Environment-Friendly Control Potential of Two Citrus Essential Oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Isolation of Essential Oils
2.3. GC–MS Analysis of Eos
2.4. Compound Identification
2.5. Insects
2.6. Bioassay
2.6.1. Toxicity Test
2.6.2. Repellence Test
2.7. Statistical Analysis
3. Results
3.1. Toxicity Assay
3.2. Repellent Activity
3.3. Chemical Compositions of Essential Oils
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balikai, R.A.; Prasanna, P.M.; Kotikal, Y.K. Status of pomegranate pests and their management strategies in India. In Proceedings of the 2nd International Conference on Pomegranate and Minor Including Mediterranean Fruits, Dharwad-Karnataka, India, 23–27 June 2009; pp. 147–148. [Google Scholar]
- Moawad, S.S.; Al-Barty, A.M. Evaluation of some medicinal and ornamental plant extracts toward pomegranate aphid, Aphis punicae (Passerini) under laboratory conditions. Afr. J. Agric. Res. 2011, 6, 2425–2429. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the Herbaceous Plants and Shrubs: The Natural History Museum; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Pfeiffer, D.G.; Schultz, P.B. Major insect and mite pests of grape in Virginia. In Virginia Cooperative Extension Service Bulletin; Natural Resource, Agriculture, and Engineering Service Pub, Cornell: Ithaca, NY, USA, 1986; pp. 444–567. [Google Scholar]
- Al-Barty, A.M. Survey and enumeration of pests on pomegranate tree with reference to its parasite in Al-Taif city. Aust. J. Basic Appl. Sci. 2011, 5, 1086–1093. [Google Scholar]
- Sugimoto, S. The taxonomic identity of Aphis punicae (Hemiptera: Aphididae) Shinji, 1922. Entomol. Sci. 2011, 14, 68–74. [Google Scholar] [CrossRef]
- Bhagat, R.C. Aphids (Insecta) of agricultural importance. In J. and K. State, India: A check-list and biodiversity. Int. J. Food Agric. Vet. Sci. 2012, 2, 116–125. [Google Scholar]
- Lee, Y.; Lee, W.; Kim, H.; Lee, S. A new record of Aphis punicae Passerini, 1863 (Hemiptera: Aphididae) from Korea. J. Asia Pac. Entomol. 2015, 18, 157–163. [Google Scholar] [CrossRef]
- Cocuzza, G.E.; Mazzeo, G.; Russo, A.; Giudice, V.L.; Bella, B. Pomegranate arthropod pests and their management in the Mediterranean area. Phytoparasitica 2016, 44, 393–409. [Google Scholar] [CrossRef]
- Eser, S.I.; Gorur, G.; Tepecik, I.; Akyildirim, H. Aphid (Hemiptera: Aphidoidea) species of the Urla district of Izmir region. J. Appl. Biol. Sci. 2008, 3, 92–95. [Google Scholar]
- Tsitsipis, J.A.; Katis, N.I.; Margaritopoulos, J.T.; Lykouressis, D.P.; Avgelis, A.D.; Gargalianou, I.; Zarpas, K.D.; Perdikis, D.C.; Papapanayotou, A. A contribution to the aphid fauna of Greece. Bull. Insectology 2007, 60, 31–38. [Google Scholar]
- Heidarnia, M.; Derakhshan, A. Faunistic study of Esfarayen aphids and the new report of the species, Aphis illinoisensis Shimer, 1866 for Iran fauna. In Proceedings of the 23th Iranian Plant Protection Congress, Gorgan, Iran, 27–30 August 2018. [Google Scholar]
- Kamel-Ben Halima, M.; Mdellel, L. First record of the grapevine aphid, Aphis illinoisensis Shimer, in Tunisia. OEPP/EPPO Bull. 2010, 40, 191–192. [Google Scholar] [CrossRef]
- Seljak, G. Prva najdba črne trtne uši (Aphis illinoisensis Shimer, 1866) v Sloveniji (Hemiptera, Aphidoidea: Aphididae). Acta Entomol. Slov. 2021, 29, 1. [Google Scholar]
- Mouttet, R.; Balmès, V. Un nouveau puceron sur vigne en France: Aphis illinoisensis Shimer, 1866 (Hemiptera, Aphididae). Bull. Soc. Entomol. Fr. 2021, 126, 206–208. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on World’s Plants: An Online Identification and Information Guide. 2021. Available online: http://www.aphidsonworldsplants.info (accessed on 30 September 2021).
