Microbes, Dodonaea viscosa and Chlorantraniliprole as Components of Helicoverpa armigera IPM Program: A Three Region Open-Field Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Crop Establishment
2.2. Beauveria bassiana
2.3. Nucleopolyhedrovirus
2.4. Dodonaea viscosa
2.5. Chlorotaniliprole
2.6. Field Trials
2.7. Population Density Monitoring
2.8. Fruit Damage Assessment
2.9. Yield Evaluation and Cost-Benefit Analysis
2.10. Statistical Analysis
3. Results
3.1. Larval Population Densities
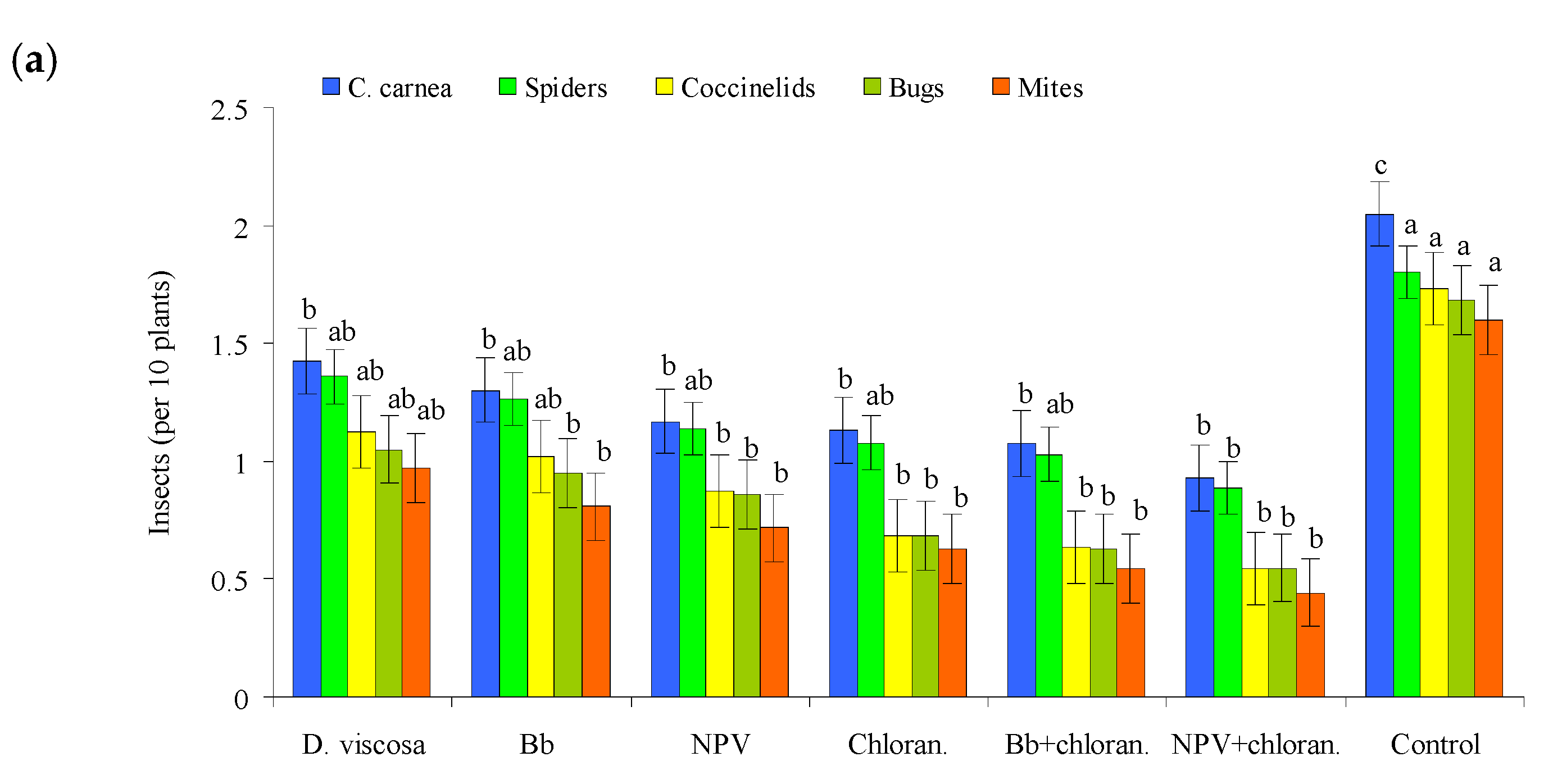
3.2. Effects on Non-Target Natural Enemies
3.3. Fruit Damage
3.4. Fruit Yield and Cost-Benefit Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirza, I. Tomato Paste Plant to be Set Up at Killa Saifullah. Available online: http://www.pakissan.com/english/news/newsDetail.php?newsid=15041 (accessed on 31 August 2007).
- FAO. Statistics, Food and Agriculture of United Nations. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor (accessed on 25 December 2012).
