Protective Effects of Systiva® Seed Treatment Fungicide for the Control of Winter Wheat Foliar Diseases Caused at Early Stages Due to Climate Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Field
- (1)
- S100: wheat seeds treated with 100 cc Systiva® per 100 kg of wheat seed
- (2)
- S125: wheat seeds treated with 125 cc Systiva® per 100 kg of wheat seed
- (3)
- S150: wheat seeds treated with 150 cc Systiva® per 100 kg of wheat seed and
- (4)
- S0, control: wheat seeds without Systiva® treatment
2.2. Climatic Anomalies
2.3. Residue Sampling
2.4. Visual Disease Assessment
2.5. Remote Sensing Assessment
2.6. Metagenomics Analysis of Disease Assessment
2.7. Statistics
2.7.1. Bioinformatics
2.7.2. ANOVA and Principal Component Analysis (PCA)
3. Results
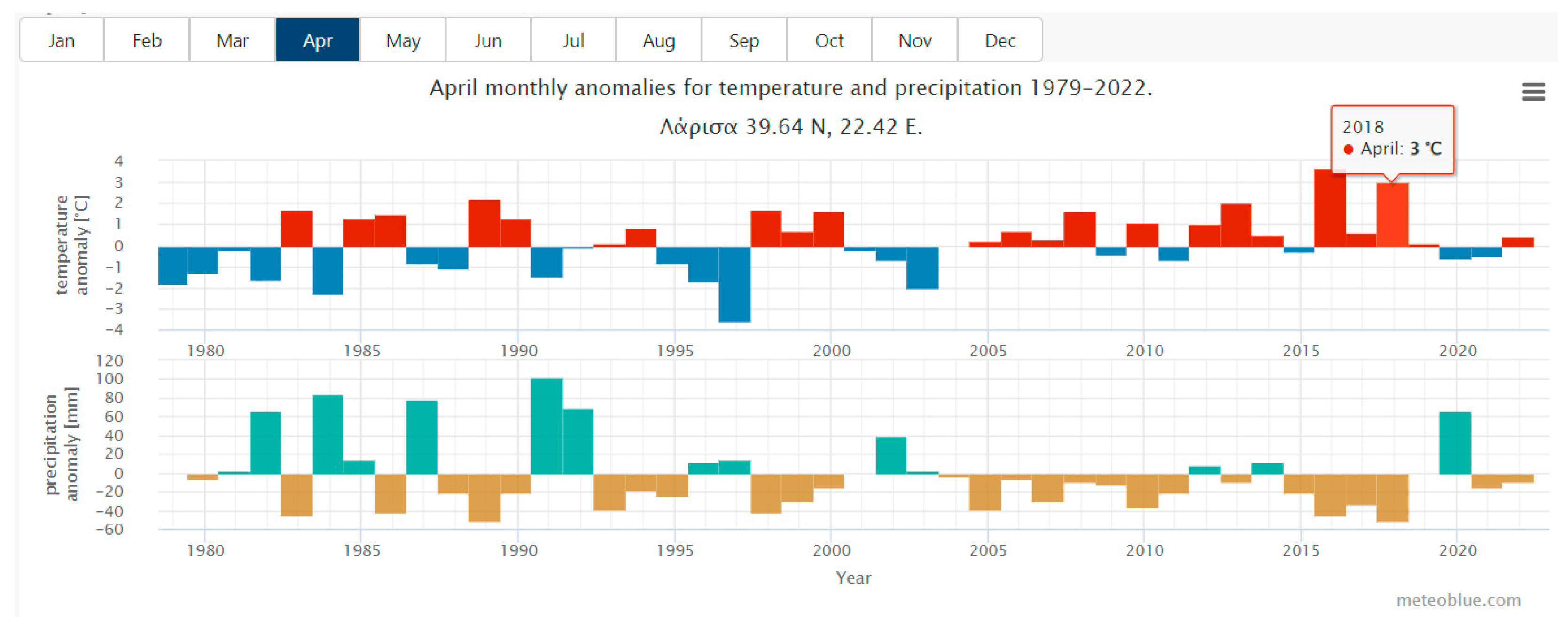
3.1. Clmate Anomalies for Monthly Temperature and Precipitation
3.2. Leaf Blotch Diseases Progression for Years 2016–2018 in Relation to Changing Climate Conditions
3.3. Evaluation of Systiva® Treatments for Growing Period 2021–2022
3.3.1. Crop Residue Sampling
3.3.2. Visual Winter Wheat Infections
3.3.3. Remote Sensing Results
3.3.4. Metagenomics Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curtis, B.C.; Rajaram, S.; Macpherson, H.G. Bread Wheat Improvement and Production. In FAO Plant Production and Protection Series; No. 30; FAO: Rome, Italy, 2002; Volume XI. [Google Scholar]
- Simón, M.R.; Börner, A.; Struik, P.C. Fungal wheat diseases: Etiology, breeding, and integrated management. Front. Plant Sci. 2021, 12, 671060. [Google Scholar] [CrossRef] [PubMed]
- Juroszek, P.; Racca, P.; Link, S.; Farhumand, J.; Kleinhenz, B. Overview on the review articles published during the past 30 years relating to the potential climate change effects on plant pathogens and crop disease risks. Plant Pathol. 2020, 69, 179–193. [Google Scholar] [CrossRef]
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe. Theor. Appl. Genet. 2021, 134, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G. Cell biology of Zymoseptoria tritici: Pathogen cell organization and wheat infection. Fungal Genet. Biol. 2015, 79, 17–23. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Vagelas, I. Important Foliar Wheat Diseases and their Management: Field Studies in Greece. Mod. Concepts Dev. Agron. 2021, 8, 783–786. [Google Scholar] [CrossRef]
- Laribi, M.; Yahyaoui, A.H.; Abdedayem, W.; Kouki, H.; Sassi, K.; Ben M’Barek, S. Characterization of Mediterranean Durum Wheat for Resistance to Pyrenophora tritici-repentis. Genes 2022, 13, 336. [Google Scholar] [CrossRef]