- Cocuzza, G.E.; Barbagallo, S. The appearance of the American aphid, Aphis illinoisensis, in grapes in Sicily. Informatore Agrario 2011, 67, 81–83. [Google Scholar]
- Casiraghi, A. First record of the invasive grapevine aphid Aphis illinoisensis Shimer, 1866 (Hemiptera: Aphididae) in mainland Italy. Arch. Biol. Sci. 2021, 70, 373–375. [Google Scholar]
- Hussain, S.; Aldryhim, Y.; Al-Dhafer, H.; Halbert, S.E.; Thomas, J. New aphid records for Saudi Arabia (Hemiptera: Aphidoidea). Zool. Middle East 2015, 61, 368–371. [Google Scholar] [CrossRef]
- Sayed, S.; El-Shehawi, A.; Al-Otaibi, S.; El-Shazly, S.; Al-Otaibi, S.; Ibrahim, R.; Alorabi, M.; Baazeem, A.; Elseehy, M. Isolation and efficacy of the endophytic fungus, Beauveria bassiana (Bals.) Vuillemin on grapevine aphid, Aphis illinoisensis under laboratory conditions. Egypt. J. Biol. Pest Control 2020, 30, 38. [Google Scholar] [CrossRef]
- Li, F.; Han, Z. Mutations in acetylcholinesterase associated with insecticide resistance in the cotton aphid, Aphis gossypii Glover. Insect Biochem. Mol. Biol. 2004, 34, 397–405. [Google Scholar] [CrossRef]
- Abd-Ella, A.A. Effect of several insecticides on pomegranate aphid, Aphis punicae Passerini (Homoptera: Aphididae) and its predators under field conditions. OEPP/EPPO Bull. 2015, 45, 90–98. [Google Scholar] [CrossRef]
- Juan, P.; Martìnez, J.; Martìnez, J.J.; Oltra, M.A.; Ferràndez, M. Current situation of pomegranate growing (Punica granatum L.) in southern Alicante: Chemical control of pests and diseases and financial cost. In Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology, Proceedings of the Symposium Jointly Organized by CIHEAM and Escuela Politécnica Superior de Orihuela of the Universidad Miguel Hernández (EPSO-UMH), Orihuela, Spain, 15–17 October 1998; Malgarejo, P., Martìnez-Nicolàs, J.J., Martìnez-Tomé, J., Eds.; CIHEAM: Paris, France, 2000; pp. 157–161. [Google Scholar]
- Delfan, A.; Tous, A.H.D. Determine the changes and the peak population of pomegranate aphid, Aphis punicae (Hem.: Aphididae) for integrated management it in Khorramabad city. In Proceedings of the 23rd Iranian Plant Protection Congress, Gorgan, Iran, 27–30 August 2018. [Google Scholar]
- Ga’Al, H.; Yang, G.; Fouad, H.; Guo, M.; Mo, J. Mannosylerythritol lipids mediated biosynthesis of silver nanoparticles: An eco-friendly and operative approach against chikungunya vector Aedes albopictus. J. Clust. Sci. 2021, 32, 17–25. [Google Scholar] [CrossRef]
- Hao, D.; Xiao, P. Genomics and evolution in traditional medicinal plants: Road to a healthier life. Evol. Bioinform. 2015, 11, 197–212. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia 2004, 75, 149–161. [Google Scholar] [CrossRef]
- Pandey, A.K.; Tripathi, S.; Singh, P. Plant essential oils: A substitute for conventional insecticides against Tribolium species (Coleoptera: Tenebrionidae)-achievements and challenges. Arch. Phytopathol. Plant Prot. 2018, 51, 696–728. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects–a review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crop. Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Nathan, S.S.; Kalaivani, K.; Chung, P.G. The effects of azadirachtin and nucleopolyhedrovirus on midgut enzymatic profile of Spodoptera litura Fab. (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2005, 83, 46–57. [Google Scholar] [CrossRef]