- Saleem, M.Y.; Asghar, M.; Iqbal, Q. Augmented analysis for yield and some yield components in tomato (Lycopersicon esculentum Mill.). Pak. J. Bot. 2013, 45, 215–218. [Google Scholar]
- Sajjad, M.; Ashfaq, M.; Suhail, A.; Akhtar, S. Screening of tomato genotypes for resistance to tomato fruit borer (Helicoverpa armigera Hubner) in Pakistan. Pak. J. Agric. Sci. 2011, 48, 49–52. [Google Scholar]
- Dhandapani, N.; Shekhar, U.; Murugan, M. Bio-intensive pest management (BIPM) in major vegetable crops: An Indian perspective. Food Agric. Environ. 2003, 1, 333–339. [Google Scholar]
- Selvanarayanan, V.; Narayanasamy, P. Factors of resistance in tomato accessions against the fruit worm, Helicoverpa armigera (Hubner). Crop Prot. 2006, 25, 1075–1079. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Y. Control of pod borers on pigeonpea. Indian J. Entomol. 2001, 63, 356–359. [Google Scholar]
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Bhatnagar, V.S.; Davies, J.C. Cropping Entomology, Progress Report. 1977–1978; ICRISAT: Hyderabad, India, 1978; p. 30. [Google Scholar]
- Mohyuddin, A.I. Distribution and economic importance of Heliothis spp. In Pakistan and Their Natural Enemies and Host Plants, Proceedings of the Workshop on Biological Control of Heliothis: Increasing the Effectiveness of Natural Enemies, New Delhi, India, 11–15 November 1985; King, E.G., Jackson, R.D., Eds.; Far Eastern Regional Research Office, United States Department of Agriculture: New Delhi, India, 1989; pp. 229–240. [Google Scholar]
- Reed, W.; Pawar, C.S. Heliothis: A Global Problem. In Proceedings of the International Workshop on Heliothis Management, Patancheru, India, 15–20 November 1982; Reed, W., Kumble, V., Eds.; ICRISAT Centre: Patancheru, Andhra Pradesh, India, 1982; pp. 9–14. [Google Scholar]
- Saleem, M.; Younas, M. Host plants, and nature and extent of damage of Heliothis armigera (Hb.). Pak. J. Agric. Res. 1982, 3, 54–58. [Google Scholar]
- Norris, R.F. Ecological bases of interactions between weeds and organisms in other pest categories. Weed Sci. 2005, 53, 909–913. [Google Scholar] [CrossRef]
- Wang, Q.; Rui, C.; Wang, C.L.; Nahiyoon, S.A.; Huang, W.; Zhu, J.; Ji, X.; Yang, W.; Yuan, H.; Cui, L. Field-evolved resistance to 11 insecticides and the mechanisms involved in Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2021, 77, 5086–5095. [Google Scholar] [CrossRef]
- Bai, L.S.; Zhao, C.X.; Xu, J.J.; Feng, C.; Li, Y.Q.; Dong, Y.L.; Ma, Z.Q. Identification and biochemical characterization of carboxylesterase 001G associated with insecticide detoxification in Helicoverpa armigera. Pestic. Biochem. Physiol. 2019, 157, 69–79. [Google Scholar] [CrossRef]
- Bird, L.J. Pyrethroid and carbamate resistance in Australian Helicoverpa armigera (Lepidoptera: Noctuidae) from 2008 to 2015: What has changed since the introduction of Bt cotton? Bull. Entomol. Res. 2018, 108, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Yan, R.; Wu, S.; Wu, Y.; Yang, Y. Reverse genetics reveals contrary effects of two Rdl-homologous GABA receptors of Helicoverpa armigera on the toxicity of cyclodiene insecticides. Pestic. Biochem. Physiol. 2020, 170, 104699. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.C.; Saari, S.; Moran-Diez, M.E.; Meyling, N.V.; Raad, M.; Glare, T.R. Beauveria bassiana as an endophyte: A critical review on associated methodology and biocontrol potential. Bio. Control 2017, 62, 1–17. [Google Scholar] [CrossRef]
- Klieber, J.; Reineke, A. The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016, 140, 580–589. [Google Scholar] [CrossRef]
- Sönmez, E.; Sevim, A.; Demirbağ, Z.; Demir, İ. Isolation, characterization and virulence of entomopathogenic fungi from Gryllotalpa gryllotalpa (Orthoptera: Gryllotalpidae). Appl. Entomol. Zool. 2016, 51, 213–223. [Google Scholar] [CrossRef]
- Imoulan, A.; Elmeziane, A. Pathogenicity of Beauveria bassiana isolated from Moroccan Argan forests soil against larvae of Ceratitis capitata (Diptera: Tephritidae) in laboratory conditions. World J. Microbiol. Biotechnol. 2014, 30, 959–965. [Google Scholar] [CrossRef]
- Almeida, J.E.M.; Alves, S.B.; Pereira, R.M. Selection of Beauveria spp. isolates for control of the termite Heterotermes tenuis (Hagen, 1858). J. Appl. Entomol. 1997, 121, 539–543. [Google Scholar] [CrossRef]
- Wakil, W.; Ghazanfar, M.U.; Usman, M.; Hunter, D.; Shi, W. Fungal-based biopesticide formulations to control nymphs and adults of the desert locust, Schistocerca gregaria Forskål (Orthoptera: Acrididae): A laboratory and field cage study. Agronomy 2022, 12, 1160. [Google Scholar] [CrossRef]