- Zhang, W.; Pfender, W.F. Effect of residue management on wetness duration and ascocarp production by Pyrenophora tritici-repentis in wheat residue. Phytopathology 1992, 82, 1434–1439. [Google Scholar] [CrossRef]
- Adee, S.; Pfender, W.F.; Hartnett, D.C. Competition between Pyrenophora tritici-repentis and Septoria nodorum in the wheat leaf as measured with de Wit replacement series. Phytopathology 1990, 80, 1177–1182. [Google Scholar] [CrossRef]
- Stadlmeier, M.; Jørgensen, L.N.; Corsi, B.; Cockram, J.; Hartl, L.; Mohler, V. Genetic Dissection of Resistance to the Three Fungal Plant Pathogens Blumeria graminis, Zymoseptoria tritici, and Pyrenophora tritici-repentis Using a Multiparental Winter Wheat Population. G3 Genes Genomes Genet. 2019, 9, 1745–1757. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.S.; Gibberd, M.R.; Hamblin, J. Co-infection of wheat by Pyrenophora tritici-repentis and Parastagonospora nodorum in the wheatbelt of Western Australia. Crop Pasture Sci. 2020, 71, 119–127. [Google Scholar] [CrossRef]
- Hjelkrem, A.G.R.; Ficke, A.; Abrahamsen, U.; Hofgaard, I.S.; Brodal, G. Prediction of leaf Bloch disease risk in Norwegian spring wheat based on weather factors and host phenology. Eur. J. Plant Pathol. 2021, 160, 199–213. [Google Scholar] [CrossRef]
- Andersson, B.; Djurle, A.; Ørum, J.E.; Jalli, M.; Ronis, A.; Ficke, A.; Jørgensen, L.N. Comparison of models for leaf blotch disease management in wheat based on historical yield and weather data in the Nordic-Baltic region. Agron. Sustain. Dev. 2022, 42, 42. [Google Scholar] [CrossRef]
- Shaw, M.W. Effects of temperature, leaf wetness and cultivar on the latent period of Mycosphaerella graminicola on winter wheat. Plant Pathol. 1990, 39, 255–268. [Google Scholar] [CrossRef]
- Wiik, L.; Ewaldz, T. Impact on temperature and precipitation on yield and plant diseases of winter wheat in southern Sweden 1993–2007. Crop. Prot. 2009, 28, 82–89. [Google Scholar] [CrossRef]
- Jalli, M.; Kaseva, J.; Andersson, B.; Ficke, A.; Jørgensen, L.N.; Ronis, A.; Kaukoranta, T.; Ørum, J.E.; Djurle, A. Yield increases due to fungicide control of leaf blotch diseases in wheat and barley as a basis for IPM decision-making in the Nordic-Baltic region. Eur. J. Plant Pathol. 2020, 158, 315–333. [Google Scholar] [CrossRef]
- Shaw, M.W.; Royle, D.J. Factors determining the severity of epidemics of Mycosphaerella graminicola (Septoria tritici) on winter wheat in the UK. Plant Pathol. 1993, 42, 882–899. [Google Scholar] [CrossRef]