- Gioffrè, G.; Ursino, D.; Labate, M.L.; Giuffrè, A.M. The peel essential oil composition of bergamot fruit (Citrus bergamia, Risso) of Reggio Calabria (Italy): A review. Emir. J. Food Agric. 2020, 32, 835–845. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Nobile, R. Citrus bergamia, Risso: The peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (South Italy). Emir. J. Food Agric. 2020, 32, 522–532. [Google Scholar]
- Luro, F.; Garcia Neves, C.; Costantino, G.; da Silva Gesteira, A.; Paoli, M.; Ollitrault, P.; Tomi, F.; Micheli, F.; Gibernau, M. Effect of environmental conditions on the yield of peel and composition of essential oils from citrus cultivated in Bahia (Brazil) and Corsica (France). Agronomy 2020, 10, 1256. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Boukhatem, M.N.; Hazzit, M.; Meklati, B.Y.; Chemat, F. Cold pressing, hydrodistillation and microwave dry distillation of citrus essential oil from Algeria: A comparative study. Electron. J. Biol. 2016, S1, 30–41. [Google Scholar]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmentally friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Damian-Reyna, A.A.; Gonzalez-Hernandez, J.C.; Maya-Yescas, R.; de Jesus Cortes-Penagos, C.; Del Carmen Chavez-Parga, M. Polyphenolic content and bactericidal effect of Mexican Citrus limetta and Citrus reticulata. J. Food Sci. Technol. 2017, 54, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Khelifa, S.; M’Hamdi, M.; Rejeb, H.; Belbahri, L.; Souayeh, N. Relation between catalase activity, salt stress and urban environment in Citrus aurantium L. J. Hortic. For. 2011, 3, 186–189. [Google Scholar]
- Kostopoulou, Z.; Therios, I.; Roumeliotis, E.; Kanellis, A.K.; Molassiotis, A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol. Biochem. 2015, 86, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Loza-Tavera, H. Monoterpenes in essential oils. In Chemicals via Higher Plant Bioengineering. Advances in Experimental Medicine and Biology; Shahidi, F., Kolodziejczyk, P., Whitaker, J.R., Munguia, A.L., Fuller, G., Eds.; Springer: Boston, MA, USA, 1999; Volume 464, pp. 49–62. [Google Scholar]
- Ladaniya, M.S. Citrus Fruit: Biology, Technology and Evaluation; Elsevier Inc.: Atlanta, GA, USA, 2008; pp. 1–10. [Google Scholar]
- Yang, Y.; Isman, M.B.; Tak, J.H. Insecticidal activity of 28 essential oils and a commercial product containing Cinnamomum cassia bark essential oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef]
- Alghamdi, A.S. Insecticidal effect of four plant essential oils against two aphid species under laboratory conditions. J. Appl. Boil. 2018, 6, 27–30. [Google Scholar]
- Abdelaal, K.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Hassan, F.A.S.; Hafez, Y. Toxicity of essential oils nanoemulsion against Aphis craccivora and their inhibitory activity on insect enzymes. Processes 2021, 9, 624. [Google Scholar] [CrossRef]