- Wakil, W.; Ghazanfar, M.U.; Yasin, M. Naturally occurring entomopathogenic fungi infecting stored grain insect species in Punjab, Pakistan. J. Insect Sci. 2014, 14, 182. [Google Scholar] [CrossRef]
- Wakil, W.; Kavallieratos, N.G.; Ghazanfar, M.U.; Usman, M. Laboratory and field studies on the combined application of Beauveria bassiana and fipronil against four major stored-product coleopteran insect pests. Environ. Sci. Pollut. Res. 2022, 29, 34912–34929. [Google Scholar] [CrossRef]
- Wakil, W.; Schmitt, T.; Kavallieratos, N.G. Mortality and progeny production of four stored-product insect species on three grain commodities treated with Beauveria bassiana and diatomaceous earths. J. Stored Prod. Res. 2021, 93, 101738. [Google Scholar] [CrossRef]
- Wakil, W.; Schmitt, T.; Kavallieratos, N.G. Persistence and efficacy of enhanced diatomaceous earth, imidacloprid and Beauveria bassiana against three coleopteran and one psocid stored-grain insects. Environ. Sci. Pollut. Res. 2021, 28, 23459–23472. [Google Scholar] [CrossRef]
- Tahir, T.; Wakil, W.; Ali, A.; Sahi, S.T. Pathogenicity of Beauveria bassiana and Metarhizium anisopliae isolates against larvae of the polyphagous pest Helicoverpa armigera. Entomol. Gen. 2019, 38, 225–242. [Google Scholar] [CrossRef]
- Usman, M.; Wakil, W.; Piñero, J.C.; Wu, S.; Toews, M.D.; Shapiro-Ilan, D.I. Evaluation of locally isolated entomopathogenic fungi against multiple life stages of Bactrocera zonata and Bactrocera dorsalis (Diptera: Tephritidae): Laboratory and field study. Microorganisms 2021, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Wakil, W.; Kavallieratos, N.G.; Ghazanfar, M.U.; Usman, M.; Habib, A.; El-Shafie, H.A.F. Efficacy of different entomopathogenic fungal isolates against four key stored-grain beetle species. J. Stored Prod. Res. 2021, 93, 101845. [Google Scholar] [CrossRef]
- Wakil, W.; Schmitt, T. Field trials on the efficacy of Beauveria bassiana, diatomaceous earth and imidacloprid for the protection of wheat grains from four major stored grain insect pests. J. Stored Prod. Res. 2015, 64, 160–167. [Google Scholar] [CrossRef]
- Yasin, M.; Wakil, W.; Ghazanfar, M.U.; Qayyum, M.A.; Tahir, M.; Bedford, G.O. Virulence of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against red palm weevil, Rhynchophorus ferrugineus (Olivier). Entomol. Res. 2019, 49, 3–12. [Google Scholar] [CrossRef]
- Qayyum, A.M.; Wakil, W.; Arif, M.J.; Sahi, S.T.; Dunlap, C.A. Infection of Helicoverpa armigera by endophytic Beauveria bassiana colonizing tomato plants. Biol. Control 2015, 90, 200–207. [Google Scholar] [CrossRef]
- Younas, A.; Wakil, W.; Khan, Z.; Shaaban, M.; Prager, M.S. The efficacy of Beauveria bassiana, jasmonic acid and chlorantraniliprole on larval populations of Helicoverpa armigera in chickpea crop ecosystems. Pest Manag. Sci. 2017, 73, 418–424. [Google Scholar] [CrossRef]
- Kaur, G.; Padmaja, V. Relationships among activities of extracellular enzyme production and virulence against Helicoverpa armigera in Beauveria bassiana. J. Basic Microbiol. 2009, 49, 264–274. [Google Scholar] [CrossRef]
- Gillespie, A.T.; Moorhouse, E.R. The Use of Fungi to Control Pests of Agricultural and Horticultural Importance, in Biotechnology of Fungi for Improving Plant Growth; Whipps, J.M., Lumsden, R.D., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 55–84. [Google Scholar]
- Sandhu, S.S. Evaluation and Development of Entomogenous Fungal Isolates through Biotechnical Approaches for Management of Chick Pea Borer Helicoverpa armigera. Ph.D. Thesis, R.D. University, Jabalpur, India, 1999. [Google Scholar]
- Broadley, H.J.; Boucher, M.; Burand, J.P.; Elkinton, J.S. The phylogenetic relationship and cross-infection of nucleopolyhedroviruses between the invasive winter moth (Operophtera brumata) and its native congener, Bruce spanworm (O. bruceata). J. Invertebr. Pathol. 2017, 143, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Rowley, D.L.; Mowery, J.; Bauchan, G.R.; Theilmann, D.A.; Rohrmann, G.F.; Erlandson, M.A. The complete genome sequence of a second distinct betabaculovirus from the true armyworm, Mythimna unipuncta. PLoS ONE 2017, 12, e0170510. [Google Scholar] [CrossRef] [PubMed]
- Arrizubieta, M.; Simón, O.; Williams, T.; Caballero, P. Determinant factors in the production of a co-occluded binary mixture of Helicoverpa armigera alphabaculovirus (HearNPV) genotypes with desirable insecticidal characteristics. PLoS ONE 2016, 11, e0164486. [Google Scholar] [CrossRef]
