Genetic Evaluation of a Diverse Rice Panel for Direct Seeded Adapted Traits Using Kompetitive Allele Specific Primer Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Irrigation
2.2. Fertilizer
2.3. Weeding
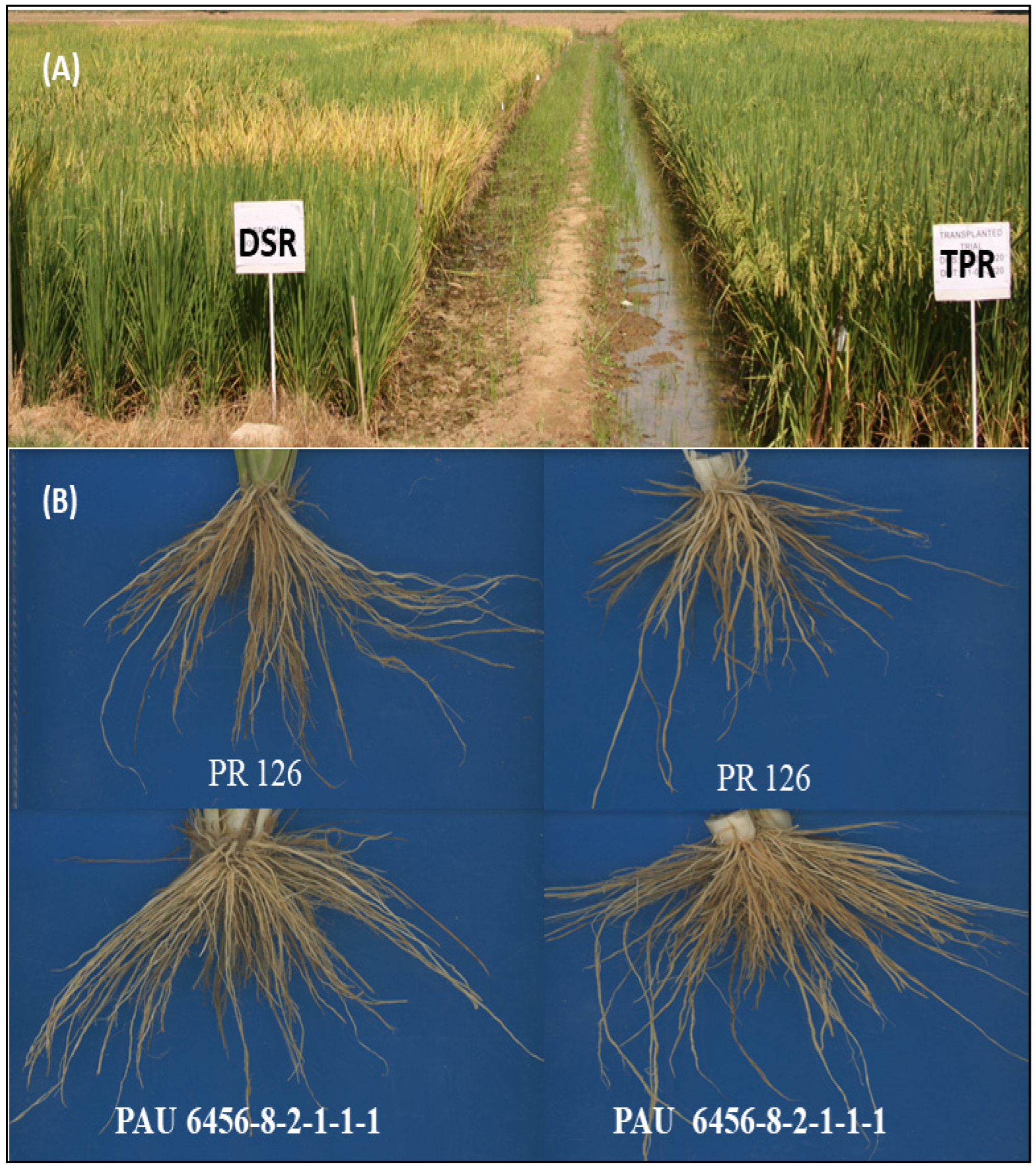
2.4. Phenotypic Characterization of the Breeding Panel
2.5. Statistical Analysis
2.6. Genotyping
2.7. DNA Extraction and Quantification
2.8. KASP Assay
2.9. KASP Assay
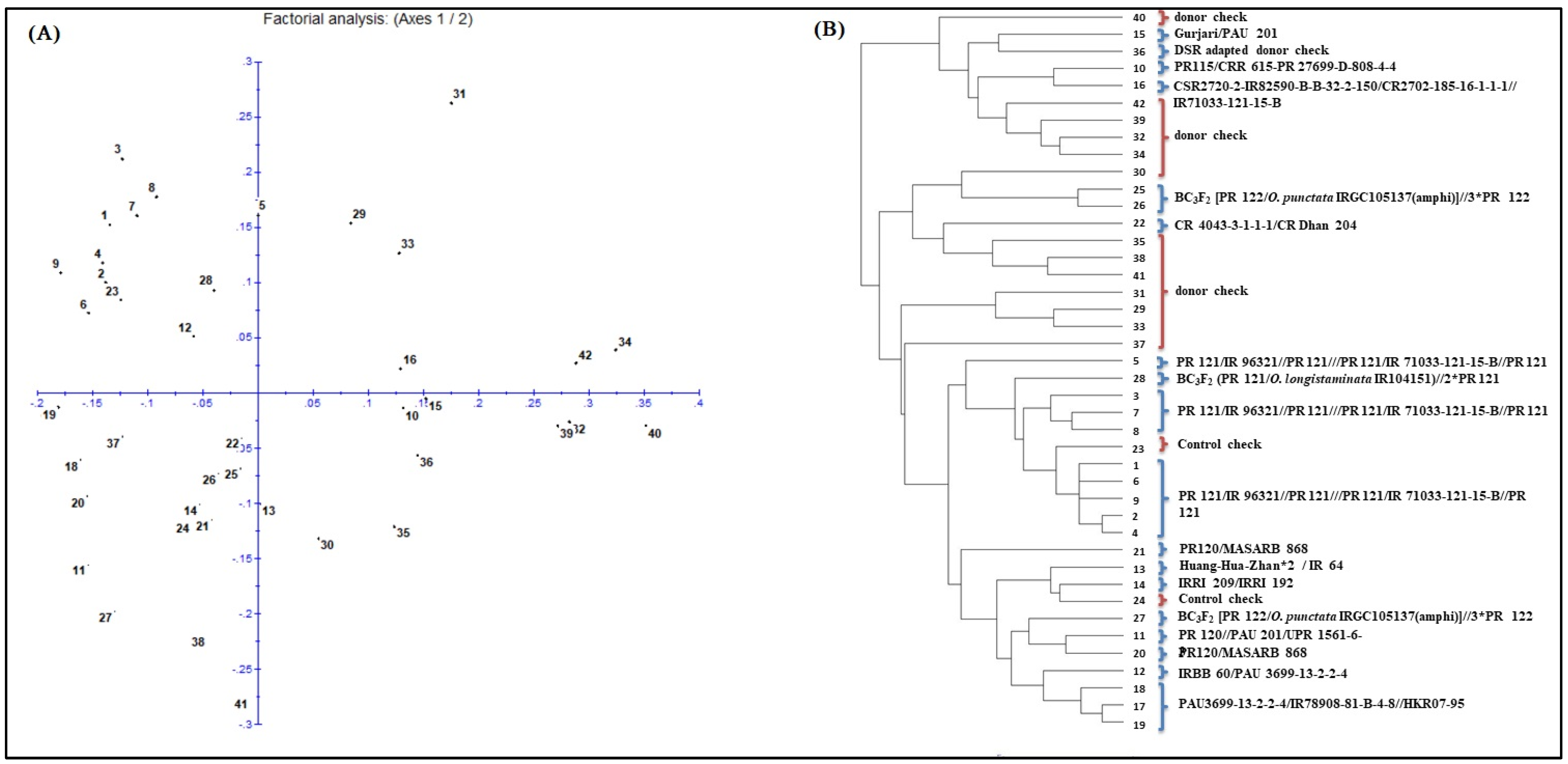
2.10. Diversity Studies of Breeding Lines
3. Results
3.1. Phenotypic Characterization of Breeding Panel
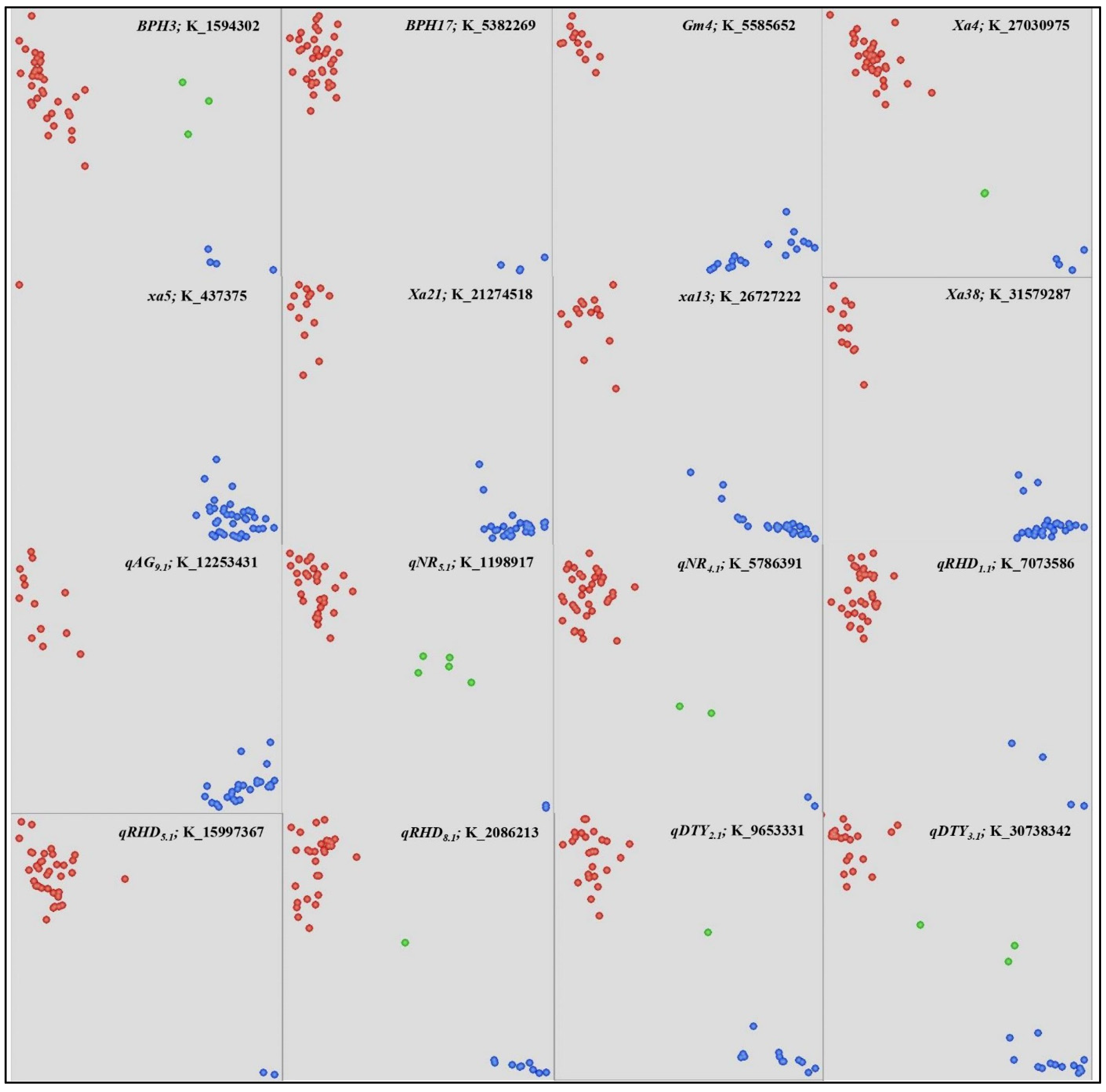
3.2. DNA Fingerprinting of the Breeding Panel
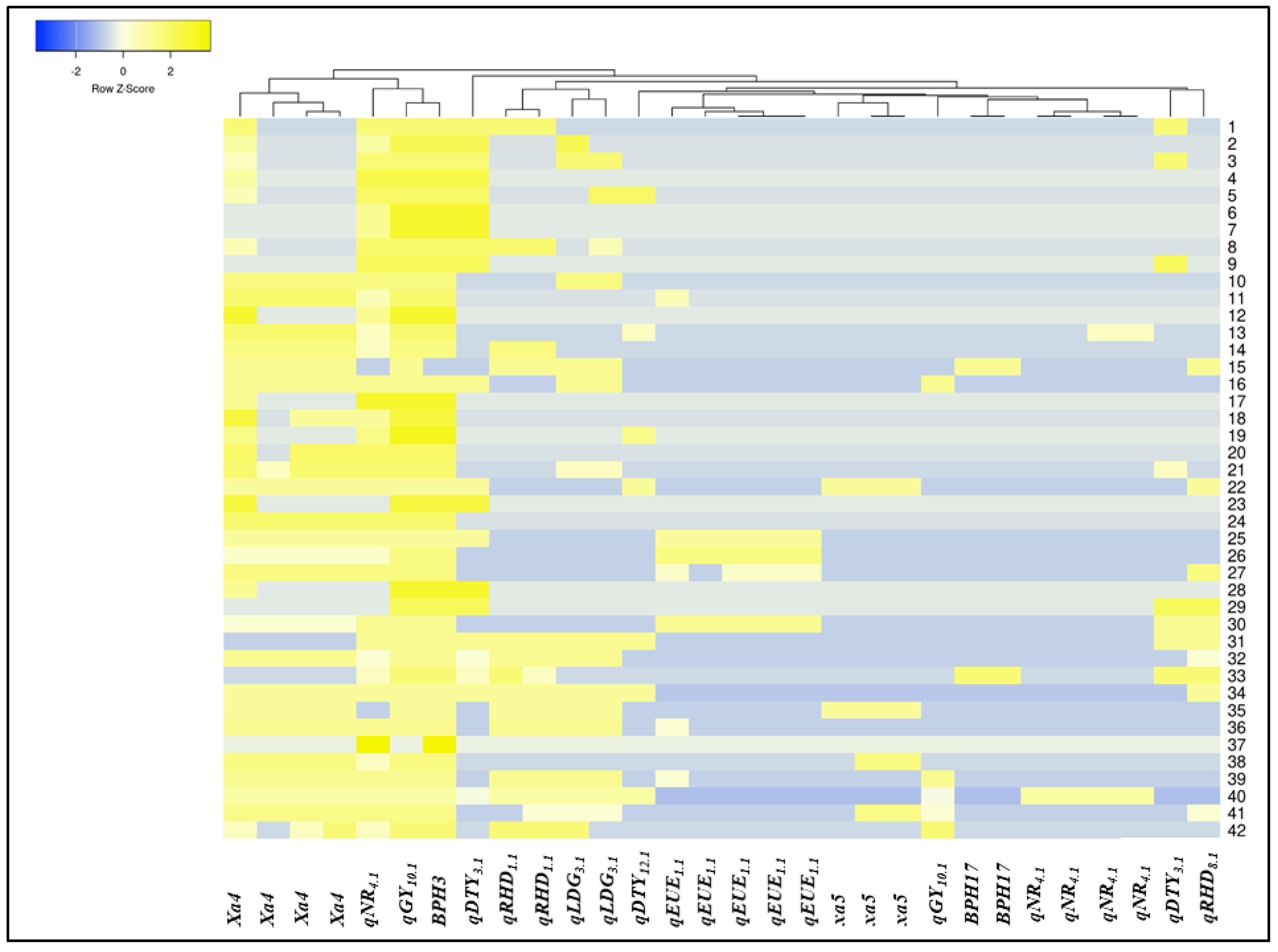
3.3. Molecular Profiling of the Breeding Panel
3.4. Selection of Promising Breeding Lines from the Breeding Panel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IRRI. Rice Almanac: Source Book for the Most Important Economic Activity on Earth; CABI: Wallingford, UK, 2002. [Google Scholar]