- Sayed, S.; Soliman, M.M.; Al-Otaibi, S.; Hassan, M.M.; Elarrnaouty, S.A.; Abozeid, S.M.; El-Shehawi, A.M. Toxicity, deterrent and repellent activities of four essential oils on Aphis punicae (Hemiptera: Aphididae). Plants 2022, 11, 463. [Google Scholar] [CrossRef]
- Al-Harbi, N.A.; Al Attar, N.M.; Hikal, D.M.; Mohamed, S.E.; Abdel Latef, A.H.; Ibrahim, A.A.; Abdein, M.A. Evaluation of insecticidal effects of plants essential oils extracted from basil, black seeds and lavender against Sitophilus oryzae. Plants 2021, 10, 829. [Google Scholar] [CrossRef]
- Demeter, S.; Lebbe, O.; Hecq, F.; Nicolis, S.; Kemene, T.K.; Martin, H.; Fauconnier, M.L.; Hance, T. Insecticidal activity of 25 essential oils on the stored product pest, Sitophilus granarius. Foods 2021, 10, 200. [Google Scholar] [CrossRef]
- Gupta, G.; Agarwal, U.; Kaur, H.; Kumar, N.R.; Gupta, P. Aphicidal effects of terpenoids present in Citrus limon on Macrosiphum roseiformis and two generalist insect predators. J. Asia Pac. Entomol. 2017, 20, 1087–1095. [Google Scholar] [CrossRef]
- Yazdgerdian, A.R.; Akhtar, Y.; Isman, M.B. Insecticidal effects of essential oils against woolly beech aphid, Phyllaphis fagi (Hemiptera: Aphididae) and rice weevil, Sitophilus oryzae (Coleoptera: Curculionidae). J. Entomol. Zool. Stud. 2015, 3, 265–271. [Google Scholar]
- Tunı, I.; Sahinkaya, S. Sensitivity of two greenhouse pest to vapours of essential oils. Entomol. Exp. Appl. 1998, 86, 183–187. [Google Scholar]
- Sampson, B.J.; Tabanca, N.; Kirimer, N.E.; Demirci, B.; Baser, K.H.C.; Khan, I.A.; Spiers, J.M.; Wedge, D.E. Insecticidal activity of 23 essential oils and their major compounds against adult Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera). Pest Manag. Sci. 2005, 61, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Murovhi, J.; Phophi, M.M.; Mafongoya, P. Efficacy of plant materials in controlling aphids on okra (Abelmoschus esculentus L. Moench) in Limpopo Province of South Africa. Agronomy 2020, 10, 1968. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Adam, W.; Stegmann, V. Hydroxy-group directivity in the regioselective and diastereoselective [2+ 2] photocycloaddition (Paterno-Büchi reaction) of aromatic carbonyl compounds to chiral and achiral allylic substrates: The preparation of oxetanes with up to three stereogenic centers as synthetic building blocks. Chem. Inform. 2001, 112, 1203–1214. [Google Scholar]
- Eidy, M.; Rafiee-Dastjerdi, H.; Zargarzadeh, F.; Golizadeh, A.; Mahdavi, V. Pathogenicity of the entomopathogenic fungi Beauveria bassiana (Balsamo) and Verticillium lecanii (Zimmerman) against aphid Macrosiphum rosae, Linnaeus (Hemiptera: Aphididae) under laboratory conditions. Jordan J. Biol. Sci. 2016, 9, 25–28. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Isman, M.B.; Machial, C.M. Pesticides based on plant essential oils: From traditional practice to commercialization. Adv. Phytomed. 2006, 3, 29–44. [Google Scholar]
- Iqbal, M.F.; Kahloon, M.H.; Nawaz, M.R.; Javid, M.J. Effectiveness of some botanical extracts on wheat aphids. J. Anim. Plant Sci. 2011, 21, 114–115. [Google Scholar]