- Mane, P.N.; Moharil, M.P.; Satpute, N.S.; Thakare, S.M.; Giri, G.K.; Gaikwad, S.; Gade, A.K.; Rai, M.K. Storage Stability and performance of aqueous and dry formulations of Helicoverpa armigera nuclear polyhedrosis virus. J. Biol. Control 2016, 30, 34–39. [Google Scholar] [CrossRef]
- Popham, H.J.; Ellersieck, M.R.; Li, H.; Bonning, B.C. Evaluation of the insecticidal efficacy of wild type and recombinant baculoviruses. Baculovirus Insect Cell Expr. Protoc. 2016, 1350, 407–444. [Google Scholar]
- Yu, H.; Zhou, B.; Meng, J.; Xu, J.; Liu, T.X.; Wang, D. Recombinant Helicoverpa arimgera nucleopolyhedrovirus with arthropod-specific neurotoxin gene RjAa17f from Rhopalurus junceus enhances the virulence against the host larvae. Insect Sci. 2017, 24, 397–408. [Google Scholar] [CrossRef]
- Fernandes, M.E.; Alves, F.M.; Pereira, R.C.; Aquino, L.A.; Fernandes, F.L.; Zanuncio, J.C. Lethal and sublethal effects of seven insecticides on three beneficial insects in laboratory assays and field trials. Chemosphere 2016, 156, 45–55. [Google Scholar] [CrossRef]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M. A retrospective look at anthranilic diamide insecticides: Discovery and lead optimization to chlorantraniliprole and cyantraniliprole. Pest Manag. Sci. 2017, 73, 658–665. [Google Scholar] [CrossRef]
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and selective ryanodine receptor activators for insect control. Bioorganic Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Phys. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Wakil, W.; Ghazanfar, M.U.; Nasir, F.; Qayyum, M.A.; Tahir, M. Insecticidal efficacy of Azadirachta indica, nucleopolyhedrovirus and chlorantraniliprole single or combined against field populations of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Chil. J. Agric. Res. 2012, 72, 52–62. [Google Scholar] [CrossRef]
- Díaz, S.; Demissew, S.; Carabias, J.; Joly, C.; Lonsdale, M.; Ash, N.; Larigauderie, A.; Adhikari, J.R.; Arico, S.; Báldi, A.; et al. The IPBES Conceptual Framework—Connecting nature and people. Curr. Opin. Environ. Sustain. 2015, 14, 1–16. [Google Scholar] [CrossRef]
- Praveena, R.; Venkatasubbu, G.D.; Jegadeesan, M. Antifeedant activity of selected medicinal plants on Earias vittella. J. Biopest. 2012, 5, 96–99. [Google Scholar]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Laboratory Techniques used for Entomopathogenic Fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 189–253. [Google Scholar]
- Usman, M.; Gulzar, S.; Wakil, W.; Wu, S.; Piñero, J.C.; Leskey, T.C.; Nixon, L.J.; Oliveira-Hofman, C.; Toews, M.D.; Shapiro-Ilan, D. Virulence of entomopathogenic fungi to Rhagoletis pomonella (Diptera: Tephritidae) and interactions with entomopathogenic nematodes. J. Econ. Entomol. 2020, 113, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Green, T.B.; Shapiro, A.; White, S.; Rao, S.; Mertens, P.P.C.; Carner, G.; Becnel, J.J. Biological and molecular studies of a cypovirus from the black fly Simulium ubiquitum (Diptera: Simuliidae). J. Invertebr. Pathol. 2006, 95, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.; Green, T.B.; Rao, S.; White, S.; Carner, G.; Mertens, P.P.C.; Becnel, J.J. Morphological and molecular characterization of a Cypovirus (Reoviridae) from the Mosquito Uranotaenia sapphirina (Diptera: Culicidae). J. Virol. 2005, 79, 9430–9438. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S.; Myers, J.H. Adaptation in an insect host–plant pathogen interaction. Ecol. Lett. 2004, 7, 632–639. [Google Scholar] [CrossRef]
- Jaganathan, R.; Narasimhan, V. Effect of plant extracts/ products on two fungal pathogens of finger millet. Indian J. Mycol. Plant Pathol. 1988, 18, 250–254. [Google Scholar]
- Walker, G.P.; Herman, T.J.B.; Kale, A.J.; Wallace, A.R. An adjustable action threshold using larval parasitism of Helicoverpa armigera (Lepidoptera: Noctuidae) in IPM for processing tomatoes. Biol. Control 2010, 52, 30–36. [Google Scholar] [CrossRef]
- Wakil, W.; Ashfaq, M.; Ghazanfar, M.U.; Afzal, M.; Riasat, T. Integrated management of Helicoverpa armigera in chickpea in rainfed areas of Punjab, Pakistan. Phytoparasitica 2009, 37, 415–420. [Google Scholar] [CrossRef]
- Gittinger, J.P. Economic Analysis of Agricultural Projects; Economic Development Institute of the World Bank: Baltimore, MD, USA; The John Hopkins University Press: Maryland, MD, USA, 1982. [Google Scholar]
- Minitab. MINITAB Release 14 for Windows; Minitab Inc.: State College, PA, USA, 2003. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; Freeman: New York, NY, USA, 1995; p. 880. [Google Scholar]
