Metabolites, Nutritional Quality and Antioxidant Activity of Red Radish Roots Affected by Gamma Rays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Irradiation Treatments
2.3. Culture Experiment
2.4. Vegetative Growth
2.5. Photosynthetic Pigments Determination
2.6. Nutritional and Metabolite Components of Roots
2.6.1. Vitamin C Content
2.6.2. Anthocyanin Content
2.6.3. Root Extracts
2.6.4. Total Soluble Protein
2.6.5. Total Phenolic Content
2.6.6. Flavonoid Content
2.6.7. Antioxidant Activity
2.6.8. Nutrient Concentrations
2.6.9. Organic Acid Contents
2.6.10. Profile of Amino Acids
2.7. Statistical Analysis
3. Results and Discussion
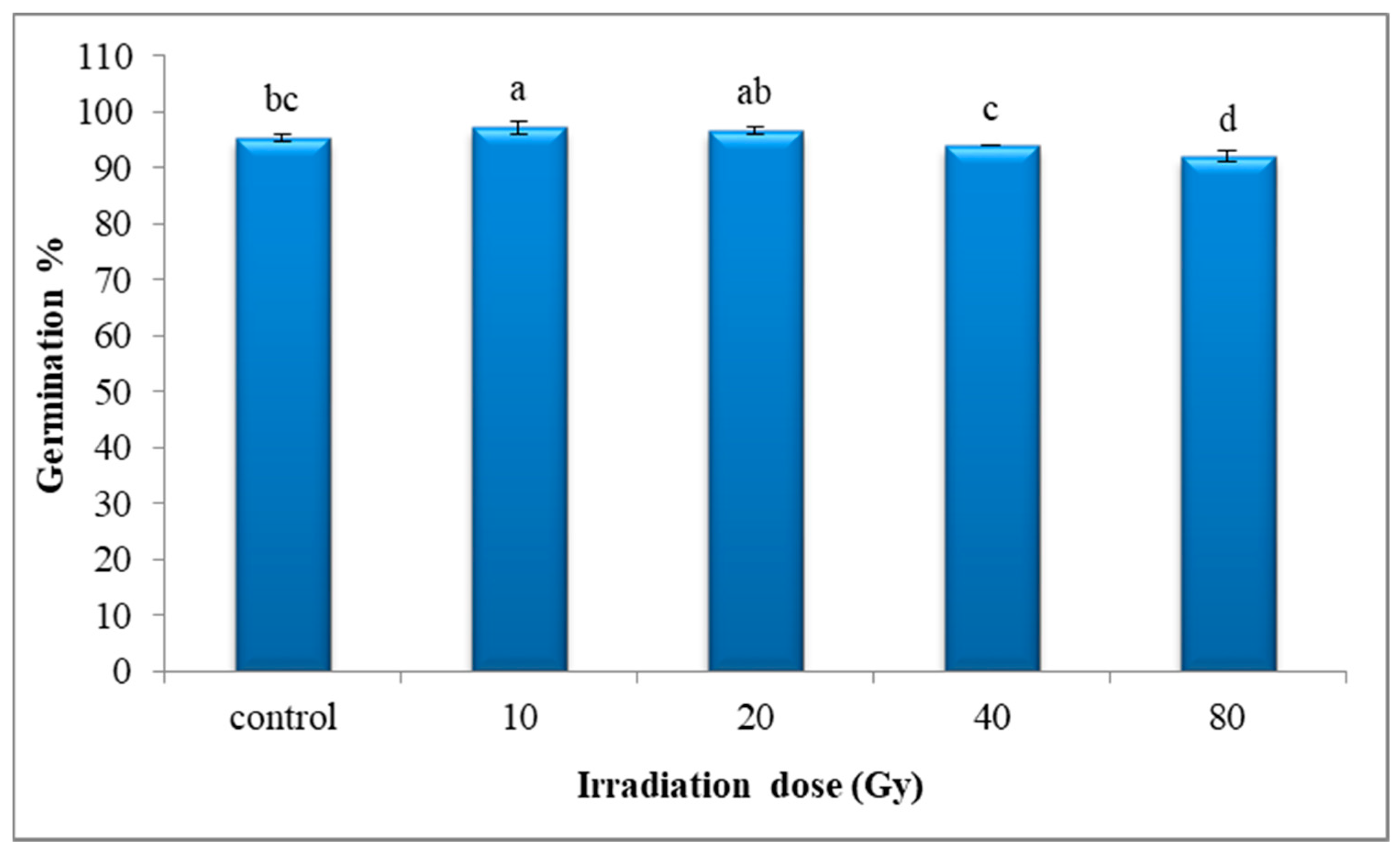
3.1. The Germination Percentage
3.2. Vegetative Growth
3.3. Photosynthetic Pigments
3.4. Nutritional and Metabolite Components of Roots
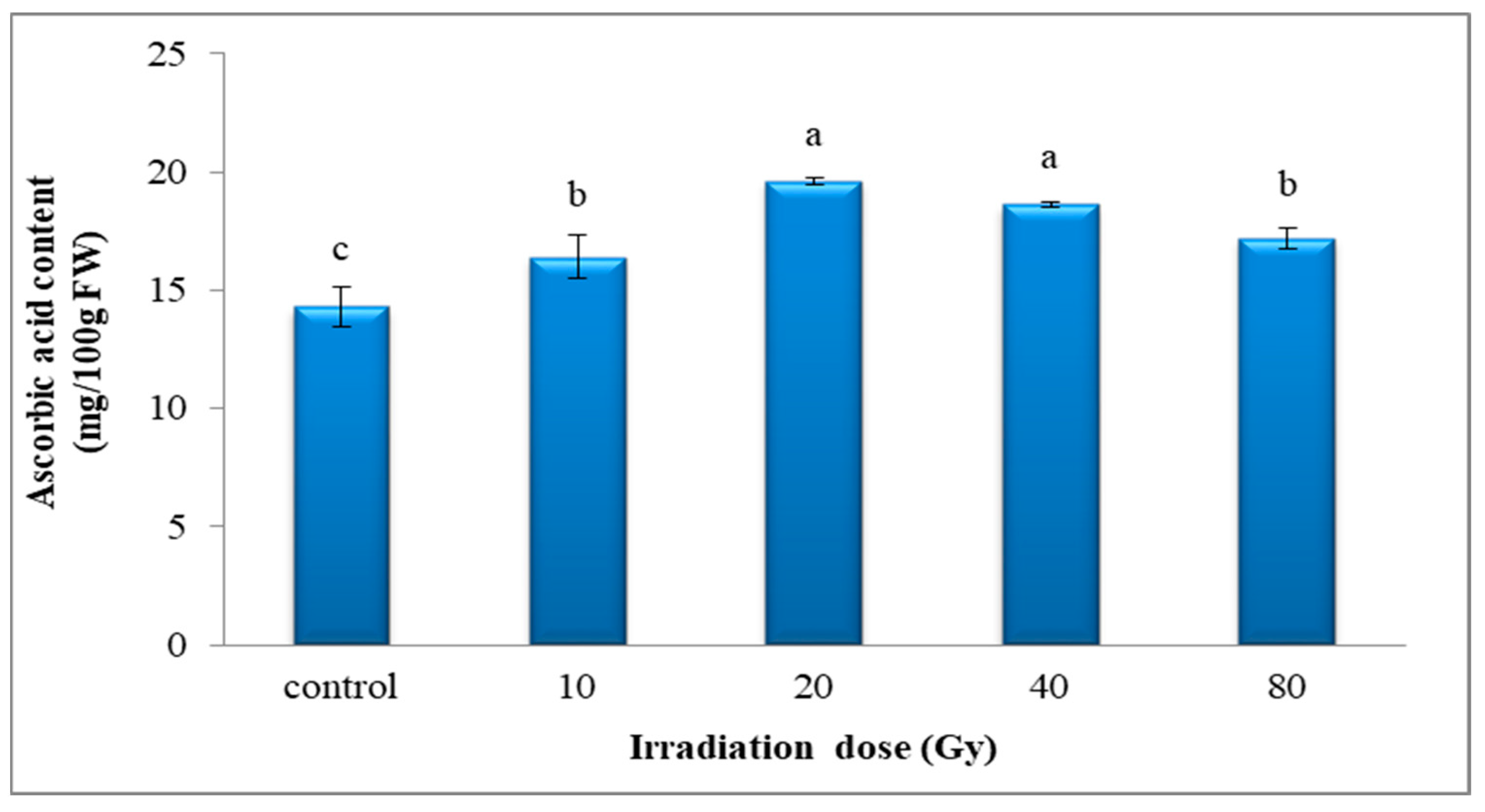
3.4.1. Vitamin C Content (Ascorbic Acid)
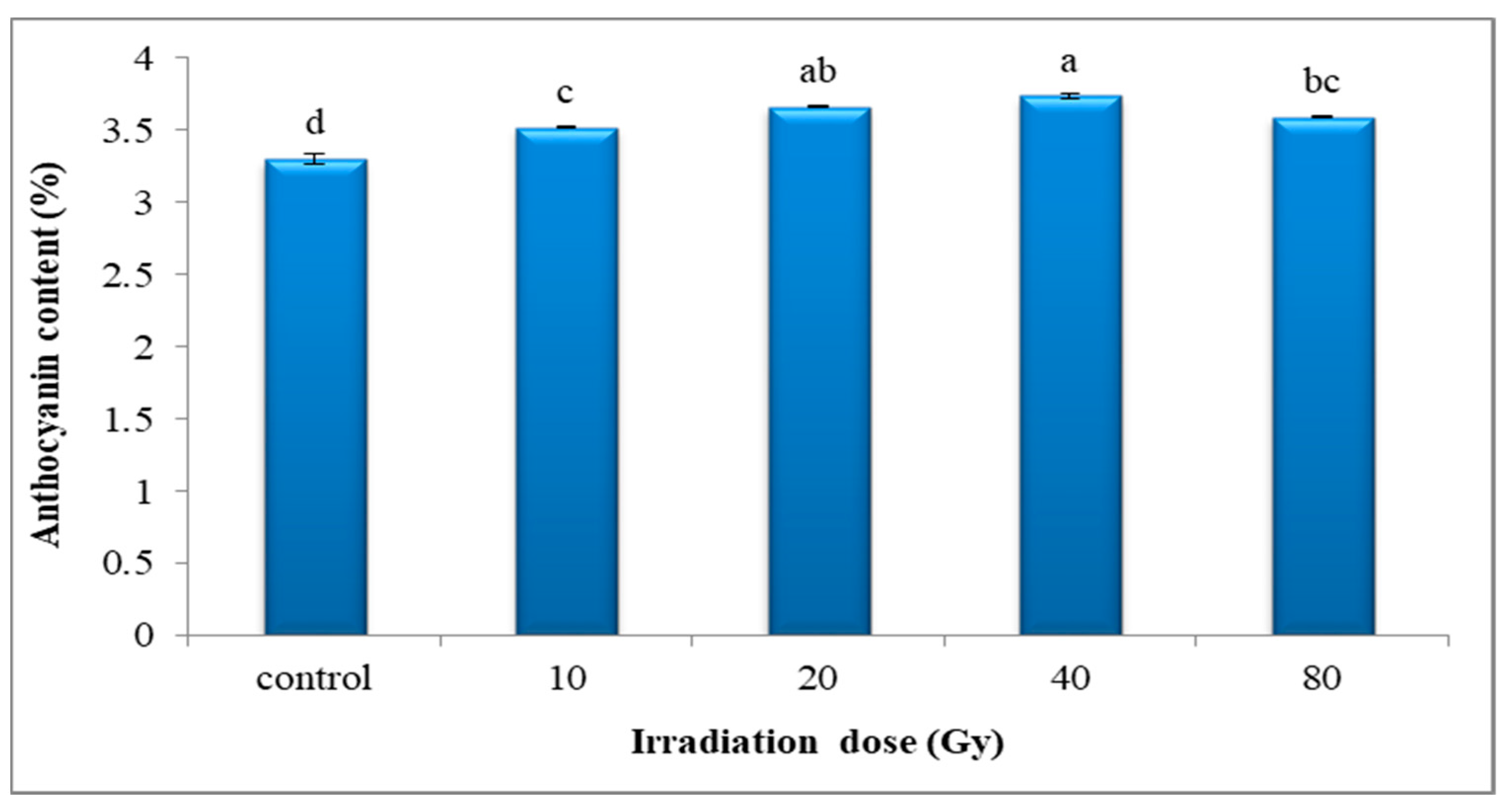
3.4.2. Anthocyanin Content
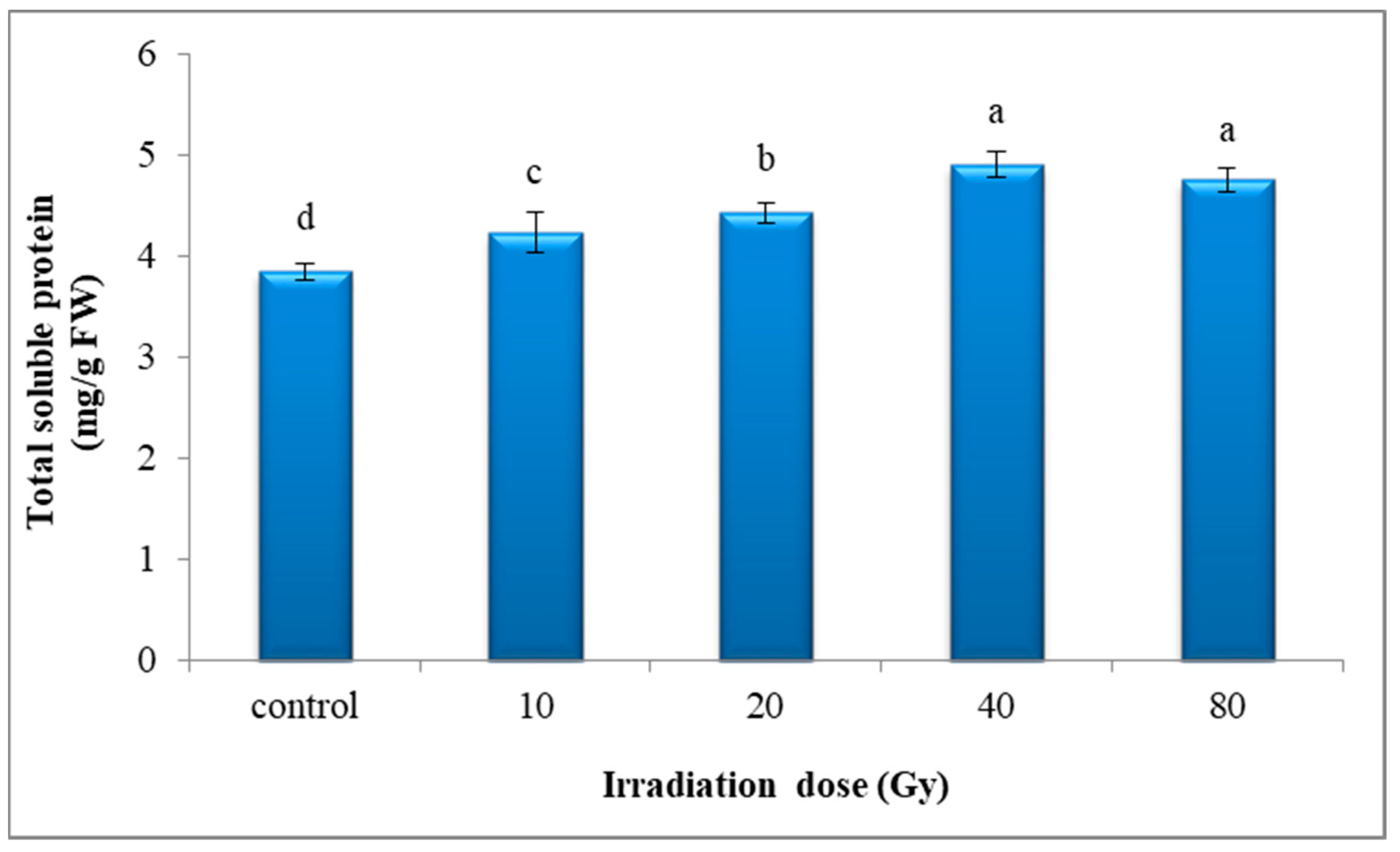
3.4.3. Total Soluble Protein Content
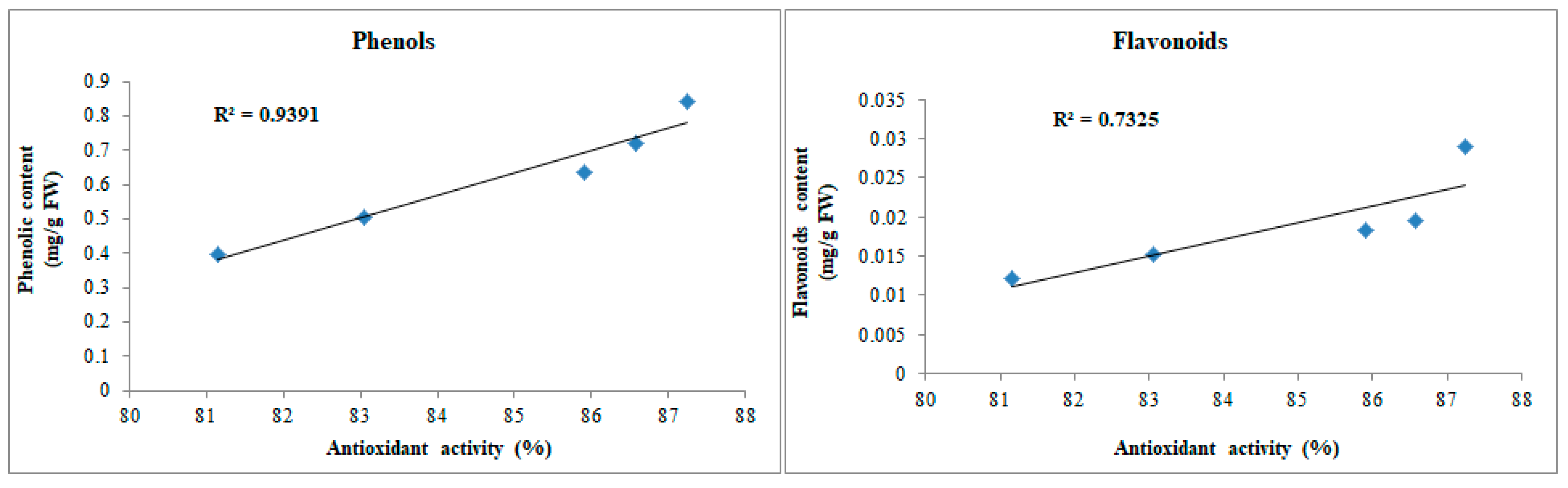
3.4.4. Total Phenolic and Flavonoid Contents
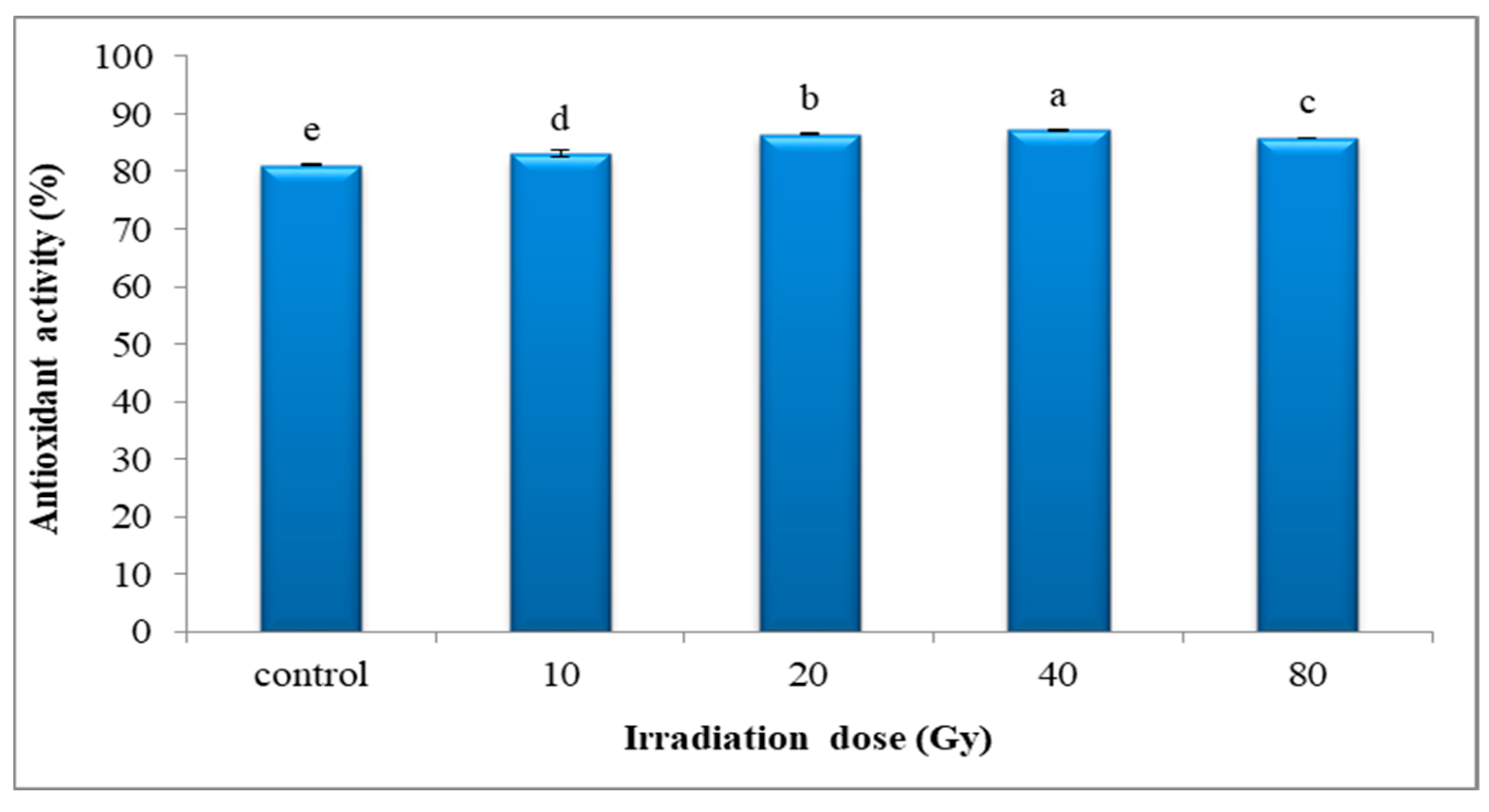
3.4.5. Antioxidant Activity by DPPH Assay
3.4.6. Elemental Composition
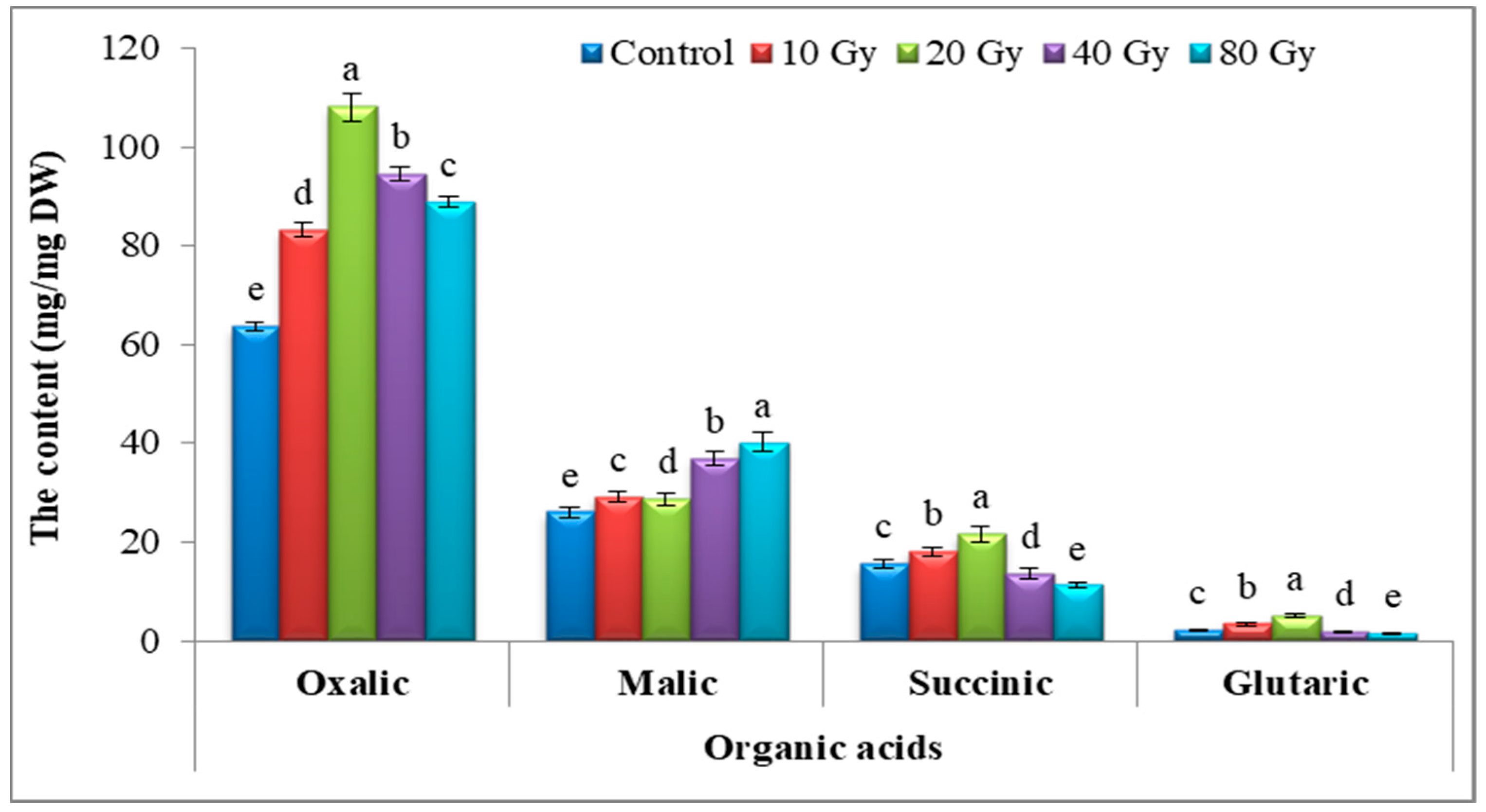
3.4.7. Organic Acid Content
3.4.8. Amino Acid Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soengas, P.; Sotelo, T.; Velasco, P.; Cartea, M.E. Antioxidant properties of brassica vegetables. Funct. Plant Sci. Biotechnol. 2011, 5, 43–55. [Google Scholar]
- Bors, M.D.; Semeniuc, C.A.; Socaci, S.; Vârva, L.; Moldovan, O.; Vlaic, R.; Tofană, M. Total phenolic content and antioxidant capacity of radish as influenced by the variety and vegetative stage. Bull. UASVM Food Sci. Technol. 2015, 72, 77–81. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWTFood Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Lu, G.; Jiashu, C.; Xiaolin, Y.; Xun, X.; Hang, C. Mapping QTLs for root morphological traits in Brassica rapa L. based on AFLP and RAPD markers. J. Appl. Genet. 2008, 49, 23–31. [Google Scholar] [CrossRef]
- Tamura, S.; Tsuji, K.; Yongzhen, P.; Ohnishi-Kameyama, M.; Murakami, N. Six new acylated anthocyanins from red radish (Raphanus sativus). Chem. Pharm. Bull. Tokyo 2010, 58, 1259–1262. [Google Scholar] [CrossRef]
- Jaafar, N.A.; Ahmed, A.S.; Al-Sandooq, D.L. Detection of active compounds in Radish (Raphanus sativus L.) and their various biological effects. Plant Arch. 2020, 20, 1647–1650. [Google Scholar]
- Capus, A.; Monnerat, M.; Ribeiro, L.C.; de Souza, W.; Martins, J.L.; Anna, C. Application of high-content image analysis for quantitatively estimating lipid accumulation in oleaginous yeasts with potential for use in biodiesel production. Bioresour. Technol. 2016, 203, 309–317. [Google Scholar] [CrossRef]