- Amiri, M.P.; Rouhani, M.; Mostafavi, H.; Aminizadeh, M.R. Enetomotoxic effect of plant extracts against the cowpea aphid, Aphis craccivora (Hem: Aphididae). Int. J. Agric. 2013, 3, 569–573. [Google Scholar]
- Abdel-Aziz, N.F.; Abdou, W.L.; Abdel-Hakim, E.A.; El-Hawarya, F.M.; El-Bakry, A.M.; Sammour, E.A. The effect of some green insecticides from essential oils on Aphis craccivora, and their side effects. J. Entomol. Res. 2015, 39, 275–286. [Google Scholar] [CrossRef]
- Fiaz, M.; Hameed, A.; Hasan, M.; Wakil, W. Efficacy of plant extracts on some cotton (Gossypium hirsutum) pests: Amrasca bigutulla bigutulla Ishida and Thrips tabaci Limdeman. Pak. J. Zool. 2012, 44, 277–283. [Google Scholar]
- Rajashekar, Y.; Bakthavatsalam, N.; Shivanandappa, T. Botanicals as grain protectants. Psyche 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Yousuf, I.; Buhroo, A.A.; Nisa, G. Repellent activities of some plant extracts against rose aphid, Macrosiphum rosae (Hemiptera: Aphididae). Munis Entomol. Zool. 2021, 16, 1045–1055. [Google Scholar]
- Sreepian, A.; Popruk, S.; Nutalai, D.; Phutthanu, C.; Sreepian, P.M. Antibacterial activities and synergistic interaction of citrus essential oils and limonene with gentamicin against clinically isolated methicillin-resistant Staphylococcus aureus. Sci. World J. 2022, 2022, 8418287. [Google Scholar] [CrossRef]
- Lin, X.; Cao, S.; Sun, J.; Lu, D.; Zhong, B.; Chun, J. The chemical compositions, and antibacterial and antioxidant activities of four types of citrus essential oils. Molecules 2021, 26, 3412. [Google Scholar] [CrossRef]
- Boughendjioua, H.; Mezedjeri, N.; Idjouadiene, I. Chemical constituents of Algerian mandarin (Citrus reticulata) essential oil by GC-MS and FT-IR analysis. Curr. Issues Pharm. Med. Sci. 2020, 33, 197–201. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Aouadi, K.; Snoussi, M.; Noumi, E.; Kadri, A. GC-MS profile, α-glucosidase inhibition potential, antibacterial and antioxidant evaluation of peels Citrus aurantium (L.), essential oil. J. Pharm. Res. Int. 2021, 33, 1580–1591. [Google Scholar] [CrossRef]
- Fadilah, N.Q.; Jittmittraphap, A.; Leaungwutiwong, P.; Pripdeevech, P.; Dhanushka, D.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Virucidal activity of essential oils from Citrus aurantium against Influenza A Virus H1N1: Limonene as a potential household disinfectant against virus. Nat. Prod. Commun. 2022, 17, 1–7. [Google Scholar] [CrossRef]
- Sevindik, E.; Aydın, S.; Sujka, M.; Apaydın, E.; Yıldırım, K.; Palas, G. GC-MS analysis and evaluation of antibacterial and antifungal activity of essential oils extracted from fruit peel of Citrus aurantium L. (Rutaceae) grown in the west Anatolian area. Erwerbs-Obstbau 2021, 63, 135–142. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Villafañe, E.; Tolosa, D.; Bardón, A.; Neske, A. Toxic effects of Citrus aurantium and C. limon essential oils on Spodoptera frugiperda (Lepidoptera: Noctuidae). Nat. Prod. Commun. 2011, 6, 1389–1392. [Google Scholar] [CrossRef]