- Rao, G.V.R.; Kumar, C.S.; Sireesha, K.; Kumar, P.L. Role of nucleopolyhedroviruses (NPVs) in the management of lepidopteran pests in Asia. In Biocontrol of Lepidopteran Pests: Use of Soil Microbes and their Metabolites; Sree, K.S., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 11–52. [Google Scholar]
- Huang, J.M.; Zhao, Y.X.; Sun, H.; Ni, H.; Liu, C.; Wang, X.; Gao, C.F.; Wu, S.F. Monitoring and mechanisms of insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae), with special reference to diamides. Pestici. Biochem. Phys. 2021, 174, 104831. [Google Scholar] [CrossRef] [PubMed]
- Kalvnadi, E.; Mirmoayedi, A.; Alizadeh, M.; Pourian, H. Sub-lethal concentrations of the entomopathogenic fungus, Beauveria bassiana increase fitness costs of Helicoverpa armigera (Lepidoptera: Noctuidae) offspring. J. Invertebr. Pathol. 2018, 158, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Phukon, M.; Sarma, I.; Borgohain, R.; Sarma, B.; Goswami, J. Efficacy of Metarhizium anisopliae, Beauveria bassiana and neem oil against tomato fruit borer, Helicoverpa armigera under field condition. Asian J. Bio Sci. 2014, 9, 151–155. [Google Scholar] [CrossRef]
- Jarrahi, A.; Safavi, S.A. Fitness costs to Helicoverpa armigera after exposure to sub-lethal concentrations of Metarhizium anisopliae sensu lato: Study on F1 generation. J. Invertebr. Pathol. 2016, 138, 50–56. [Google Scholar] [CrossRef]
- Rijal, J.P.; Dhoj, Y.G.C.; Thapa, R.B.; Kafle, L. Efficacy of Metarhizium anisopliae and Beauveria bassiana against Helicoverpa armigera in chickpea, under field conditions in Nepal. Formos. Entomol. 2008, 28, 249–258. [Google Scholar]
- Savitha, P.; Nandish, M.S.; Shivaprakash, M.K. Pathogenesis of entomopathogenic fungal isolates against Helicoverpa armigera. Indian J. Plant Prot. 2015, 43, 178–181. [Google Scholar]
- Kankale, M.D.; Kelwatkar, N.M.; Das, S.B.; Sontakke, B.K. Bioefficacy of Beauveria bassiana with neem derivatives against Helicoverpa armigera infesting chickpea. J. Appl. Zool. Res. 2015, 26, 161–166. [Google Scholar]
- Ali, S.; Li, Y.; Haq, I.U.; Abbas, W.; Shabbir, M.Z.; Khan, M.M.; Mamay, M.; Niaz, Y.; Farooq, T.; Skalicky, M.; et al. The impact of different plant extracts on population suppression of Helicoverpa armigera (Hub.) and tomato (Lycopersicon esculentum Mill) yield under field conditions. PLoS ONE 2021, 16, e0260470. [Google Scholar] [CrossRef]
- Subashini, H.D.; Malarvannan, S.; Renjith, R.P. Dodonaea angustifolia—A potential biopesticide against Helicoverpa armigera. Curr. Sci. 2004, 86, 26–28. [Google Scholar]
- Souza, C.M.; Baldin, E.L.L.; Ribeiro, L.P.; Silva, I.F.; Morando, R.; Bicalho, K.U.; Vendramim, J.D.; Fernandes, J.B. Lethal and growth inhibitory activities of Neotropical annonaceae-derived extracts, commercial formulation, and an isolated acetogenin against Helicoverpa armigera. J. Pest Sci. 2017, 90, 701–709. [Google Scholar] [CrossRef]
- Kumar, N.S.; Murugan, K.; Zhang, W. Additive interaction of Helicoverpa armigera nucleopolyhedrovirus and Azadirachtin. BioControl 2008, 53, 869–880. [Google Scholar] [CrossRef]
- Karabhantanal, S.S.; Awaknavar, J.S. Bio intensive approach for the management of tomato fruit borer, Helicoverpa armigera (Hubner). Pest Manag. Hortic. Ecosyst. 2012, 18, 135–138. [Google Scholar]
- Pugalenthi, P.; Dhanasekaran, S.; Elumali, K.; Krishnappa, K. Bio-efficacy of NPV tested against American bollworm, Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae) and protection of cotton boll damage. Int. J. Renew. Environ. Sci. 2013, 1, 22–26. [Google Scholar]
- Eroğlu, G.B.; Nalçacioğlu, R.; Demirbağ, Z. A new Helicoverpa armigera Nucleopolyhedrovirus isolate from Heliothis peltigera (Denis & Schiffermuller) (Lepidoptera: Noctuidae) in Turkey. Turkish J. Biol. 2019, 43, 340–348. [Google Scholar]
- Ravi, M.; Santharam, G.; Sathiah, N. Ecofriendly management of tomato fruit borer, Helicoverpa armigera (Hubner). J. Biopest. 2008, 1, 134–137. [Google Scholar]
- Jia, M.; Cao, G.; Li, Y.; Tu, X.; Wang, G.; Nong, X.; Whitman, D.W.; Zhang, Z. Biochemical basis of synergism between pathogenic fungus Metarhizium anisopliae and insecticide chlorantraniliprole in Locusta migratoria (Meyen). Sci. Rep. 2016, 6, 28424. [Google Scholar] [CrossRef]
- Simón, O.; Torres-Vila, L.M.; Figueiredo, E.; Mendiola, J.; Mexia, A.; Caballero, P.; Williams, T. Insecticidal efficacy and persistence of a co-occluded binary mixture of Helicoverpa armigera ucleopolyhedrovirus (HearNPV) variants in protected and field-grown tomato crops on the Iberian Peninsula. Pest Manag. Sci. 2016, 72, 660–670. [Google Scholar]