- Jing, P.; Song, L.-H.; Shen, S.-Q.; Zhao, S.-J.; Pang, J.; Qian, B.-J. Characterization of phytochemicals and antioxidant activities of red radish brines during lactic acid fermentation. Molecules 2014, 19, 9675–9688. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Beltagi, H.S.; Rashed, M.M. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Electron. J. Environ. Agric. Food Chem. 2009, 8, 969–983. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, A.A.; Rashed, M.M. Response of antioxidative enzymes to cadmium stress in leaves and roots of radish. Not. Sci. Biol. 2010, 2, 76–82. [Google Scholar] [CrossRef]
- Chen, W.; Karangwa, E.; Yu, J.; Xia, S.; Feng, B.; Zhang, X.; Jia, C. Characterizing red radish pigment off-odor and aroma-active compounds by sensory evaluation, gas chromatography-mass spectrometry/olfactometry and partial least square regression. Food Bioprocess Technol. 2017, 10, 1337–1353. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, S.; Ruan, S.; Sui, Z.; Chen, L.; Jiang, L.; Qian, B. Quantitative studies on structure—ORAC relationships of anthocyanins from eggplant and radish using 3D-QSAR. Food Chem. 2014, 145, 365–371. [Google Scholar] [CrossRef]
- Sakr, M.T.; Ibrahim, H.M.; ElAwady, A.E.; Abo, E.L.; Makarem, A.A. Effect of humic acid, seaweed extract and essential oils as antioxidants on pre-and post-harvest quality of red radish plants. Horticult. Int. J. 2019, 3, 129–138. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods. 2015, 16, 256–264. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A. Changes in non protein thiols, some antioxidant enzymes activity and ultrastructural alteration in radish plant (Raphanus sativus L.) grown under lead toxicity. Not. Bot. Horti. Agrobot. Cluj-Napoca 2010, 38, 76–85. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, O.K.; El-Desouky, W. Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of Rosemary (Rosmarinus officinalis L.) callus culture. Rad. Physics Chem. 2011, 80, 965–973. [Google Scholar] [CrossRef]
- Rezk, A.A.; Al-Khayri, J.M.; Al-Bahrany, A.M.; El-Beltagi, H.S.; Mohamed, H.I. X-ray irradiation changes germination and biochemical analysis of two genotypes of okra (Hibiscus esculentus L.). J. Radiat. Res. Appl. Sci. 2019, 12, 393–402. [Google Scholar] [CrossRef]
- Hanafy, R.S.; Akladious, S.A. Physiological and molecular studies on the effect of gamma radiation in fenugreek (Trigonella foenum-graecum L.) plants. J. Genet. Eng. Biotechnol. 2018, 16, 683–692. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Beltagi, H.E.S. Influence of ionizing irradiation on the antioxidant enzymes of Vicia faba L. Grasas Y Aceites 2010, 61, 288–294. [Google Scholar] [CrossRef]
- Vernon, L.P.; Seely, G.R. The Chlorophylls, Physical, Chemical and Biological Properties; Academic Press: New York, NY, USA; London, UK, 1966. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Officinal Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, Food Composition; Additives; Natural Contaminate, 17th ed.; Official Method 982; Association of Official Analytical Chemists (AOAC): Washington, DC, USA, 2000; Volume 2. [Google Scholar]