- Suffert, F.; Sache, I.; Lannou, C. Early stages of septoria tritici blotch epidemics of winter wheat: Build-up, overseasoning, and release of primary inoculum. Plant Pathol. 2011, 60, 166–177. [Google Scholar] [CrossRef]
- Kumar, N.; Rana, M.; Kumar, B.; Chand, S.; Shiv, A.; Wani, S.H.; Kumar, S. Genomic Selection for Wheat Improvement. In Physiological, Molecular, and Genetic Perspectives of Wheat Improvement; Wani, S.H., Mohan, A., Singh, G.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-030-59577-7. [Google Scholar]
- Toropova, E.Y.; Kazakova, O.A.; Piskarev, V.V. Septoria blotch epidemic process on spring wheat varieties. Vavilov J. Genet. Breed. 2020, 24, 139. [Google Scholar] [CrossRef]
- Kerdraon, L.; Barret, M.; Laval, V.; Suffert, F. Differential dynamics of microbial community networks help identify microorganisms interacting with residue-borne pathogens: The case of Zymoseptoria tritici in wheat. Microbiome 2019, 7, 125. [Google Scholar] [CrossRef]
- Barroso-Bergadà, D.; Massot, M.; Vignolles, N.; Faivre d’Arcier, J.; Chancerel, E.; Guichoux, E.; Walker, A.; Vacher, C.; Bohan, D.A.; Laval, V.; et al. Metagenomic next-generation sequencing (mNGS) data reveals the phyllosphere microbiome of wheat plants infected by the fungal pathogen Zymoseptoria tritici. Phytobiomes J. 2022, 102, 290–297. [Google Scholar] [CrossRef]
- Liu, K.; Wen, Z.; Ma, Z.; Shao, W. Biological and molecular characterizations of fluxapyroxad-resistant isolates of Botrytis cinerea. Phytopathol. Res. 2022, 4, 2. [Google Scholar] [CrossRef]
- Lin, H.; Lin, F.; Yuan, J.; Cui, F.; Chen, J. Toxic effects and potential mechanisms of Fluxapyroxad to zebrafish (Danio rerio) embryos. Sci. Total Environ. 2021, 769, 144519. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance fluxapyroxad (BAS 700 F). EFSA J. 2012, 10, 2522. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Yuan, M.; Liu, X. Fluxapyroxad induces developmental delay in zebrafish (Danio rerio). Chemosphere 2020, 256, 127037. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, E.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- James, W.E. An illustrated series of assessment keys for plant diseases, their preparation, and usage. Can. Plant Dis. Surv. 1971, 51, 39–65. [Google Scholar]
- Eyal, Z.; Scharen, A.L.; Prescott, J.M.; Ginkel, M.V. The Septoria Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT: Texcoco, Mexico, 1987; p. 52. Available online: http://hdl.handle.net/10883/1113 (accessed on 18 August 2022).