- Cherry, A.J.; Rabindra, R.J.; Parnell, M.A.; Geetha, N.; Kennedy, J.S.; Grzywacz, D. Field evaluation of Helicoverpa armigera nucleopolyhedrovirus formulations for control of the chickpea pod-borer, H. armigera (Hubn.), on chickpea (Cicer arietinum var. Shoba) in southern India. Crop Prot. 2000, 19, 51–60. [Google Scholar] [CrossRef]
- Gupta, R.K.; Raina, J.C.; Arora, R.K.; Bali, K. Selection and field effectiveness of nucleopolyhedrovirus isolates against Helicoverpa armigera (Hübner). Int. J. Virol. 2007, 3, 45–59. [Google Scholar]
- Byasigideri, D.; Chakravarthy, A.; Narabenchi, G.B.; Devi, S.S.G.; Rajagopal, D. Evaluation and validation of HearSNPV for fruit borer, Helicoverpa armigera (Noctuidae: Lepidoptera) management on tomato. Indian J. Agric. Sci. 2013, 83, 106–112. [Google Scholar]
- Kumar, B.; Singh, S.; Verma, R.A. Management of Helicoverpa armigera in chickpea through synthetic and bio-rational insecticides. Ann. Plant Prot. Sci. 2011, 19, 205–206. [Google Scholar]
- Reddy, G.P.V.; Miller, R.H. Biorational versus conventional insecticides–Comparative field study for managing red spider mite and fruit borer on tomato. Crop Prot. 2014, 64, 88–92. [Google Scholar] [CrossRef]
- Raj, S.S.; Jai, S.; Dhermandra, S.P.; Rampratap, B. Bio-efficacy of insecticides for management of Helicoverpa armigera in chickpea and their economics. Ann. Plant Prot. Sci. 2016, 24, 71–73. [Google Scholar]
- Tadavi, M.; Saindane, Y.; Patil, R.; Deore, B. Evaluation of some microbial and botanical pesticides against Helicoverpa armigera (Hubner) in chickpea. Adv. Life Sci. 2016, 5, 1030–1033. [Google Scholar]
- Wyckhuys, K.; O’Neil, R.J. Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot. 2006, 25, 1180–1190. [Google Scholar] [CrossRef]
- Singh, S.P. Compatible natural enemies and synthethic pesticides for use in integrated pest management in India. Pestic. Res. J. 1995, 7, 8–28. [Google Scholar]
- Lucas, E.; Giroux, S.; Demougeot, S.; Duchesne, R.M.; Coderre, D. Compatibility of a natural enemy, Coleomegilla maculata lengi (Col., Coccinellidae) and four insecticides used against the Colorado potato beetle (Col., Chrysomelidae). J. Appl. Entomol. 2004, 128, 233–239. [Google Scholar] [CrossRef]
- Sharma, H.C.; Arora, R.; Pampapathy, G. Influence of transgenic cottons with Bacillus thuringiensis cry1Ac gene on the natural enemies of Helicoverpa armigera. BioControl 2007, 52, 469–489. [Google Scholar] [CrossRef]




| Region | Rawalpindi | Faisalabad | Bahawalpur | |||||
|---|---|---|---|---|---|---|---|---|
| Source | df | F | p | F | p | F | p | |
| Between variables | Treatment | 6 | 18.68 | <0.01 | 4.10 | 0.0045 | 17.89 | <0.01 |
| Spray application | 1 | 18.33 | <0.01 | <0.01 | 1.00 | 31.10 | <0.01 | |
| Treatment × spray application | 6 | 5.03 | <0.01 | <0.01 | 1.00 | 5.16 | <0.01 | |
| Within variables | Interval | 3 | 30.11 | <0.01 | 39.22 | 0.00 | 39.48 | <0.01 |
| Treatment × interval | 18 | 3.26 | <0.01 | 4.41 | 0.00 | 3.64 | <0.01 | |
| Spray application×interval | 3 | 0.46 | 0.71 | <0.01 | 1.00 | 0.02 | 0.99 | |
| Treatment × spray application × interval | 18 | 0.34 | 0.99 | <0.01 | 1.00 | 0.22 | 0.99 | |
| Region | Treatment | 1st Spray | 2nd Spray | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment | 3 DAT | 5 DAT | 10 DAT | Pre-Treatment | 3 DAT | 5 DAT | 10 DAT | ||
| Rawalpindi | B. bassiana | 6.47 ± 1.26 a | 4.78 ± 1.05 b | 3.76 ± 0.81 ab | 3.50 ± 1.31 b | 4.35 ± 0.79 b | 2.70 ± 0.71 b | 1.81 ± 0.40 b | 0.84 ± 0.11 b |
| HaNPV | 7.35 ± 0.85 a | 4.12 ± 1.11 bc | 3.38 ± 0.60 ab | 2.90 ± 0.94 b | 3.55 ± 0.59 ab | 2.35 ± 0.94 b | 1.46 ± 1.04 b | 0.74 ± 0.34 b | |
| D. viscosa | 5.89 ± 0.41 ab | 5.20 ± 1.21 ab | 4.58 ± 0.85 ab | 3.85 ± 0.96 ab | 4.53 ± 1.15 ab | 3.31 ± 0.77 b | 2.26 ± 0.74 b | 1.70 ± 0.50 b | |
| Chloran. | 6.59 ± 0.50 a | 3.97 ± 1.06 c | 3.10 ± 0.77 b | 2.18 ± 0.54 b | 3.15 ± 0.90 b | 2.20 ± 0.77 c | 0.98 ± 0.80 c | 0.58 ± 0.14 c | |
| B. bassiana + chloran. | 5.32 ± 0.91 ab | 3.70 ± 1.06 c | 2.72 ± 0.84 b | 1.92 ± 0.62 c | 3.05 ± 0.94 c | 1.41 ± 0.54 c | 0.70 ± 0.26 c | 0.48 ± 0.09 c | |
| HaNPV + chloran. | 5.38 ± 1.16 ab | 3.37 ± 1.23 c | 2.16 ± 0.95 c | 1.57 ± 0.88 c | 2.90 ± 1.24 c | 1.13 ± 0.73 c | 0.54 ± 0.30 c | 0.35 ± 0.06 c | |
| Control | 4.92 ± 0.46 b | 6.02 ± 1.13 a | 6.55 ± 0.67 a | 7.20 ± 0.73 a | 8.15 ± 1.33 a | 8.30 ± 1.01 a | 9.79 ± 1.37 a | 10.29 ± 1.07 a | |
| F6,20 | 0.94 | 2.55 | 4.79 | 7.23 | 4.48 | 12.45 | 25.25 | 89.02 | |