- Sobhy, M.; Abdalla, M.; Ammar, S. Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chem. 2009, 112, 595–598. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Methods of Analysis and Quantification of Phenolic Compounds. Food Phenolic, Sources, Chemistry, Effects and Applications; Technomic Publishing Company, Inc.: Lancaster, UK, 1995; p. 287. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolic and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Tech. Metal. 2005, 40, 255–260. [Google Scholar]
- Gulluce, M.; Sokmen, M.; Sahin, F.; Sokmen, A.; Adiguzel, A.; Ozer, H. Biological activities of the essential oil and methanolic extract of Micromeria fruticose (L.) Druce ssp serpyllifolia (Bieb) PH Davis plants from the Eastern Anatolia region of Turkey. J. Sci. Food Agric. 2004, 84, 735–741. [Google Scholar] [CrossRef]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; Food and Agricultural Organisation of the United Nations, FAO Fertilizer and Plant Nutrition Bulletin: Rome, Italy, 2008; p. 219. [Google Scholar]
- Duncan, D.B. Multiple range and multiple ’F’ tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Stagnari, F.; Galieni, A.; D’egidio, S.; Pagnani, G.; Pisante, M. Responses of radish (Raphanus sativus) to drought stress. Annal. Appl. Biol. 2017, 172, 170–186. [Google Scholar] [CrossRef]
- Aly, A.A.; Eliwa, N.E.; AbdEl-Megid, M.H. Stimulating effect of gamma radiation on some active compounds in eggplant fruits. Egypt. J. Rad. Sci. Appl. 2019, 32, 61–73. [Google Scholar] [CrossRef]
- Zanzibar, M.; Sudrajat, D.J. Effect of gamma irradiation on seed germination, storage, and seedling growth of Magnolia champaca L. J. For. Res. 2016, 3, 95–106. [Google Scholar] [CrossRef]
- Waje, C.K.; Park, J.; Kim, G.; Kim, Y.R.; Han, B.; Lee, Y.; Moon, K.; Kwon, J. Effect of irradiation of red radish seeds on the seed viability and functional properties of sprouts. Food Sci. Biotechnol. 2009, 18, 390–395. [Google Scholar]
- De Micco, V.; Arena, C.; Pignalosa, D.; Durante, M. Effects of sparsely and densely ionizing radiation on plants. Radiat. Environ. Biophys. 2011, 50, 1–19. [Google Scholar] [CrossRef]
- Songsri, P.; Jogloy, S.; Holbrook, C.C.; Puangbut, D. Determination of lethal dose and effect of gamma rays on growth and tuber yield of Jerusalem artichoke mutant. SABRAO J. Breed Genet. 2019, 51, 1–11. [Google Scholar]
- Aly, A.A.; El-Desouky, W.; Abou El-Leel, O.F. Micropropagation, phytochemical content and antioxidant activity of gamma-irradiated blackberry (Rubus fruticosus L.) plantlets. In Vitr. Cell. Dev. Biol. Plant 2022, 58, 457–469. [Google Scholar] [CrossRef]
- Aly, A.A.; Eliwa, N.E.; Borik, Z.M.; Safwat, G. Physiological variation of irradiated red radish plants and their phylogenic relationship using SCoT and CDDP markers. Not. Bot. Horti Agrobot. Cluj-Napocaj 2021, 49, 12396. [Google Scholar] [CrossRef]
- Jaipo, N.; Kosiwikul, M.; Panpuang, N.; Prakrajang, K. Low dose gamma radiation effects on seed germination and seedling growth of cucumber and okra. J. Phys. 2019, 1380, 012106. [Google Scholar] [CrossRef]