- FAOSTAT. FAO Statistical Databases; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Farooq, M.; Siddique, K.H.; Rehman, H.; Aziz, T.; Lee, D.J.; Wahid, A. Rice direct seeding, experiences, challenges and opportunities. Soil Tillage. Res. 2011, 111, 87–98. [Google Scholar] [CrossRef]
- Bandillo, N.; Raghavan, C.; Muyco, P.A.; Sevilla, M.A.L.; Lobina, I.T.; Dilla-Ermita, C.J. Multi-parent advanced generation inter-cross (MAGIC) populations in rice: Progress and potential for genetics research and breeding. Rice 2013, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Kobayashi, N.; Xu, D.; Fukuta, Y. Resistance genes and selection DNA markers for blast disease in rice (Oryza sativa L.). Jpn. Agric. Res. 2009, 43, 255–280. [Google Scholar] [CrossRef]
- Sandhu, N.; Subedi, S.R.; Singh, V.K.; Sinha, P.; Kumar, S.; Singh, S.P. Deciphering the genetic basis of root morphology, nutrient uptake, yield, and yield-related traits in rice under dry direct-seeded cultivation systems. Sci. Rep. 2019, 9, 9334. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ladha, J.K. Direct seeding of rice: Recent developments and future research needs. Adv. Agron. 2011, 111, 297–313. [Google Scholar]
- Department of Agriculture Chandigarh. 2021. Available online: https://chandigarh.gov.in/dept_agri.htm (accessed on 20 June 2022).
- Comas, L.; Becker, S.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Sandhu, N.; Raman, K.A.; Torres, R.O.; Audebert, A.; Dardou, A.; Kumar, A.; Henry, A. Rice root architectural plasticity traits and genetic regions for adaptability to variable cultivation and stress conditions. Plant Physiol. 2016, 171, 2562–2576. [Google Scholar] [CrossRef]
- Sandhu, N.; Rolando, O.T.; Teresa, S.C.; Maturan, P.C.; Jain, R.; Kumar, A.; Henry, A. Traits and QTLs for development of dry direct-seeded rainfed rice varieties. J. Exp. Bot. 2015, 66, 225–244. [Google Scholar] [CrossRef]
