208, 197–208. [Google Scholar] [CrossRef]
- Ali, I.; Tang, L.; Dai, J.; Kang, M.; Mahmood, A.; Wang, W.; Zhu, Y. Responses of Grain Yield and Yield Related Parameters to Post-Heading Low-Temperature Stress in Japonica Rice. Plants 2021, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, D.; Zhang, H.; Meng, C.; Zhang, X.; Hou, J.; Wei, C. Low soil temperature reducing the yield of drip irrigated rice in arid area by influencing anther development and pollination. J. Arid. Land 2019, 11, 419–430. [Google Scholar] [CrossRef]
- Huang, M.; Fang, S.; Shuanglü, S.; Zou, Y. Delayed transplanting reduced grain yield due to low temperature stress at anthesis in machine-transplanted late-season rice. Exp. Agric. 2019, 55, 843–848. [Google Scholar] [CrossRef]
- Siddik, M.A.; Zhang, J.; Chen, J.; Qian, H.; Jiang, Y.; Raheem, A.K.; Zhang, W. Responses of indica rice yield and quality to extreme high and low temperatures during the reproductive period. Eur. J. Agron. 2019, 106, 30–38. [Google Scholar] [CrossRef]
- .Pengyuan, X.; Dongxue, L.; Yuguang, L.; Xiaoli, Q. Research status and countermeasures of rice barrier chilling injury in cold region of china. IOP Conference Series. Earth Environ. Sci. 2018, 199, 022027. [Google Scholar] [CrossRef]
- Cai, C.; Yin, X.; He, S.; Jiang, W.; Si, C.; Struik, P.C.; Luo, W.; Li, G.; Xie, Y.; Xiong, Y.; et al. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 2016, 22, 856–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, J.; Zhang, H.; Meng, C.; Zhang, X.; Wei, C. Low soil temperature inhibits yield of rice under drip irrigation. J. Soil Sci. Plant Nutr. 2019, 19, 228–236. [Google Scholar] [CrossRef]
- Wang, W.; Quan, C.; Zheng, S.; Yu, W.W.; Mo, Y.; Ma, C.; Chen, R. OsPM1 is a positive regulator of rice tolerance to drought stress but a negative regulator of rice tolerance to salt stress. J. Plant Interact. 2021, 16, 213–221. [Google Scholar] [CrossRef]
- Menghao, Z.; He, Y.; Mingqiang, Z.; Ayaz, A.; Xu, S.; Zijun, H.; Zhang, Z. ipa1 improves rice drought tolerance at seedling stage mainly through activating abscisic acid pathway. Plant Cell Rep. 2022, 41, 221–232. [Google Scholar] [CrossRef]
- Han, B.; Wang, J.; Li, Y.; Ma, X.; Jo, S.; Cui, D.; Han, L. Identification of quantitative trait loci associated with drought tolerance traits in rice (Oryza sativa L.) under PEG and field drought stress. Euphytica 2018, 214, 74. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Y.; Chen, C.; Lai, M.; Yen, H.; Yang, C. Physiological and molecular responses of seedlings of an upland rice (‘Tung Lu 3’) to total submergence compared to those of a submergence-tolerant lowland rice (‘FR13A’). Rice 2017, 10, 42. [Google Scholar] [CrossRef]
- Tran, D.X.; Do, T.K. Effects of Exogenous Application of Protocatechuic Acid and Vanillic Acid to Chlorophylls, Phenolics and Antioxidant Enzymes of Rice (Oryza sativa L.) in Submergence. Molecules 2018, 23, 620. [Google Scholar] [CrossRef]
- Yong-Pei, W.; Wang, S.; Yu-Chi, C.; Ho, C.; Yu-Chia, H. Submergence Gene Sub1A Transfer into Drought-Tolerant japonica Rice DT3 Using Marker-Assisted Selection. Int. J. Mol. Sci. 2021, 22, 13365. [Google Scholar] [CrossRef]