- Piri, I.; Babayan, M.; Tavassoli, A.; Javaheri, M. The use of gamma irradiation in agriculture. Afr.J. Microbiol. Res. 2011, 5, 5806–5811. [Google Scholar] [CrossRef]
- Strid, A.; Chow, W.S.; Anderson, J.M. Effects of supplementary gamma irradiation on photosynthesis in Pisum sativum. Biochemistry 1990, 1020, 260–268. [Google Scholar]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Jan, S.; Parween, T.; Hameed, R.; Siddiqi, T.O.; Mahmooduzzafar. Effects of presowing gamma irradiation on the photosynthetic pigments, sugar content and carbon gain of Cullen corylifolium (L.) Medik. Chil. J. Agric. Res. 2013, 73, 345–350. [Google Scholar] [CrossRef]
- Marcu, D.; Cristea, V.; Daraban, L. Dose-dependent effects of gamma radiation on lettuce (Lactuca sativa var. capitata) seedlings. Int. J. Radiat. Biology. 2013, 89, 219–223. [Google Scholar] [CrossRef]
- Davey, M.W.; Van, M.M.; Inz, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid, chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Dietary vitamin C in human health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A. Biosynthesis of phenolic compounds and water soluble vitamins in culantro (Eryngium foetidum L.) plantlets as affected by low doses of gamma irradiation. An. Univ. Din Oradea Fasc. Biol. 2010, 2, 356–361. [Google Scholar]
- Kiong, A.L.P.; Lai, A.G.; Hussein, S.; Harun, A.R. Physiological responses of Orthosiphon Stamineus plantlets to gamma irradiation. Am. -Eurasian J. Sustain. Agric. 2008, 2, 135–149. [Google Scholar]
- Sanni, T.A.; Ogundele, J.O.; Ogunbusola, E.M.; Oladimeji, O. Effect of Gamma Irradiation on Mineral, Vitamins and Cooking Properties of Sorrel (Hibiscus Sabdariffa L.) Seeds. In Proceedings of the 2nd International Conference on Chemical, Biological, and Environmental Sciences (ICCBES’15), Dubai, United Arab Emirates, 20–21 May 2015. [Google Scholar]
- Bandekar, J.R.; Dhokane, V.S.; Shashidhar, R.; Hajare, S.; Saroj, S.; Sharma, A. Use of irradiation to ensure hygienic quality of fresh, pre-cut fruits and vegetables and other minimally processed foods of plant origin. In Proceedings of the a Final Research Coordination Meeting, Islamabad, Pakistan, 22–30 July 2005; The Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture. International Atomic Energy Agency: Vienna, Austria, 2006; pp. 170–187. [Google Scholar]
- Sajilata, M.G.; Singhal, R.S. Effect of irradiation and storage on the antioxidative activity of cashew nuts. Radiat. Phys. Chem. 2006, 75, 297–300. [Google Scholar] [CrossRef]
- Park, C.H.; Baskar, T.B.; Park, S.; Kim, S.; Arasu, M.V.; Al-Dhabi, N.A.; Kim, J.K.; Park, S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus). Molecules 2016, 21, 157. [Google Scholar] [CrossRef]
- Lee, D.S.; Jeon, D.S.; Park, S.G.; Arasu, M.V.; Al-Dhabi, N.A.; Kim, S.C.; Kim, S.J. Effect of cold storage on the contents of glucosinolates in Chinese cabbage (Brassica rapa L. ssp. pekinensis). South Ind. J. Biol. Sci. 2015, 1, 38–42. [Google Scholar] [CrossRef]