- Khan, F.A.; Abdeltawab, A.A.; Al-Deyab, S.S.; Ali, J.; Ullah, R.; Qureshi, M.N.; Ziaurrahman, Q.; Siddique, M.; Ullah, N. Comparative evaluation of physiochemical and GC-MS analysis of sour oranges and sweet oranges peels oil. Life Sci. J. 2013, 10, 205–209. [Google Scholar]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.; Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Marzouk, B. Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. Biomed. Res. Int. 2013, 2013, 345415. [Google Scholar]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Suryawanshi, J.A.S. An overview of Citrus aurantium used in treatment of various diseases. Afr. J. Plant Sci. 2011, 5, 390–395. [Google Scholar]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Chhetri, B.K.; Dosoky, N.S.; Shari, K.; Al-Fahad, A.; Wessjohann, L.; Setzer, W.N. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines 2017, 4, 17. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Yan, J.; Sun, Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Li, X.J.; Qin, Q.F.; Li, Y.S.; Zhang, W.K.; Tang, H.B. Anti-inflammatory and antinociceptive effects of active ingredients in the essential oils from Gynura procumbens, a traditional medicine and a new and popular food material. J. Ethnopharmacol. 2019, 239, 111916. [Google Scholar] [CrossRef]
- Eirini, S.; Paschalina, C.; Loannis, T.; Kortessa, D.T. Effect of drought and salinity on volatile organic compounds and other secondary metabolites of Citrus aurantium leaves. Nat. Prod. Commun. 2017, 12, 1193–1196. [Google Scholar] [CrossRef]
- Cámara-Zapata, J.; García-Sánchez, F.; Martinez, V.; Nieves, M.; Cerdá, A. Effect of NaCl on citrus cultivars. Agronomie 2004, 24, 155–160. [Google Scholar] [CrossRef]
- Gimeno, V.; Gimeno, V.; Syvertsen, J.P.; Rubio, F.; Martínez, V.; García-Sánchez, F. Growth and mineral nutrition are affected by substrate type and salt stress in seedlings of two contrasting citrus rootstocks. J. Plant Nutr. 2010, 33, 1435–1447. [Google Scholar] [CrossRef]
- Sievert, B. Cereal Based Food Product Comprising DHA and/or EPA. U.S. Patent Application No. 10/523,765, 11 May 2006. [Google Scholar]
- Ezeonu, F.C.; Chidume, G.I.; Udedi, S.C. Insecticidal properties of volatile extracts of orange peels. J. Biochem. Technol. 2001, 76, 273–274. [Google Scholar] [CrossRef]
- Mansour, F.; Azaizeh, H.; Saad, B.; Tadmor, Y.; Abo-Moch, F.; Said, O. The potential of middle eastern flora as a source of new bio-acaricides to control Tetranychus cinnabarinus, the carmine spider mite. Phytoparasitica 2004, 32, 66–72. [Google Scholar] [CrossRef]
- Akram, W.; Khan, H.A.A.; Hafeez, F.; Bilal, H.; Kim, Y.K.; Lee, J.J. Potential of Citrus seed extracts against dengue fever mosquito, Aedes albopictus (Skuse) (Culicidae: Diptera). Pak. J. Bot. 2010, 42, 3343–3348. [Google Scholar]
- Abad, M.R.; Besheli, B.A. Insecticidal potential of essential oil from the leaves of Citrus aurantium L. against Oryzaephilus surinamensis (F.), Lasioderma serricorne (L.) and Sitophilus oryzae (L.). J. Entomol. Zool. Stud. 2016, 4, 865–869. [Google Scholar]
- Estrela, J.L.V.; Fazolin, M.; Catani, V.; Alécio, M.R.; de Lima, M.S. Toxicidade de óleos essenciais de Piper aduncum e Piper hispidinervum em Sitophilus zeamais. Pesqui. Agropecu. Bras. 2006, 41, 217–222. [Google Scholar] [CrossRef]