- Guonan, F.; Shenglong, Y.; Banpu, R.; Chaolei, L.; Anpeng, Z.; Hongzhen, J.; Qian, Q. Isolation of TSCD11 Gene for Early Chloroplast Development under High Temperature in Rice. Rice 2020, 13, 49. [Google Scholar] [CrossRef]
- Luo, R.; Jiang, H.; Lv, Y.; Hu, S.; Sheng, Z.; Shao, G.; Wei, X. Chlorophyll deficient 3, Encoding a Putative Potassium Efflux Antiporter, Affects Chloroplast Development Under High Temperature Conditions in Rice (Oryza sativa L.). Plant Mol. Biol. Report. 2018, 36, 675–684. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Shujun, O.; Ruci, W.; Wang, Y.; Chengcai, C.; Shanguo, Y. Natural variations of SLG1 confer high-temperature tolerance in indica rice. Nat. Commun. 2020, 11, 5441. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, J.; Fang, J.; Guo, Z.; Lu, S. Down-regulation of S-adenosylmethionine decarboxylase genes results in reduced plant length, pollen viability, and abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2014, 116, 311–322. [Google Scholar] [CrossRef]
- Liu, C.; Schläppi, M.R.; Mao, B.; Wang, W.; Wang, A.; Chu, C. The bZIP 73 transcription factor controls rice cold tolerance at the reproductive stage. Plant Biotechnol. J. 2019, 17, 1834–1849. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Lee, Y.; Kwon, Y.; Kim, J.H.; Ham, T.-H.; Kwon, S.-W.; Lee, J. Genome-wide association study reveals the genetic basis of chilling tolerance in rice at the reproductive stage. Plants 2021, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, W.; Lu, Q.; Huang, J.; Peng, S.; Cui, K. Abnormal anther development leads to lower spikelet fertility in rice (Oryza sativa L.) under high temperature during the panicle initiation stage. BMC Plant Biol. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Koumoto, T.; Saito, N.; Aoki, N.; Iwasaki, T.; Kawai, S.; Yokoi, S.; Shimono, H. Effects of salt and low light intensity during the vegetative stage on susceptibility of rice to male sterility induced by chilling stress during the reproductive stage. Plant Prod. Sci. 2016, 19, 497–507. [Google Scholar] [CrossRef]
- Pereira, D.C.R.; Sperotto, R.A.; Cargnelutti, D.; Adamski, J.M.; de FreitasTerra, T.; Janette, P.F. Avoiding damage and achieving cold tolerance in rice plants. Food Energy Secur. 2013, 2, 96–119. [Google Scholar] [CrossRef]
- Dingkuhn, M.; Sow, A.; Ramantsoanirina, A.; Alpha, B.B.; Manneh, B.; Courtois, B.; .Radanielina, T. Field phenomics for response of a rice diversity panel to ten environments in senegal and madagascar. 2. chilling-induced spikelet sterility. Field Crops Res. 2015, 183, 282–293. [Google Scholar] [CrossRef]
- Xu, D.; Qu, S.; Tucker, M.R.; Zhang, D.; Liang, W.; Shi, J. Ostkpr1 functions in anther cuticle development and pollen wall formation in rice. BMC Plant Biol. 2019, 19, 104. [Google Scholar] [CrossRef]