- Mohajer, S.; Taha, R.M.; Lay, M.M.; Esmaeili, A.K.; Khalili, M. Stimulatory effects of gamma irradiation on phytochemical properties, mitotic behavior, and nutritional composition of Sainfoin (Onobrychis viciifolia Scop.). Sci. World J. 2014, 2014, 854093. [Google Scholar] [CrossRef]
- Kim, J.K.; Bong, S.J.; Park, S.U. Amino Acids Content in Different Cultivars of Young Radish (Raphanus sativus). Asian J. Chem. 2017, 29, 1427–1430. [Google Scholar] [CrossRef]
- Al-Rumaih, M.M.; Al-Rumaih, M.M. Influence of ionizing radiation on antioxidant enzymes in three species of Trigonella. Am. J. Environ. Sci. 2008, 4, 151–156. [Google Scholar] [CrossRef]
- Maity, J.P.; Chakraborty, A.; Saha, A.; Santra, S.C.; Chanda, S. Radiation induced effects on some common storage edible seeds in India infested with surface microflora. Radiat. Phys. Chem. 2004, 71, 1065–1072. [Google Scholar] [CrossRef]
- Hieng, B.; Ugrinovic, K.; Sustar-vozlic, J.; Kidric, M. Different classes of proteases are involved in the response to drought of Phaseolus vulgaris L. cultivars differing in sensitivity. J. Plant Physiol. 2004, 161, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.M.R.; Rashed, M.M.; Mahmoud, E.A.; El-Beltagi, H.S. Effect of gamma radiation on protein profile, protein fraction and solubility of three oil seeds. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 90–98. [Google Scholar] [CrossRef]
- Yusuf, E.; Tkacz, K.; Turkiewicz, I.P.; Wojdyło, A.; Nowicka, P. Analysis of chemical compounds’ content in different varieties of carrots, including qualification and quantification of sugars, organic acids, minerals, and bioactive compounds by UPLC. Eur. Food Res. Technol. 2021, 247, 3053–3062. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitr. Cell. Dev. Biol. Plant 2022. [Google Scholar] [CrossRef]
- Aly, A.A.; Mohamed, A.A. Natural antioxidants in maize callus tissue as enhanced by gamma irradiation. Egypt J. Radiat. Sci. Appl. 2005, 18, 221–236. [Google Scholar]
- Variyar, P.S.; Limaye, A.; Sharma, A. Radiation-induced enhancement of antioxidant contents of soybean (Glycine max Merrill). J. Agric. Food Chem. 2004, 52, 3385–3388. [Google Scholar] [CrossRef]
- Wei, X.; Liu, F.; Chen, C.; Ma, F.; Li, M. The Malus domestica sugar transporter gene family, identifications based on genome and expression profiling related to the accumulation of fruit sugars. Front. Plant Sci. 2014, 5, 569. [Google Scholar] [CrossRef]
- Fan, X.; Toivonen, P.M.A.; Rajkowski, K.T.; Sokorai, K.J.B. Warm water treatment in combination with modified atmosphere packaging reduces undesirable effects of irradiation on the quality of fresh-cut iceberg lettuce. J. Agric. Food Chem. 2003, 51, 1231–1236. [Google Scholar] [CrossRef]
- Gitz, D.C.; Lui-Gitz, L.; McClure, J.W.; Huerta, A.J. Effects of a PAL inhibitor on phenolic accumulation and UV-B tolerance in Spirodela intermedia (Koch.). J. Exp. Bot. 2004, 55, 919–927. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, J.H.; Kim, J.K.; Kim, D.H.; Yook, H.S.; Byun, M.W. Combined effects of irradiation and modified atmosphere packaging on minimally processed Chinese cabbage (Brassica rapa L.). Food Chem. 2005, 89, 589–597. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, Q. Effect of lanthanum on the flavonoids in the soybean seeding under ultraviolet-B stress, II effect on content of flavonoids. J. Agro-Environ. Sci. 2008, 27, 462–466. [Google Scholar]