- Shahzad, Z.; Amtmann, A. Food for thought: How nutrients regulate root system architecture. Curr. Opin. Plant Biol. 2017, 39, 80–87. [Google Scholar] [CrossRef]
- Dhillon, B.S.; Mangat, G.S. Direct seeded rice in Punjab: Opportunities and challenges. Technol. Rice Prod. 2018, 72, 535–538. [Google Scholar]
- Anonymous. Package and Practices for Kharif Crops; Punjab Agricultural University: Ludhiana, India, 2022; pp. 1–17. [Google Scholar]
- Uddin, M.R.; Wade, L.J.; Pyon, J.Y.; Mazid, M.A. Rooting behavior of rice cultivars under different planting methods. J. Crop Sci. Biotechnol. 2009, 12, 17–23. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Liu, X. Optimal leaf positions for SPAD meter measurement in rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Li, X.; Wang, X.; Xiong, D.; Wang, F. Comparing the grain yields of direct-seeded and transplanted rice: A meta-analysis. Agronomy 2019, 9, 767. [Google Scholar] [CrossRef]
- Parimala, K.; Raju, C.S.; Kumar, A.H.P.S.S. Evaluation of genotypes for identification of potential restorers and maintainers for development of hybrid rice (Oryza sativa L.). J. Pharmacogn. Phytochem. 2020, 9, 1283–1285. [Google Scholar]
- Sharma, N.; Singh, N.; Singh, M.; Bharaj, T.S. Quality characteristics of aromatic fine-grained rice (Oryza sativa) genotypes for utilization in basmati improvement. Ind. J. Agric. Sci. 2008, 78, 42–49. [Google Scholar]
- SAS. The SAS System for Windows; Version 9.2; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- Sandhu, N.; Singh, J.; Singh, G.; Sethi, M.; Singh, M.P.; Pruthi, G.; Kumar, A. Development and validation of a novel core set of KASP markers for the traits improving grain yield and adaptability of rice under direct seeded cultivation conditions. Genomics 2022, 114, 110269. [Google Scholar] [CrossRef]
- Murray, M.; Thopson, W.F. Rapid isolation of molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. 2006. Available online: http://darwin.cirad.fr/ (accessed on 24 June 2022).