- Sato, Y.; Masuta, Y.; Saito, K.; Murayama, S.; Ozawa, K. Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep. 2011, 30, 399–406. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Xu, W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the molecular mechanism of anther development under abiotic stresses. Plant Mol. Biol. 2021, 105, 1–10. [Google Scholar] [CrossRef]
- Gan, P.; Liu, F.; Li, R.; Wang, S.; Luo, J. Chloroplasts—Beyond energy capture and carbon fixation: Tuning of photosynthesis in response to chilling stress. Int. J. Mol. Sci. 2019, 20, 5046. [Google Scholar] [CrossRef]
- Sritama, M.; Abhishek, M.; Das, P.; Subhendu, B.; Debapriya, C.; Jolly, C.; Majumder, A.L. A salt-tolerant chloroplastic FBPase from Oryza coarctata confers improved photosynthesis with higher yield and multi-stress tolerance to indica rice. Plant Cell Tissue Organ Cult. 2021, 145, 561–578. [Google Scholar] [CrossRef]
- Wang, W.; Cui, K.; Hu, Q.; Wu, C.; Li, G.; Huang, J.; Peng, S. Response of spikelet water status to high temperature and its relationship with heat tolerance in rice. Crop J. 2020, 9, 1344–1356. [Google Scholar] [CrossRef]
- Li, Z.; Umar Khan, M.; Yan, X.; Mu, D.; Xie, Y.; Waqas, M.; Wu, X.; Letuma, P.; Fang, C.; Lin, W. Deciphering the Molecular Mechanisms of Chilling Tolerance in Lsi1-Overexpressing Rice. Int. J. Mol. Sci. 2022, 23, 4667. [Google Scholar] [CrossRef]
- Jia, M.; Meng, X.; Song, X.; Zhang, D.; Kou, L.; Zhang, J.; Jing, Y.; Liu, G.; Liu, H.; Huang, X.; et al. Chilling-induced phosphorylation of IPA1 by OsSAPK6 activates chilling tolerance responses in rice. Cell Discov. 2022, 8, 71. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Xu, S.; Lyu, M.-J.; Wang, J.; Wang, H.; Zheng, H.; Xin, W.; Liu, J.; Zou, D. OsWRKY115 on qCT7 links to cold tolerance in rice. Theor. Appl. Genet. 2022, 135, 2353–2367. [Google Scholar] [CrossRef]

| Variety Name | Growth Duration (d) | Released Year |
|---|---|---|
| Xiangwanxian2 | 109 | 1988 |
| Xiangwanxian8 | 118 | 1998 |
| Xiangwanxian17 | 117 | 2008 |
| Variety | Temperature Treatments | Panicle Number (No. plant−1) | Spikelet Per Panicle | Grain Weight (g) | Grain Filling Percentage (%) |
|---|---|---|---|---|---|
| XWX2 | NT | 12.2 ± 0.97 a | 120.1 ± 6.05 a | 24.46 ± 0.54 a | 75.13 ± 0.97 a |
| MCS | 11.8 ± 1.80 a | 108.2 ± 8.08 a | 24.30 ± 0.48 a | 65.26 ± 1.34 b | |
| ECS | 11.8 ± 1.43 a | 101.0 ± 2.95 a | 24.24 ± 0.84 a | 51.72 ± 2.81 c | |
| XWX8 | NT | 12.6 ± 0.98 a | 126.0 ± 3.39 a | 27.64 ± 0.45 a | 77.44 ± 2.61 a |
| MCS | 12.2 ± 1.43 a | 123.2 ± 2.91 a | 26.58 ± 0.53 a | 69.87 ± 1.55 b | |
| ECS | 11.8 ± 1.16 a | 114.2 ± 6.82 a | 26.58 ± 1.10 a | 57.96 ± 1.77 c | |
| XWX17 | NT | 13.6 ± 1.57 a | 136.6 ± 5.56 a | 27.76 ± 0.59 a | 81.29 ± 2.73 a |
| MCS | 13.2 ± 1.24 a | 131.0 ± 3.58 a | 27.06 ± 0.64 a | 78.91 ± 1.07 a | |
| ECS | 13.0 ± 1.22 a | 131.6 ± 5.71 a | 27.68 ± 1.22 a | 62.44 ± 4.11 b |
| Variety | Temperature | Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Chlorophyll (a + b) (mg/g) | Chlorophyll (a/b) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0DAT | 5DAT | 10DAT | 0DAT | 5DAT | 10DAT | 0DAT | 5DAT | 10DAT | 0DAT | 5DAT | 10DAT | ||