- Taheri, S.; Abdullah, T.L.; Karimi, E.; Oskoueian, E.; Ebrahimi, M. Antioxidant capacities and total phenolic contents enhancement with acute gamma irradiation in Curcuma alismatifolia (Zingiberaceae) leaves. Int. J. Mol. Sci. 2014, 15, 13077–13090. [Google Scholar] [CrossRef] [PubMed]
- Said, A.H.A.; Sarhan, A.M.Z.; Abou Dahab, A.D.M.; Abou-Zeid, E.N.; Ali, M.S.; Naguib, N.Y. Bio-fertilizer and gamma radiation influencing flavonoids content at different parts of dill herb. Int. J. Life Sci. Eng. 2015, 1, 145–149. [Google Scholar]
- Karaca, H.; Velioglu, Y.S. Effects of ozone treatments on microbial quality and some chemical properties of lettuce, spinach, and parsley. Postharvest Biol. Technol. 2014, 88, 46–53. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Baltrusaityte, V.; Venskutonis, P.R.; Ceksteryt, V. Radical scavenging activity of different floral origin honey and bee bread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Vijayarengan, P.; Uthayam, M.C. Changes in nutrients content of Radish (Raphanus sativus) under copper toxicity. Int. J. Adv. Res. Biol. Sci. 2017, 4, 88–93. [Google Scholar] [CrossRef]
- Osman, A.; Rady, M.M. Ameliorative effects of sulphur and humic acid on the growth, anti-oxidant levels and yields of and pea (Pisum sativum L.) plants grown in reclaimed saline soil. J. Hortic. Sci. Biotechnol. 2012, 87, 626–632. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Pérez Gutiérrez, R.M.; Perez, R.L. Raphanus sativus (Radish), Their Chemistry and Biology. Sci. World J. 2004, 4, 811–837. [Google Scholar] [CrossRef]
- Pereiraa, C.; Calhelha, R.C.; Antonio, A.L.; Queiroz, M.J.R.P.; Barros, L.; Ferreira, I.C.F.R. Effects of gamma radiation on chemical and antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of borututu. Innov. Food Sci. Emerg. 2014, 26, 271–277. [Google Scholar] [CrossRef]
- Kmiecik, W.; Słupski, J.; Lisiewska, Z. Comparison of amino acid content and protein quality in raw broccoli and in broccoli after technological and culinary processing. J. Food Process. Preserv. 2010, 34, 639–652. [Google Scholar] [CrossRef]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics, and amino acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef]
- Moemen, A.M.E. Effect of Gamma Rays and Salinity on Growth and Chemical Composition of Ambrosia maritima L. Plant. Master’s Thesis, Cairo University, Giza, Egypt, 2021; 159p. [Google Scholar]
- Aly, A.A.; Maraei, R.W.; Ayadi, S. Some biochemical changes in two Egyptian bread wheat cultivars in response to gamma irradiation and salt stress. Bulg. J. Agric. Sci. 2018, 24, 50–59. [Google Scholar]
- Afify, A.E.M.M.; El-Beltagi, H.S. Effect of insecticide cyanophos on liver function in adult male rats. Fresenius Environ. Bull. 2011, 20, 1084–1088. [Google Scholar]