- Restello, R.M.; Menegatt, C.; Mossi, A.J. Efeito do óleo essencial de Tagetes patula L. (Asteraceae) sobre Sitophilus zeamais Motschulsky (Coleoptera, Curculionidae). Rev. Bras. Entomol. 2009, 53, 304–307. [Google Scholar] [CrossRef]
- Changbunjong, T.; Boonmasawai, S.; Sungpradit, S.; Weluwanarak, T.; Leesombun, A. Contact and fumigant activities of Citrus aurantium essential oil against the stable fly Stomoxys calcitrans (Diptera: Muscidae). Plants 2022, 11, 1122. [Google Scholar] [CrossRef]
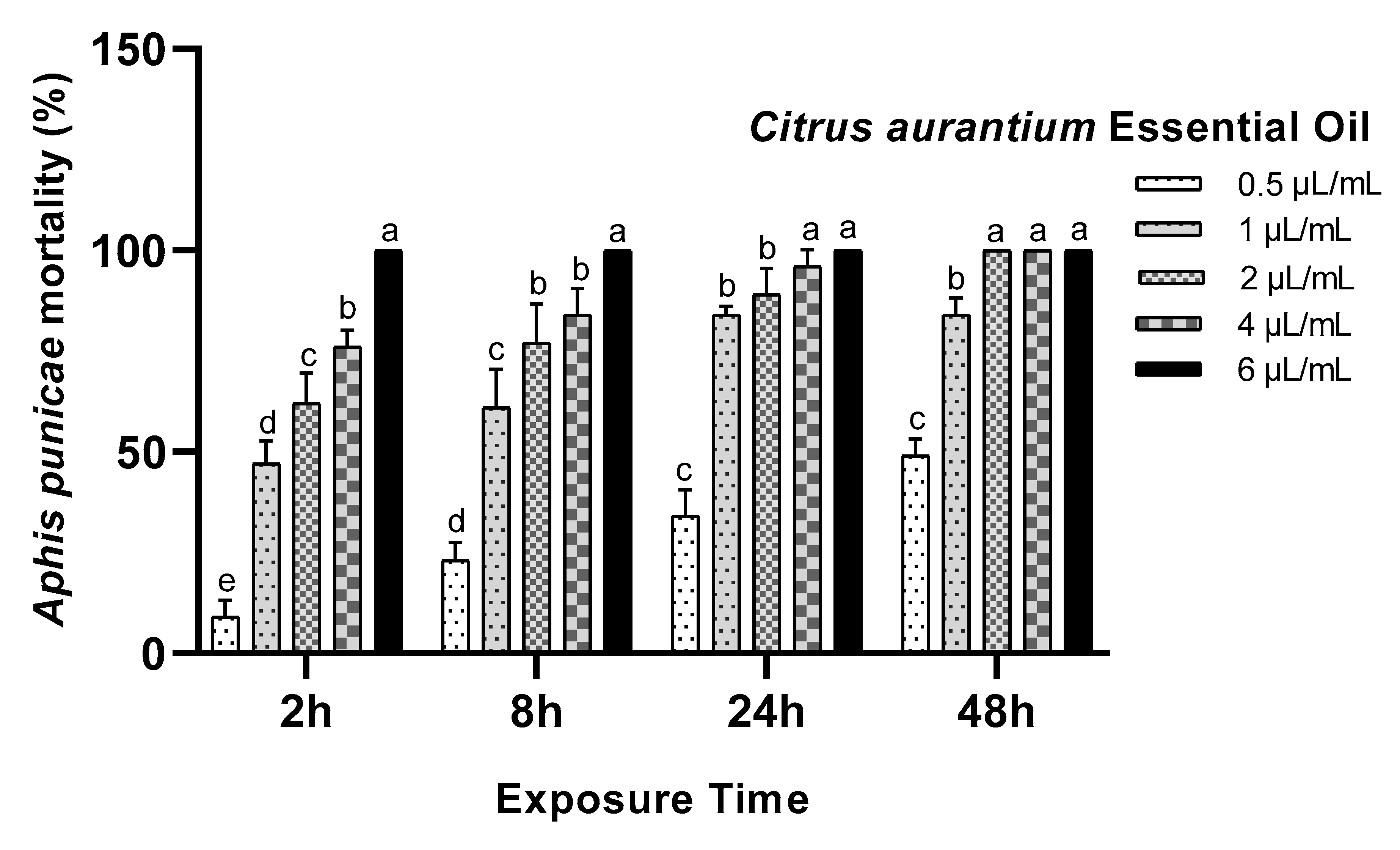
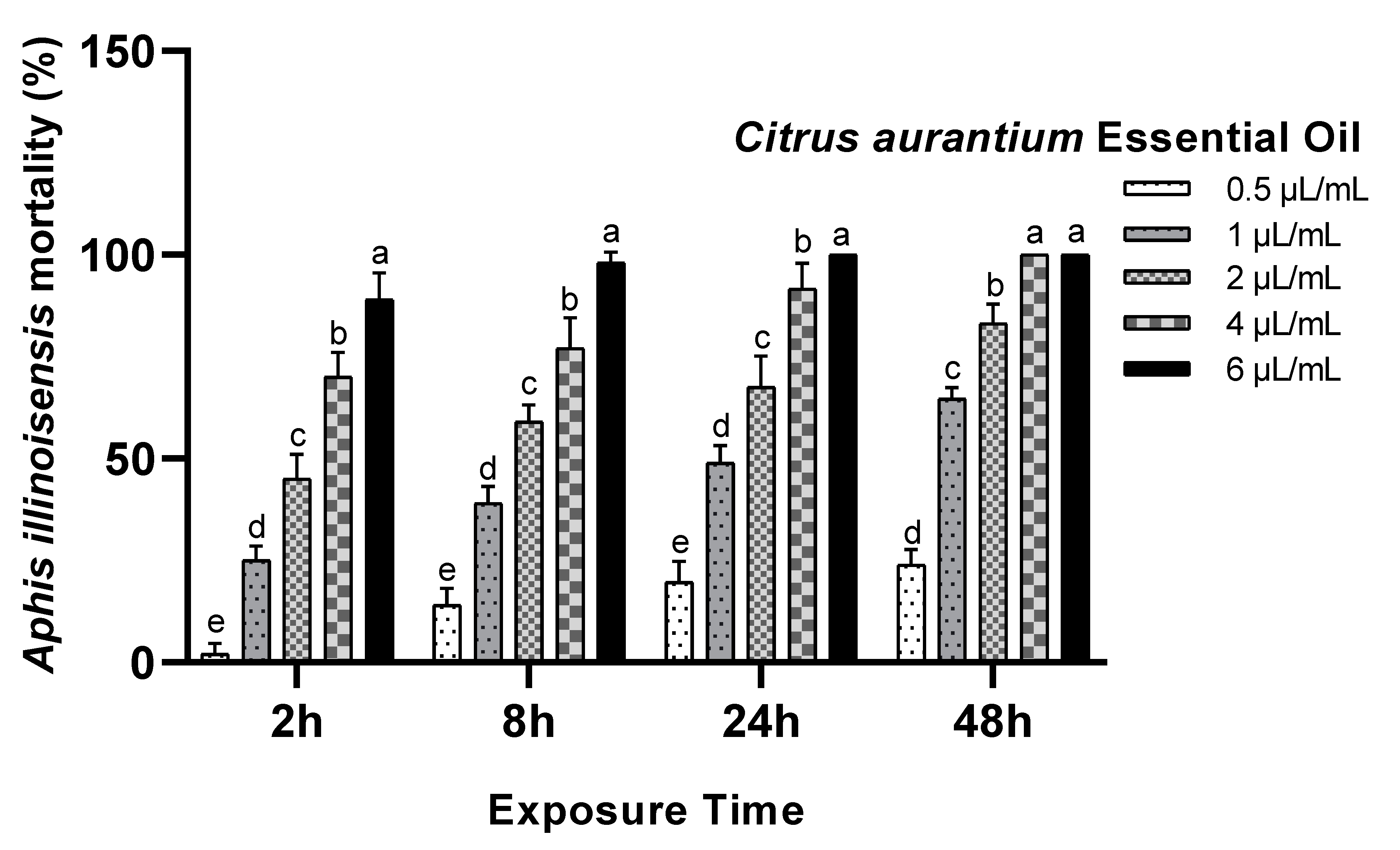

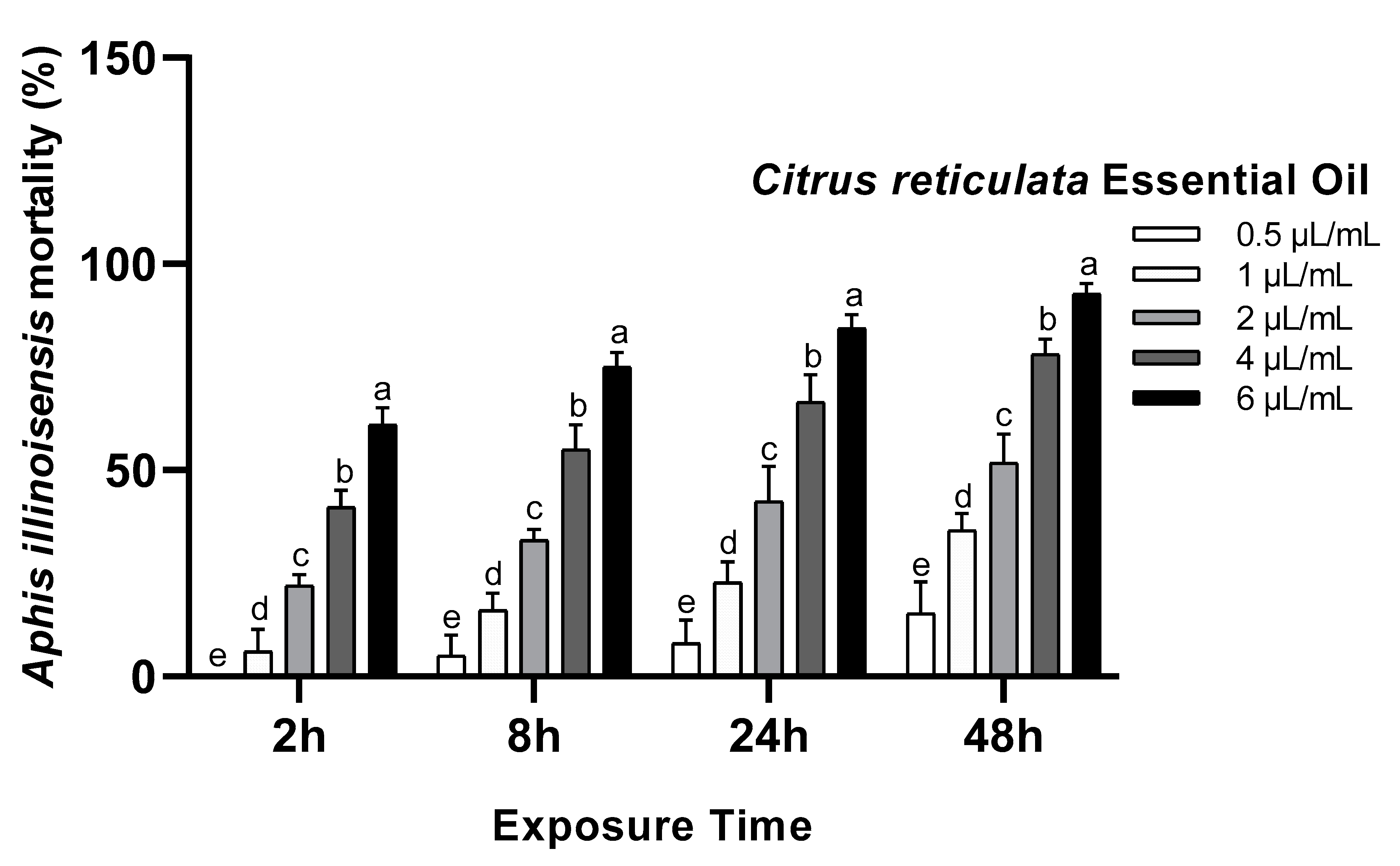
| Essential Oils | Exposure Time (h) | LC50 µL/mL (95% LCL–UCL) | LC90 µL/mL (95% LCL–UCL) | Slope ± SE | Intercept | X2 | p-Value |
|---|---|---|---|---|---|---|---|
| Citrus aurantium | 2 | 3.95 (2.32–5.98) | 4.8 (2.98–6.69) | 2.42 ± 0.46 | 5.05 | 6.06 | 0.0131 |
| 8 | 2.31 (1.04–4.69) | 3.87 (1.6–5.1) | 2.87 ± 0.57 | 4.59 | 7.01 | 0.0154 | |
| 24 | 1.74 (1.11–3.4) | 2.89 (1.36–4.47) | 2.26 ± 0.36 | 5.55 | 7.86 | 0.0082 | |
| 48 | 0.37 (0.047–4.04) | 1.13 (0.89–2.33) | 2.25 ± 0.56 | 5.97 | 0.02 | 0.0276 | |
| Citrus reticulata | 2 | 4.28 (3.37–5.79) | 8.47 (6.63–12.97) | 2.11 ± 0.24 | 1.11 | 1.99 | 0.0030 |
| 8 | 2.92 (2.01–3.97) | 7.14 (5.58–10.91) | 2.20 ± 0.38 | 3.50 | 2.42 | 0.0110 | |
| 24 | 1.85 (0.99–2.56) | 5.1 (4.07–7.28) | 2.44 ± 0.42 | 5.21 | 1.37 | 0.0100 | |
| 48 | 1.03 (−0.09–1.73) | 4.13 (3.21–6.21) | 2.12 ± 0.49 | 8.03 | 1.28 | 0.0220 |
| Essential Oils | Exposure Time (h) | LC50 µL/mL (95% LCL–UCL) | LC90 µL/mL (95% LCL–UCL) | Slope ± SE | Intercept | X2 | p-Value |
|---|---|---|---|---|---|---|---|
| Citrus aurantium | 2 | 2.29 (1.91–3.69) | 5.71 (4.74–7.51) | 2.8 ± 0.28 | 4.0 | 4.03 | 0.0022 |
| 8 | 1.41 (1.23–3.92) | 4.69 (3.8–6.44) | 2.57 ± 0.42 | 4.62 | 2.74 | 0.0088 | |
| 24 | 1.09 (1.88–3.68) | 3.47 (2.77–5.0) | 2.78 ± 0.28 | 4.9 | 1.83 | 0.0022 | |
| 48 | 0.82 (2.0–3.87) | 2.15 (1.69–3.44) | 2.94 ± 0.29 | 5.25 | 2.12 | 0.0021 | |
| Citrus reticulata | 2 | 4.89 (4.06–6.31) | 8.33 (6.77–11.81) | 2.2 ± 0.12 | −0.75 | 2.55 | 0.0050 |
| 8 | 3.82 (3.06–4.89) | 7.35 (5.96–10.29) | 2.48 ± 0.19 | 0.67 | 1.62 | 0.0010 | |
| 24 | 3.05 (2.34–3.89) | 6.31 (5.16–8.32) | 2.68 ± 0.30 | 1.72 | 1.82 | 0.0030 | |
| 48 | 2.21 (1.49–2.91) | 5.24 (4.25–7.22) | 2.67 ± 0.38 | 3.73 | 1.32 | 0.0060 |
| Essential Oils | Dose (µL/cm2) | a Repellence % | ||||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 180 min | ||
| Citrus aurantium | 0.156 | b 8 ± 3.7 | 20 ± 3.2 | 30 ± 3.2 | 36 ± 2.4 | 40 ± 0 |
| 0.312 | 12 ± 3.7 | 26 ± 2.4 | 40 ± 3.2 | 50 ± 3.2 | 52 ± 3.7 | |
| 0.625 | 28 ± 2 | 44 ± 2.4 | 56 ± 2.4 | 60 ± 0 | 64 ± 2.4 | |
| 1.25 | 50 ± 3.2 | 66 ± 2.4 | 72 ± 3.7 | 76 ± 2.4 | 86 ± 2.4 | |
| 2.5 | 70 ± 3.2 | 82 ± 2 | 92 ± 3.7 | 98 ± 2 | 100 ± 0 | |
| Citrus reticulata | 0.156 | 0 ± 0 | 4 ± 2.4 | 8 ± 2 | 22 ± 4.9 | 36 ± 2.4 |
| 0.312 | 2 ± 2 | 8 ± 2 | 16 ± 2.4 | 34 ± 2.4 | 46 ± 2.4 | |
| 0.625 | 6 ± 2.4 | 24 ± 2.4 | 38 ± 2 | 46 ± 2.4 | 50 ± 0 | |
| 1.25 | 16 ± 2.4 | 38 ± 2 | 46 ± 2.4 | 52 ± 2 | 58 ± 3.7 | |