- Del Ponte, E.M.; Pethybridge, S.J.; Bock, C.H.; Michereff, S.J.; Machado, F.J.; Spolti, P. Standard Area Diagrams for Aiding Severity Estimation: Scientometrics, Pathosystems, and Methodological Trends in the Last 25 Years. Phytopathology 2017, 107, 1161–1174. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS (Earth Resources Technology Satellite). In Proceedings of the 3rd Earth Resources Technology Satellite Symposium, Greenbelt, MD, USA, 10–14 December 1973; Volume SP-351, pp. 309–317. [Google Scholar]
- Huete, A.R. A Soil Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afgan, E.; Baker, D.; Batut, B.; Beek, M.V.; Bouvier, D.; Čech, M.; Chilton, J.M.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Friesen, T.L.; Ali, S.; Stack, R.W.; Francl, L.J.; Rasmussen, J.B. Rapid and efficient production of the Pyrenophora tritici-repentis teleomorph. Can. J. Bot. 2003, 81, 890–895. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Kefauver, S.C.; Fernandez-Gallego, J.A.; Vergara-Díaz, O.; Nieto-Taladriz, M.T.; Araus, J.L. UAV and ground image-based phenotyping: A proof of concept with durum wheat. Remote Sens. 2019, 11, 1244. [Google Scholar] [CrossRef]
- Vagelas, I. Latest Application Technology in Wheat Crop Simulation and Disease Prediction Models. Mod. Concepts Dev. Agron. 2021, 7, 767–768. [Google Scholar] [CrossRef]
- Vagelas, I. A Simple Infection Model for Foliar Pathogens of Wheat. Nov. Res. Sci. 2021, 6, NRS.000635.2021. Available online: https://crimsonpublishers.com/nrs/pdf/NRS.000635.pdf (accessed on 18 August 2022).
- Vagelas, I. A Tool for Early Detection of Septoria Tritici Blotch of Wheat. Nov. Res. Sci. 2021, 7, NRS.000651.2021. Available online: https://crimsonpublishers.com/nrs/pdf/NRS.000651.pdf (accessed on 24 August 2022).
- Adolf, B.; Schofl, U.; Verreet, J.A. Effect of infection with Septoria tritici of different growth stages of wheat (GS25 to GS59) an dry matter production, nitrogen uptake and yield. Meded. Van De Fac. Landbouwwet. 1993, 58, 1167–1174. Available online: https://www.cabdirect.org/cabdirect/abstract/19942308230 (accessed on 18 August 2022).
- Fleitas, M.C.; Castro, A.C.; Simón, M.R. Quality and yield response to the control of Mycosphaerella graminicola in wheat as affected by nitrogen rate and cultivar bread-making characteristics. Crop Pasture Sci. 2017, 68, 317327. [Google Scholar] [CrossRef]
- Hunger, R.M.; Brown, D.A. Colony color, growth, sporulation, fungicide sensitivity, and pathogenicity of Pyrenophora tritici-repentis. Plant Disease 1987, 71, 907–910. [Google Scholar] [CrossRef]
- Cook, R.J.; Yarham, D.J. Occurrence of tan spot of wheat caused by Pyrenophora tritici-repentis on wheat in England and Wales in 1987. Plant Pathol. 1989, 38, 101–102. [Google Scholar] [CrossRef]
- Mehta, Y.R.; Mehta, Â.; Riede, C.R. Molecular and pathogenic variability of Pyrenophora tritici-repentis isolates causing tan spot of wheat in the state of Paraná, Brazil. Summa Phytopathol. 2004, 30, 436–444. [Google Scholar]
- Moreno, M.V.; Stenglein, S.A.; Balatti, P.A.; Perelló, A. Pathogenic and molecular variability among isolates of Pyrenophora tritici-repentis, causal agent of tan spot of wheat in Argentina. Eur. J. Plant Pathol. 2008, 122, 239–252. [Google Scholar] [CrossRef]
- Kader, K.A.; Hunger, R.M.; Payton, M.E. Pathogenic Fitness of Pyrenophora tritici-repentis Isolates Causing Tan Spot of Winter Wheat in Oklahoma. J. Plant Pathol. Microbiol. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Moreno, M.V.; Perelló, A.; Arya, A. Occurrence of Pyrenophora tritici-repentis causing tan spot in Argentina in Management of fungal plant pathogens. In CABI International; Arya, A., Perelló, A.E., Eds.; CABI Digital Library: Wallingford, UK, 2010; pp. 275–290. [Google Scholar] [CrossRef]
- McLean, M.; Hollaway, G. Spot Form of Net Blotch Yield Loss and Management in Barley Using Fungicides and Resistance in Victoria. GRDC Update. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2015/02/spot-form-of-net-blotch-yield-loss-and-management (accessed on 15 August 2022).