- Kobeasy, M.I.; El-Beltagi, H.S.; El-Shazly, M.A.; Khattab, E.A.H. Induction of resistance in Arachis hypogaea L. against Peanut mottle virus by nitric oxide and salicylic acid. Physiol. Mol. Plant Pathol. 2011, 76, 112–118. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ali, M.R.; Ramadan, K.M.A.; Anwar, R.; Shalaby, T.A.; Rezk, A.A.; El-Ganainy, S.M.; Mahmoud, S.F.; Alkafafy, M.; El-Mogy, M.M. Exogenous postharvest application of calcium chloride and salicylic acid to maintain the quality of broccoli florets. Plants 2022, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Maraei, R.W.; Louis, Y.A.; Safwat, G. Assessment of irradiated TiO2 nanoparticles on the growth and nutritional components of broccoli. Not. Bot. Horti. Agrobot. 2021, 49, 12397. [Google Scholar] [CrossRef]


| A. Physical Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sand % | Silt % | Clay % | Texture | ||||||
| 58.4 | 18.2 | 23.4 | Sandy clay loam | ||||||
| B. Chemical Characteristics | |||||||||
| E.C. (ds/m) | pH | Cations (meq/L) | Anions (meq/L) | ||||||
| Ca2+ | Mg2+ | K+ | Na+ | CO32− | HCO3− | Cl− | SO42− | ||
| 1.8 | 7.31 | 10.1 | 3.0 | 0.41 | 9.34 | 1.2 | 4.55 | 7.5 | 9.6 |
| Irradiation Dose Levels (Gy) | Plant Height (cm) | Leaves No./Plant | Leaf Length (cm) | Leaf Width (cm) |
|---|---|---|---|---|
| Control | 23.35 ± 0.41 d | 7.80 ± 0.08 b | 13.59 ± 0.13 e | 7.53 ± 0.03 e |
| 10 | 32.65 ± 0.53 a | 7.81 ± 0.06 b | 14.3 ± 0.11 d | 7.84 ± 0.07 d |
| 20 | 29.13 ± 0.59 b | 8.04 ± 0.05 a | 15.15 ± 0.18 c | 8.52 ± 0.04 c |
| 40 | 29.10 ± 0.45 b | 8.08 ± 0.04 a | 15.90 ± 0.14 b | 8.70 ± 0.05 b |
| 80 | 27.00 ± 0.38 c | 8.02 ± 0.03 a | 16.41 ± 0.16 a | 9.04 ± 0.08 a |
| Irradiation Dose Levels (Gy) | Root Length (cm) | Root Diameter (cm) | Root Size (cm3) | Root Weight (g) |
|---|---|---|---|---|
| Control | 14.75 ± 0.05 e | 5.01 ± 0.02 c | 67.50 ± 0.43 d | 62.96 ± 0.45 e |
| 10 | 14.95 ± 0.06 d | 5.03 ± 0.01 c | 69.00 ± 0.48 c | 66.92 ± 0.58 d |
| 20 | 17.51 ± 0.15 a | 5.45 ± 0.04 a | 85.25 ± 0.67 a | 78.12 ± 0.79 a |
| 40 | 15.71 ± 0.10 b | 5.14± 0.03 b | 85.00 ± 0.65 a | 71.55 ± 0.67 b |
| 80 | 15.35 ± 0.09 c | 5.02 ± 0.02 c | 73.50 ± 0.46 b | 69.22 ± 0.51 c |
| Irradiation Dose Levels (Gy) | Concentration (%) | Concentration (ppm) | ||||
|---|---|---|---|---|---|---|
| N | K | S | P | Ca | Mg | |
| Control | 1.44 ± 0.03 d | 2.18 ± 0.03 e | 0.253 ± 0.16 e | 1245.8 ± 2.58 e | 2115.1 ± 8.61 c | 2249.9 ± 7.49 c |
| 10 | 1.52 ± 0.02 c | 2.28 ± 0.02 d | 0.363 ± 0.11 d | 1340.6 ± 3.64 d | 1949.6 ± 4.35 d | 2159.4 ± 6.42 d |
| 20 | 1.78 ± 0.05 a | 2.40 ± 0.03 c | 0.432 ± 0.29 b | 1787.5 ± 4.87 b | 2200.3 ± 7.95 b | 2414.2 ± 8.61 a |
| 40 | 1.59 ± 0.04 b | 2.88 ± 0.05 a | 0.652 ± 0.35 a | 1549.6 ± 4.56 c | 2115.8 ± 7.51 c | 2332.3 ± 7.26 b |
| 80 | 1.33 ± 0.03 e | 2.68 ± 0.06 b | 0.381 ± 0.12 c | 1942.5 ± 5.25 a | 2346.8 ± 8.29 a | 1983.6 ± 5.48 e |
| Amino Acid | Irradiation Dose Levels (Gy) | ||||
|---|---|---|---|---|---|
| Control | 10 | 20 | 40 | 80 | |
| Aspartic | 8 ± 0.15 e | 9.5 ± 0.21 d | 9.82 ± 0.14 c | 10.08 ± 0.10 b | 10.21 ± 0.09 a |