| 2.5 | 22 ± 2 | 48 ± 2 | 52 ± 2 | 56 ± 2.4 | 70 ± 3.2 | |
| Essential Oils | Dose (µL/cm2) | a Repellence % | ||||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 180 min | ||
| Citrus aurantium | 0.156 | b 2 ± 2 | 4 ± 2.4 | 14 ± 4 | 26 ± 2.4 | 32 ± 2 |
| 0.312 | 6 ± 2.4 | 10 ± 3.2 | 18 ± 2 | 30 ± 3.2 | 40 ± 0 | |
| 0.625 | 8 ± 2 | 18 ± 2 | 28 ± 2 | 36 ± 2.4 | 46 ± 2.4 | |
| 1.25 | 18 ± 2 | 28 ± 2 | 44 ± 2.4 | 44 ± 2.4 | 56 ± 4 | |
| 2.5 | 32 ± 2 | 42 ± 2 | 50 ± 3.2 | 68 ± 3.7 | 88 ± 3.7 | |
| Citrus reticulata | 0.156 | 0 ± 0 | 0 ± 0 | 2 ± 2 | 6 ± 2.4 | 14 ± 2.4 |
| 0.312 | 0 ± 0 | 2 ± 2 | 6 ± 2.4 | 6 ± 2.4 | 18 ± 2 | |
| 0.625 | 4 ± 2.4 | 8 ± 3.7 | 12 ± 3.7 | 18 ± 2 | 32 ± 2 | |
| 1.25 | 8 ± 2 | 14 ± 2.4 | 14 ± 2 | 24 ± 2.4 | 42 ± 2 | |
| 2.5 | 22 ± 2 | 26 ± 2.4 | 34 ± 2.4 | 44 ± 2.4 | 62 ± 3.7 | |
| No. | Compounds | C. aurantium | C. reticulata | ||||
|---|---|---|---|---|---|---|---|
| Detect | R.T (Min.) | Area % | Detect | R.T (Min.) | Area % | ||
| 1 | Limonene | + | 4.738 | 96.98 | + | 4.768 | 91.86 |
| 2 | α-Pinene | + | 3.020 3.930 | 0.42 0.88 | + | 3.026 3.946 | 0.33 0.40 |
| 3 | 3-Carene | + | 3.629 4.285 6.371 | 0.31 0.27 0.63 | + | 3.640 13.846 | 0.04 0.17 |
| 4 | p-Cymene | + | 4.605 | 0.06 | + | 4.620 | 0.04 |
| 5 | beta-Guaiene | + | 16.570 | 0.23 | - | - | - |
| 6 | 2,7-Bis (spirocyclopropane)bicyclo [2.2.1]heptan-5-one | + | 8.785 | 0.22 | - | - | - |
| 7 | cis-p-Mentha-2,8-dien-1-ol | - | - | - | + | 7.150 | 1.08 |
| 8 | Carveol | - | - | - | + | 7.264 9.454 9.808 | 0.94 0.86 0.41 |
| 9 | Doconexent | - | - | - | + | 8.801 8.914 13.294 13.732 | 0.19 0.34 0.53 0.72 |
| 10 | Methyl 6,8-octadeca diynoate and Methyl 8,10-octadecadiynoate | - | - | - | + | 6.887 11.851 12.193 | 0.26 0.56 0.28 |
| 11 | (-) Carvone | - | - | - | + | 10.089 | 0.29 |
| 12 | 3-Cyclohexen-1-ol, 5-methylene-6 (1methylethenyl)-, acetate | - | - | - | + | 12.466 | 0.19 |
| 13 | Oxacyclotetradeca-4,11-diyne | - | - | - | + | 13.080 | 0.13 |
| 14 | 5,8-Dimethylene bicyclo [2.2.2]oct-2-ene | - | - | - | + | 8.359 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, S.S.; Darwish, H.; Alzahrani, A.K.; Alharthi, S.; Alghamdi, A.S.; Al-Barty, A.M.; Helal, M.; Maghrabi, A.; Baazeem, A.; Alamari, H.A.; et al. Environment-Friendly Control Potential of Two Citrus Essential Oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae). Agronomy 2022, 12, 2040. https://doi.org/10.3390/agronomy12092040
Alotaibi SS, Darwish H, Alzahrani AK, Alharthi S, Alghamdi AS, Al-Barty AM, Helal M, Maghrabi A, Baazeem A, Alamari HA, et al. Environment-Friendly Control Potential of Two Citrus Essential Oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae). Agronomy. 2022; 12(9):2040. https://doi.org/10.3390/agronomy12092040
Chicago/Turabian StyleAlotaibi, Saqer S., Hadeer Darwish, Ahmed K. Alzahrani, Sarah Alharthi, Akram S. Alghamdi, Amal M. Al-Barty, Mona Helal, Amal Maghrabi, Alaa Baazeem, Hala A. Alamari, and et al. 2022. "Environment-Friendly Control Potential of Two Citrus Essential Oils against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae)" Agronomy 12, no. 9: 2040. https://doi.org/10.3390/agronomy12092040