- Simpfendorfer, S.; Street, M. Evaluation of Fungicide Management Strategies to Control Spot Form of Net Blotch in Barley. GRDC Update. 2017. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2017/02/evaluation-of-fungicide-management-strategies-to-control-spot-form-of-net-blotch-in-barley (accessed on 15 August 2022).
- Berraies, S.; Ammar, K.; Gharbi, M.; Yahyaoui, A.H.; Rezgui, S. Quantitative inheritance of resistance to Septoria tritici blotch in durum wheat in Tunisia. Chilean J. Agric. Res. 2014, 74, 35–40. [Google Scholar] [CrossRef]
- Ouaja, M.; Aouini, L.; Bahri, B.A.; Ferjaoui, S.; Medini, M.; Marcel, T.C.; Hamza, S.M. Identification of valuable sources of resistance to Zymoseptoria tritici in the Tunisian durum wheat landraces. Eur. J. Plant Pathol. 2020, 156, 647–661. [Google Scholar] [CrossRef]
- Ferjaoui, S.; Aouini, L.; Slimane, R.B.; Ammar, K.; Dreisigacker, S.; Schouten, H.J.; Sapkota, S.; Bahri, B.A.; M’barek, S.B.; Visser, R.; et al. Deciphering resistance to Zymoseptoria tritici in the Tunisian durum wheat landrace accession ‘Agili39’. BMC Genom. 2022, 23, 1–20. [Google Scholar] [CrossRef]
- Sierotzki, H.; Scalliet, G. A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef]
- Harvey, I.C.; Craigie, R.; McCloy, B.L. The control of tan spot of wheat (caused by Pyrenophora tritici-repentis): A possible emerging disease in New Zealand. N. Z. Plant Prot. 2015, 68, 428–433. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Schierenbeck, M.; Gerard, G.S.; Dietz, J.I.; Golik, S.I.; Simón, M.R. Breadmaking quality and yield response to the green leaf area duration caused by fluxapyroxad under three nitrogen rates in wheat affected with tan spot. Crop Prot. 2018, 106, 201–209. [Google Scholar] [CrossRef]
- Bearchell, S.J.; Fraaije, B.A.; Shaw, M.W.; Fitt, B.D.L. Wheat archive links long-term fungal pathogen population dynamics to air pollution. Proc. Natl. Acad. Sci. USA 2005, 102, 5438–5442. [Google Scholar] [CrossRef] [PubMed]
- Heick, T.M.; Justesen, A.F.; Jørgensen, L.N. Resistance of wheat pathogen Zymoseptoria tritici to DMI and QoI fungicides in the Nordic-Baltic region—A status. Eur. J. Plant Pathol. 2017, 149, 669–682. [Google Scholar] [CrossRef]
- Mäe, A.; Fillinger, S.; Sooväli, P.; Heick, T.M. Fungicide Sensitivity Shifting of Zymoseptoria tritici in the Finnish-Baltic Region and a Novel Insertion in the MFS1 Promoter. Front. Plant Sci. 2020, 11, 385. [Google Scholar] [CrossRef] [Green Version]
- Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.G.; Magrans, J.O.; Miron, I.; et al. Review of the existing maximum residue levels for fluxapyroxad according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2020, 18, e06059. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Reasoned opinion on the modification of the existing maximum residue levels for fluxapyroxad in grapes and potatoes. EFSA J. 2015, 13, 4223. [CrossRef]
- United Kingdom. Draft Assessment Report on the Active Substance Fluxapyroxad Prepared by the Rapporteur Member State UK in the Framework of Council Directive 91/414/EEC. 2011. Available online: http://www.efsa.europa.eu (accessed on 15 August 2022).