| Glutamic acid | 3.05 ± 0.11 e | 3.59 ± 0.15 d | 3.98 ± 0.26 c | 4.59 ± 0.22 b | 5.43 ± 0.32 a |
| Serine | 0.7 ± 0.04 d | 0.7 ± 0.03 d | 0.78 ± 0.02 c | 0.99 ± 0.03 b | 1.05 ± 0.02 a |
| Histidine | 11.38 ± 0.10 a | 11.14 ± 0.03 c | 11.19 ± 0.01 b | 11.16 ± 0.02 c | 11.16 ± 0.02 c |
| Glycine | 0.95 ± 0.03 e | 1.03 ± 0.04 d | 1.23 ± 0.08 c | 1.35 ± 0.06 b | 1.43 ± 0.04 a |
| Threonine | 0.27 ± 0.01 d | 0.37 ± 0.04 b | 0.37 ± 0.05 b | 0.47 ± 0.02 b | 0.5 ± 0.02 a |
| Arginine | 1.3 ± 0.09 d | 1.48 ± 0.08 c | 1.63 ± 0.03 b | 1.71 ± 0.05 a | 1.69 ± 0.04 a |
| Alanine | 0.79 ± 0.01 c | 0.82 ± 0.04 c | 0.89 ± 0.02 b | 1.09 ± 0.04 a | 1.1 ± 0.02 a |
| Tyrosine | 0.31 ± 0.02 c | 0.36 ± 0.01 a | 0.33 ± 0.01 bc | 0.34 ± 0.01 b | 0.34 ± 0.01 b |
| Valine | 1.48 ± 0.02 e | 1.56 ± 0.05 d | 1.79 ± 0.03 c | 1.84 ± 0.04 b | 2 ± 0.11 a |
| Cysteine | 0 | 0 | 0 | 0 | 0 |
| Methionine | 1.77 ± 0.02 e | 1.84 ± 0.04 d | 1.9 ± 0.03 c | 2.13 ± 0.01 b | 2.16 ± 0.02 a |
| Phenylalanine | 2.84 ± 0.01 c | 2.78 ± 0.02 d | 2.87 ± 0.02 b | 2.9 ± 0.01 a | 2.88 ± 0.01 b |
| Isoleucine | 0.62 ± 0.01 e | 0.66 ± 0.02 d | 0.72 ± 0.03 c | 0.87 ± 0.02 a | 0.84 ± 0.01 b |
| Leucine | 1.91 ± 0.04 e | 2.03 ± 0.06 d | 2.23 ± 0.03 c | 2.37 ± 0.05 a | 2.31 ± 0.04 b |
| Lysine | 0.61 ± 0.07 a | 0.51 ± 0.03 b | 0.45 ± 0.02 c | 0.41 ± 0.01 d | 0.39 ± 0.02 d |
| Proline | 50.2 ± 0.35 b | 48.23 ± 0.26 c | 47 ± 0.31 d | 52.8 ± 0.40 a | 45.55 ± 0.28 e |
| Total sulfur AA | 1.77 ± 0.02 e | 1.84 ± 0.03 d | 1.9 ± 0.02 c | 2.13 ± 0.01 b | 2.16 ± 0.02 a |
| Total aromatic AA | 3.15 ± 0.02 b | 3.14 ± 0.01 b | 3.2 ± 0.01 a | 3.24 ± 0.01 a | 3.22 ± 0.02 a |
| Total essential AA | 10.8 ± 0.13 d | 11.23 ± 0.20 c | 11.96 ± 0.34 b | 12.7 ± 0.16 a | 12.77 ± 0.18 a |
| Total unessential AA | 75.38 ± 0.05 d | 75.37 ± 0.03 d | 75.22 ± 0.05 c | 82.4 ± 0.19 a | 76.27 ± 0.24 b |
| Total | 86.18 ± 0.21 e | 86.6 ± 0.33 d | 87.18 ± 0.37 c | 95.1 ± 0.43 a | 89.04 ± 0.40 b |
| GLS precursors | 4.92 ± 0.02 d | 4.98 ± 0.01 c | 5.1 ± 0.01 b | 5.37 ± 0.06 a | 5.38 ± 0.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; Maraei, R.W.; Shalaby, T.A.; Aly, A.A. Metabolites, Nutritional Quality and Antioxidant Activity of Red Radish Roots Affected by Gamma Rays. Agronomy 2022, 12, 1916. https://doi.org/10.3390/agronomy12081916
El-Beltagi HS, Maraei RW, Shalaby TA, Aly AA. Metabolites, Nutritional Quality and Antioxidant Activity of Red Radish Roots Affected by Gamma Rays. Agronomy. 2022; 12(8):1916. https://doi.org/10.3390/agronomy12081916
Chicago/Turabian StyleEl-Beltagi, Hossam S., Rabab W. Maraei, Tarek A. Shalaby, and Amina A. Aly. 2022. "Metabolites, Nutritional Quality and Antioxidant Activity of Red Radish Roots Affected by Gamma Rays" Agronomy 12, no. 8: 1916. https://doi.org/10.3390/agronomy12081916
APA StyleEl-Beltagi, H. S., Maraei, R. W., Shalaby, T. A., & Aly, A. A. (2022). Metabolites, Nutritional Quality and Antioxidant Activity of Red Radish Roots Affected by Gamma Rays. Agronomy, 12(8), 1916. https://doi.org/10.3390/agronomy12081916