| XWX2 | NT | 3.82 ± 0.11 a | 3.81 ± 0.16 a | 3.88 ± 0.05 a | 0.81 ± 0.02 a | 0.79 ± 0.01 a | 0.73 ± 0.02 a | 4.63 ± 0.12 a | 4.60 ± 0.15 a | 4.60 ± 0.06 a | 4.69 ± 0.15 a | 4.83 ± 0.23 a | 5.34 ± 0.12 a |
| MCS | 3.80 ± 0.07 a | 3.25 ± 0.11 b | 3.72 ± 0.06 a | 0.82 ± 0.01 a | 0.74 ± 0.01 b | 0.69 ± 0.02 a | 4.62 ± 0.08 a | 3.99 ± 0.1 b | 4.42 ± 0.07 a | 4.66 ± 0.05 a | 4.42 ± 0.23 a b | 5.38 ± 0.16 a | |
| ECS | 3.95 ± 0.13 a | 2.93 ± 0.11 b | 3.71 ± 0.13 a | 0.82 ± 0.02 a | 0.72 ± 0.01 b | 0.68 ± 0.01 a | 4.77 ± 0.14 a | 3.65 ± 0.12 b | 4.39 ± 0.13 a | 4.81 ± 0.07 a | 4.09 ± 0.08 b | 5.46 ± 0.15 a | |
| XWX8 | NT | 3.46 ± 0.09 a | 3.52 ± 0.11 a | 3.32 ± 0.10 a | 0.80 ± 0.04 a | 0.80 ± 0.01 a | 0.74 ± 0.01 a | 4.27 ± 0.12 a | 4.32 ± 0.12 a | 4.06 ± 0.11 a | 4.32 ± 0.08 a | 4.42 ± 0.08 a | 4.48 ± 0.13 a |
| MCS | 3.43 ± 0.06 a | 3.15 ± 0.02 a b | 3.18 ± 0.04 a | 0.81 ± 0.02 a | 0.76 ± 0.01 a b | 0.70 ± 0.01 b | 4.24 ± 0.08 a | 3.91 ± 0.03 b | 3.88 ± 0.04 a | 4.26 ± 0.06 a | 4.15 ± 0.03 a | 4.52 ± 0.12 a | |
| ECS | 3.30 ± 0.09 a | 2.90 ± 0.15 b | 3.07 ± 0.16 a | 0.80 ± 0.02 a | 0.73 ± 0.01 b | 0.66 ± 0.01 c | 4.10 ± 0.09 a | 3.64 ± 0.15 b | 3.73 ± 0.16 a | 4.13 ± 0.19 a | 3.96 ± 0.23 a | 4.67 ± 0.19 a | |
| XWX17 | NT | 3.83 ± 0.11 a | 3.83 ± 0.11 a | 3.74 ± 0.05 a | 0.80 ± 0.01 a | 0.80 ± 0.02 a | 0.74 ± 0.03 a | 4.64 ± 0.12 a | 4.63 ± 0.10 a | 4.48 ± 0.05 a | 4.77 ± 0.09 a | 4.82 ± 0.25 a | 5.07 ± 0.20 a |
| MCS | 3.82 ± 0.09 a | 3.54 ± 0.08 a | 3.66 ± 0.06 a | 0.81 ± 0.01 a | 0.76 ± 0.01 a | 0.72 ± 0.02 a b | 4.63 ± 0.09 a | 4.30 ± 0.09 a | 4.38 ± 0.07 a | 4.74 ± 0.17 a | 4.66 ± 0.03 a | 5.12 ± 0.16 a | |
| ECS | 3.79 ± 0.13 a | 3.20 ± 0.10 b | 3.40 ± 0.05 b | 0.80 ± 0.02 a | 0.75 ± 0.02 a | 0.66 ± 0.01 b | 4.59 ± 0.15 a | 3.95 ± 0.1 b | 4.07 ± 0.06 b | 4.76 ± 0.05 a | 4.27 ± 0.21 a | 5.13 ± 0.08 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Wang, W.; Pu, S.; Shi, W.; Hu, T.; Tang, Q.; Xu, H. Assessing the Genetic Improvement in Inbred Late Rice against Chilling Stress: Consequences for Spikelet Fertility, Pollen Viability and Anther Characteristics. Agronomy 2022, 12, 1894. https://doi.org/10.3390/agronomy12081894
Ren M, Wang W, Pu S, Shi W, Hu T, Tang Q, Xu H. Assessing the Genetic Improvement in Inbred Late Rice against Chilling Stress: Consequences for Spikelet Fertility, Pollen Viability and Anther Characteristics. Agronomy. 2022; 12(8):1894. https://doi.org/10.3390/agronomy12081894
Chicago/Turabian StyleRen, Maofei, Weiqin Wang, Siwei Pu, Wanju Shi, Teng Hu, Qiyuan Tang, and Huaqin Xu. 2022. "Assessing the Genetic Improvement in Inbred Late Rice against Chilling Stress: Consequences for Spikelet Fertility, Pollen Viability and Anther Characteristics" Agronomy 12, no. 8: 1894. https://doi.org/10.3390/agronomy12081894
APA StyleRen, M., Wang, W., Pu, S., Shi, W., Hu, T., Tang, Q., & Xu, H. (2022). Assessing the Genetic Improvement in Inbred Late Rice against Chilling Stress: Consequences for Spikelet Fertility, Pollen Viability and Anther Characteristics. Agronomy, 12(8), 1894. https://doi.org/10.3390/agronomy12081894