| p | 0.50 | 0.08 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Faisalabad | B. bassiana | 7.66 ± 1.27 ab | 6.06 ± 1.06 ab | 5.14 ± 0.83 ab | 4.39 ± 1.33 b | 5.04 ± 0.80 b | 3.67 ± 0.70 b | 2.68 ± 0.43 b | 1.05 ± 0.08 b |
| HaNPV | 8.55 ± 0.87 a | 5.44 ± 1.13 b | 4.72 ± 0.62 ab | 3.80 ± 0.96 b | 4.24 ± 0.61 ab | 3.21 ± 0.95 b | 2.29 ± 1.01 b | 0.96 ± 0.34 b | |
| D. viscosa | 7.11 ± 0.44 ab | 6.51 ± 1.22 b | 5.93 ± 0.87 ab | 4.74 ± 0.98 ab | 5.22 ± 1.17 ab | 4.18 ± 0.81 b | 3.13 ± 0.75 b | 1.91 ± 0.51 b | |
| Chloran. | 7.78 ± 0.49 ab | 5.27 ± 0747 b | 4.44 ± 0.77 b | 3.05 ± 0.96 b | 3.84 ± 0.92 b | 2.93 ± 1.00 c | 1.82 ± 0.81 c | 0.63 ± 0.16 c | |
| B. bassiana + chloran. | 6.51 ± 0.92 b | 5.03 ± 0.10 bc | 4.08 ± 0.81 c | 2.86 ± 0.67 c | 3.73 ± 0.95 c | 2.26 ± 0.54 c | 1.09 ± 0.10 c | 0.49 ± 0.14 c | |
| HaNPV + chloran. | 6.52 ± 1.17 b | 4.66 ± 1.24 c | 3.51 ± 0.95 c | 2.44 ± 0.88 c | 3.60 ± 1.26 c | 1.92 ± 0.77 c | 0.98 ± 0.29 c | 0.28 ± 0.16 c | |
| Control | 5.78 ± 0.13 c | 7.37 ± 1.16 a | 7.92 ± 0.67 a | 8.15 ± 0.75 a | 9.08 ± 1.35 a | 9.16 ± 1.08 a | 10.61 ± 1.37 a | 10.83 ± 1.13 a | |
| F6,20 | 1.14 | 2.56 | 4.90 | 7.03 | 4.68 | 13.08 | 25.56 | 68.90 | |
| p | 0.39 | 0.08 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Bahawalpur | B. bassiana | 9.61 ± 1.29 a | 7.94 ± 1.09 ab | 7.03 ± 0.87 ab | 6.20 ± 1.33 b | 6.95 ± 0.83 b | 5.47 ± 0.73 b | 4.46 ± 0.42 b | 2.8 ± 0.10 b |
| HaNPV | 9.03 ± 0.94 a | 7.32 ± 1.13 b | 6.81 ± 0.54 b | 5.66 ± 0.90 b | 6.16 ± 0.60 ab | 4.98 ± 0.92 bc | 4.07 ± 1.03 bc | 2.71 ± 0.35 b | |
| D. viscosa | 8.95 ± 0.44 ab | 8.37 ± 1.19 b | 7.81 ± 0.85 ab | 6.59 ± 0.95 b | 7.14 ± 1.19 ab | 5.95 ± 0.79 b | 4.96 ± 0.72 b | 3.72 ± 0.50 b | |
| Chloran. | 9.58 ± 0.45 a | 7.15 ± 1.07 b | 6.36 ± 0.76 c | 4.87 ± 0.98 c | 5.78 ± 0.94 b | 4.76 ± 0.92 bc | 3.60 ± 0.80 c | 2.44 ± 0.20 c | |
| B. bassiana + chloran. | 8.40 ± 0.85 ab | 6.95 ± 1.08 c | 6.04 ± 0.76 c | 4.68 ± 0.70 c | 5.63 ± 0.97 b | 4.04 ± 0.51 c | 2.89 ± 0.13 d | 2.24 ± 0.15 c | |
| HaNPV + chloran. | 8.52 ± 1.13 ab | 6.54 ± 1.26 c | 5.39 ± 0.93 c | 4.30 ± 0.82 c | 5.52 ± 1.27 c | 3.69 ± 0.80 c | 2.75 ± 0.31 d | 2.08 ± 0.17 c | |
| Control | 7.70 ± 0.16 c | 9.29 ± 1.14 a | 9.78 ± 0.65 a | 9.97 ± 0.72 a | 10.97 ± 1.34 a | 11.53 ± 0.74 a | 12.47 ± 1.52 a | 12.87 ± 1.33 a | |
| F6,20 | 4.48 | 2.54 | 4.97 | 7.21 | 4.48 | 0.93 | 21.96 | 54.53 | |
| p | <0.01 | 0.08 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Region | Rawalpindi | Faisalabad | Bahawalpur | |||||
|---|---|---|---|---|---|---|---|---|
| Source | df | F | p | F | p | F | p | |
| Between variables | Treatment | 6 | 35.30 | <0.01 | 30.81 | <0.01 | 35.30 | <0.01 |
| Spray application | 1 | 26.50 | <0.01 | 24.89 | <0.01 | 26.50 | <0.01 | |
| Treatment × spray application | 6 | 5.81 | <0.01 | 4.91 | <0.01 | 5.81 | <0.01 | |
| Within variables | Interval | 3 | 11.97 | <0.01 | 12.57 | <0.01 | 11.97 | <0.01 |
| Treatment × interval | 18 | 2.39 | 0.01 | 2.38 | 0.01 | 2.39 | 0.01 | |
| Spray application × interval | 3 | 2.35 | 0.10 | 3.18 | 0.04 | 2.35 | 0.10 | |
| Treatment × spray application × interval | 18 | 0.07 | 1.00 | 0.11 | 0.99 | 0.07 | 1.00 | |
| Region | Treatment | Fruit Damage (%) | |||||
|---|---|---|---|---|---|---|---|
| 1st Spray | 2nd Spray | ||||||
| 3 DAT | 5 DAT | 10 DAT | 3 DAT | 5 DAT | 10 DAT | ||
| Rawalpindi | B. bassiana | 24.84 ± 4.90 b | 20.59 ± 3.86 b | 16.83 ± 4.12 b | 13.63 ± 3.29 bc | 9.09 ± 2.19 b | 8.82 ± 1.61 bc |
| NPV | 21.96 ± 5.35 b | 18.71 ± 2.88 ab | 14.45 ± 3.17 b | 12.53 ± 2.68 bc | 7.52 ± 2.75 b | 6.97 ± 2.66 bc | |
| D. viscosa | 26.94 ± 5.75 b | 24.39 ± 4.08 ab | 18.61 ± 3.02 b | 16.06 ± 2.56 b | 11.17 ± 2.56 b | 9.31 ± 1.85 c | |
| Chloran. | 21.15 ± 5.05 bc | 17.40 ± 3.61 bc | 11.62 ± 1.89 c | 10.37 ± 1.68 bc | 6.91 ± 1.53 c | 5.54 ± 1.75 bc | |
| B. bassiana + chloran. | 20.02 ± 5.22 bc | 15.25 ± 2.06 c | 10.40 ± 2.58 c | 7.69 ± 1.12 c | 2.69 ± 0.58 d | 1.73 ± 1.38 d | |
| NPV + Coragen | 18.25 ± 4.44 c | 13.14 ± 4.27 c | 8.43 ± 3.27 c | 5.39 ± 1.63 c | 1.66 ± 1.08 d | 0.96 ± 0.62 c | |
| Control | 30.42 ± 4.85 a | 34.26 ± 2.77 a | 35.51 ± 3.34 a | 40.10 ± 4.14 a | 46.68 ± 5.30 a | 51.20 ± 4.55 a | |
| F6,20 | 2.65 | 6.25 | 14.80 | 21.88 | 38.98 | 96.43 | |
| p | 0.07 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Faisalabad | B. bassiana | 28.52 ± 4.99 b | 24.11 ± 3.89 b | 20.40 ± 4.12 b | 17.04 ± 3.20 b | 12.45 ± 2.16 b | 12.21 ± 1.62 bc |
| NPV | 25.58 ± 5.38 b | 22.19 ± 2.88 ab | 18.04 ± 3.19 b | 15.94 ± 2.69 bc | 10.88 ± 2.73 bc | 10.35 ± 2.66 bc | |
| D. viscosa | 30.60 ± 5.76 ab | 27.93 ± 4.10 ab | 22.03 ± 3.97 bc | 19.55 ± 2.58 b | 14.52 ± 2.53 b | 13.30 ± 2.36 b | |