- Iqbal, M.A.; Fahim, M.A.; Zaman, Q.U.; Usam, M.; Sundus, H.; Rahman, A.U. Effect of various factors on wheat production. Sarhad J. Agric. 2014, 30, 135–143. [Google Scholar]
- Ihsan, M.Z.; El-Nakhlawy, F.S.; Ismail, S.M.; Fahad, S.; Daur, I. Wheat Phenological Development and Growth Studies As Affected by Drought and Late Season High Temperature Stress under Arid Environment. Front. Plant Sci. 2016, 7, 795. [Google Scholar] [CrossRef]
- Sanadya, A.; Kumar, M.; Nanda, H. Low productivity of wheat in Bastar region with special Enphasis on effect of temperature on phenology, growth and yield of wheat: A review. J. Pharmacogn. Phytochem. 2021, 10, 2558–2565. Available online: https://www.phytojournal.com/archives/2021/vol10issue1/PartAJ/10-1-159-152.pdf (accessed on 18 August 2022).
- Bouras, N.; Kim, Y.M.; Strelkov, S.E. Influence of water activity and temperature on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Int. J. Food Microbiol. 2009, 131, 251–2555. [Google Scholar] [CrossRef]
- Menzies, J.; Gilbert, J. Diseases of wheat. In Diseases of Field Crops in Canada, 3rd ed.; Bailey, K.L., Gossen, B.D., Gugel, R.K., Morrall, R.A.A., Eds.; Canadian Phytopathological Society: Harrow, ON, Canada, 2003; pp. 94–128. [Google Scholar]
- Hosford, R.M.; Lárez, C.R.; Hammond, J.J. Interaction of wet period and temperature on Pyrenophora tritici-repentis infection and development in wheats of differing resistance. Phytopathology 1987, 77, 1021–1027. [Google Scholar] [CrossRef]
- Mróz, M.; Sobieraj, A. Comparison of several vegetation indices calculated on the basis of a seasonal SPOT XS time series, and their suitability for land cover and agricultural crop identification. Tech. Sci 2004, 7, 39–66. Available online: http://www.uwm.edu.pl/wnt/technicalsc/ts7_2004/4_7_2004.pdf (accessed on 18 August 2022).













| RC1 | RC2 | RC3 | Uniqueness | |
|---|---|---|---|---|
| Year | 0.913 | 0.146 | ||
| Disease Score | 0.848 | 0.146 | ||
| Temperature (mean) | 0.841 | 0.291 | ||
| Rain | −0.759 | 0.434 | ||
| Calendar DAY | 0.998 | 0.018 |
| Unrotated Solution | Rotated Solution | ||||
|---|---|---|---|---|---|
| Eigen-Value | Proportion var. | Cumulative | SumSq. Loadings | Proportion var. | Cumulative |
| 1.627 | 0.325 | 0.325 | 1.559 | 0.312 | 0.312 |
| 1.320 | 0.264 | 0.589 | 1.364 | 0.273 | 0.585 |
| 1.017 | 0.203 | 0.793 | 1.041 | 0.208 | 0.793 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagelas, I.; Cavalaris, C.; Karapetsi, L.; Koukidis, C.; Servis, D.; Madesis, P. Protective Effects of Systiva® Seed Treatment Fungicide for the Control of Winter Wheat Foliar Diseases Caused at Early Stages Due to Climate Change. Agronomy 2022, 12, 2000. https://doi.org/10.3390/agronomy12092000
Vagelas I, Cavalaris C, Karapetsi L, Koukidis C, Servis D, Madesis P. Protective Effects of Systiva® Seed Treatment Fungicide for the Control of Winter Wheat Foliar Diseases Caused at Early Stages Due to Climate Change. Agronomy. 2022; 12(9):2000. https://doi.org/10.3390/agronomy12092000
Chicago/Turabian StyleVagelas, Ioannis, Chris Cavalaris, Lefkothea Karapetsi, Charalambos Koukidis, Dimitris Servis, and Panagiotis Madesis. 2022. "Protective Effects of Systiva® Seed Treatment Fungicide for the Control of Winter Wheat Foliar Diseases Caused at Early Stages Due to Climate Change" Agronomy 12, no. 9: 2000. https://doi.org/10.3390/agronomy12092000
APA StyleVagelas, I., Cavalaris, C., Karapetsi, L., Koukidis, C., Servis, D., & Madesis, P. (2022). Protective Effects of Systiva® Seed Treatment Fungicide for the Control of Winter Wheat Foliar Diseases Caused at Early Stages Due to Climate Change. Agronomy, 12(9), 2000. https://doi.org/10.3390/agronomy12092000