| Chloran. | 24.76 ± 5.07 b | 20.89 ± 3.62 bc | 14.41 ± 2.54 bc | 13.80 ± 1.70 bc | 9.053 ± 2.86 bc | 8.913 ± 1.74 c | |
| B. bassiana + chloran. | 23.61 ± 5.22 b | 19.25 ± 3.79 c | 13.63 ± 3.11 c | 11.20 ± 1.12 c | 5.29 ± 1.15 c | 5.08 ± 1.40 c | |
| NPV + chloran. | 21.87 ± 4.44 b | 16.60 ± 4.28 c | 11.67 ± 3.55 b | 8.90 ± 1.63 b | 4.64 ± 1.23 b | 4.34 ± 1.59 c | |
| Control | 34.60 ± 5.41 a | 37.60 ± 2.69 a | 38.84 ± 3.11 a | 42.80 ± 4.10 a | 49.57 ± 5.44 a | 54.71 ± 4.56 a | |
| F6,20 | 2.73 | 5.64 | 14.55 | 22.30 | 37.39 | 103.13 | |
| p | 0.06 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Bahawalpur | B. bassiana | 32.66 ± 5.22 bc | 28.62 ± 3.75 b | 24.87 ± 3.08 b | 21.48 ± 3.19 b | 16.87 ± 2.23 b | 16.37 ± 1.57 bc |
| NPV | 30.05 ± 5.36 bc | 26.74 ± 2.74 ab | 22.54 ± 3.09 bc | 20.44 ± 2.68 b | 15.34 ± 2.82 b | 14.56 ± 2.56 bc | |
| D. viscosa | 35.58 ± 5.31 ab | 32.33 ± 4.16 ab | 26.46 ± 3.94 b | 23.93 ± 2.44 bc | 18.92 ± 2.50 bc | 17.70 ± 2.39 b | |
| Chloran. | 29.33 ± 5.11 bc | 25.30 ± 3.58 bc | 18.86 ± 2.60 bc | 18.24 ± 1.68 bc | 13.46 ± 2.86 bc | 12.85 ± 1.39 bc | |
| B. bassiana + chloran. | 24.65 ± 2.01 c | 23.64 ± 3.81 c | 18.28 ± 3.04 c | 15.60 ± 1.12 c | 9.64 ± 1.15 c | 8.94 ± 0.96 c | |
| NPV + chloran. | 26.28 ± 4.49 c | 21.02 ± 4.25 c | 16.22 ± 3.40 b | 13.39 ± 1.63 c | 9.08 ± 1.19 c | 8.10 ± 1.07 c | |
| Control | 38.83 ± 5.56 a | 41.76 ± 2.51 a | 43.32 ± 3.09 a | 46.73 ± 3.70 a | 53.10 ± 4.64 a | 59.21 ± 4.57 a | |
| F6,20 | 3.12 | 5.78 | 16.02 | 23.69 | 44.89 | 106.59 | |
| p | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Region | Treatment | Yield (kg/ha.) | Cost (ha.) USD | Increased Yield Over Control (kg/ha.) | Increased Yield Over Control (%) | Income Increased/ha. (USD) | Cost-Benefit Ratio |
|---|---|---|---|---|---|---|---|
| Rawalpindi | B. bassiana | 3466 ± 2.20 ab | 24.09 | 21.72 | 153.61 | 49.36 | 1.05 |
| HaNPV | 3519 ± 2.64 ab | 24.09 | 22.25 | 167.83 | 50.57 | 1.10 | |
| D. viscosa | 3282 ± 3.13 b | 22.72 | 19.88 | 171.95 | 45.18 | 0.99 | |
| Chloran. | 3726 ± 2.21 ab | 25.45 | 24.33 | 187.97 | 55.29 | 1.17 | |
| B. bassiana + chloran. | 4112 ± 2.86 ab | 27.27 | 28.18 | 217.75 | 64.04 | 1.35 | |
| HaNPV + chloran. | 4659 ± 1.90 a | 27.27 | 33.6 | 260.10 | 76.50 | 1.81 | |
| Control | 1294 ± 1.79 c | - | - | - | - | - | |
| F6,20 | 18.14 | - | - | - | - | - | |
| p | <0.01 | - | - | - | - | - | |
| Faisalabad | B. bassiana | 3350 ± 1.68 ab | 19.99 | 54.98 | 156.30 | 25.89 | 1.07 |
| HaNPV | 3419 ± 1.89 ab | 20.61 | 56.70 | 191.05 | 27.45 | 1.14 | |
| D. viscosa | 2950 ± 3.62 b | 16.35 | 44.98 | 197.05 | 18.16 | 0.80 | |
| Chloran. | 3626 ± 2.14 ab | 25.45 | 24.75 | 215.03 | 56.25 | 1.21 | |
| B. bassiana + chloran. | 4037 ± 2.16 ab | 27.27 | 28.87 | 250.80 | 65.60 | 1.41 | |
| HaNPV + chloran. | 4451 ± 2.97 a | 27.27 | 33 | 286.74 | 75.00 | 1.75 | |
| Control | 1151 ± 1.47 c | - | - | - | - | - | |
| F6,20 | 17.42 | - | - | - | - | - | |
| p | <0.01 | - | - | - | - | - | |
| Bahawalpur | B. bassiana | 3062 ± 2.57 ab | 18.86 | 51.88 | 162.24 | 23.07 | 0.96 |
| HaNPV | 3280 ± 1.48 ab | 20.84 | 57.33 | 210.27 | 28.02 | 1.16 | |
| D. viscosa | 2588 ± 3.25 b | 14.55 | 40.03 | 232.35 | 13.66 | 0.60 | |
| Chloran. | 3469 ± 3.65 ab | 25.45 | 24.82 | 251.50 | 56.40 | 1.22 | |
| B. bassiana + chloran. | 3889 ± 3.47 ab | 27.27 | 29.03 | 294.12 | 65.97 | 1.42 | |
| HaNPV + chloran. | 4279 ± 3.68 a | 27.27 | 32.92 | 333.60 | 74.82 | 1.74 | |
| Control | 987 ± 1.34 c | - | - | - | - | - | |
| F6,20 | 11.86 | - | - | - | - | - | |
| p | <0.01 | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakil, W.; Tahir, M.; Ghazanfar, M.U.; Qayyum, M.A.; Yasin, M.; Maqsood, S.; Asrar, M.; Shapiro-Ilan, D.I. Microbes, Dodonaea viscosa and Chlorantraniliprole as Components of Helicoverpa armigera IPM Program: A Three Region Open-Field Study. Agronomy 2022, 12, 1928. https://doi.org/10.3390/agronomy12081928
Wakil W, Tahir M, Ghazanfar MU, Qayyum MA, Yasin M, Maqsood S, Asrar M, Shapiro-Ilan DI. Microbes, Dodonaea viscosa and Chlorantraniliprole as Components of Helicoverpa armigera IPM Program: A Three Region Open-Field Study. Agronomy. 2022; 12(8):1928. https://doi.org/10.3390/agronomy12081928
Chicago/Turabian StyleWakil, Waqas, Muhammad Tahir, Muhammad Usman Ghazanfar, Mirza Abdul Qayyum, Muhammad Yasin, Sumaira Maqsood, Muhammad Asrar, and David I. Shapiro-Ilan. 2022. "Microbes, Dodonaea viscosa and Chlorantraniliprole as Components of Helicoverpa armigera IPM Program: A Three Region Open-Field Study" Agronomy 12, no. 8: 1928. https://doi.org/10.3390/agronomy12081928