- Kaur, J.; Singh, A. Direct seeded rice: Prospects, problems/constraints and researchable issues in India. Curr. Agric. Res. J. 2017, 5, 13. [Google Scholar] [CrossRef]
- Anandan, A.; Meher, J.; Sah, R.P.; Samantaray, S.; Parameswaran, C.P.; Panneerselvam; Dash, S.K.; Swain, P.; Kartikeyan, P.; Annamalai, M.; et al. Enhancing input use efficiency in direct seeded rice with classical and molecular breeding. In Rice Research for Enhancing Productivity, Profitability and Climate Resilience; Pathak, H., Nayak, A.K., Jena, M., Singh, O.N., Samal, P., Sharma, S.G., Eds.; ICAR-National Rice Research Institute: Cuttack, Odisha, India, 2018; pp. 73–89. [Google Scholar]
- Panda, S.; Majhi, P.K.; Anandan, A.; Mahender, A.; Veludandi, S.; Bastia, D.; Ali, J. Proofing Direct-Seeded Rice with better root plasticity and architecture. Int. J. Mol. Sci. 2021, 22, 6058. [Google Scholar] [CrossRef]
- Bhadru, D.; Reddy, D.L.; Ramesha, M.S. Correlation and path coefficient analysis of yield and yield contributing traits in rice hybrids and their parental lines. Electron. J. Plant Breed. 2011, 2, 112–116. [Google Scholar]
- Chen, Y.T.; Song, J.Y.; Chuan, Y.; Zhu, G.L.; Hong, X.F. Comparison of yield performance and rice quality between direct-seeded and hand-transplanted rice under different nitrogen rates in Eastern China. Afr. J. Agric. Res. 2020, 16, 875–883. [Google Scholar]
- Sagare, D.B.; Abbai, R.; Jain, A.; Jayadevappa, P.K.; Dixit, S.; Singh, A.K.; Challa, V.; Alam, S.; Singh, U.M.; Yadav, S.; et al. More and more of less and less: Is genomics-based breeding of dry direct-seeded rice (DDSR) varieties the need of hour? Plant Biotechnol. J. 2020, 18, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
| Genotypes | Number | PARENTAGE | Combination of Gene/QTL |
|---|---|---|---|
| PAU 7180-36-5-0-0-0 | 1 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | xa13 + Gm4 + qGY10.1 + qDTY3.1 + qDTY2.1 + BPH3 |
| PAU 7180-8-13-0-0-0 | 2 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | xa13 + Gm4 + qGY10.1 + BPH3 |
| PAU 7180-9-17-0-0-0 | 3 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | Xa21 + xa13 + Gm4 + qGY10.1 + qGY1.1 + qDTY3.1 + qDTY2.1 + qAG9.1 + BPH3 |
| PAU 7180-3-9-0-0-0 | 4 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | Xa21 + Gm4 + qGY10.1 + qDTY3.1 + BPH3 |
| PAU 7180-3-15-0-0-0 | 5 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | xa13 + Gm4 + qGY10.1 + qDTY12.1 + BPH3 + qLDG3.1 + qEUE11.1 |
| PAU 7180-4-2-0-0-0 | 6 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | Xa21 + Gm4 + qGY10.1 + BPH3 |
| PAU 7180-113-14-0-0-0 | 7 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | Xa21 + xa13 + Gm4 + qGY10.1 + qGY1.1 + qDTY2.1 + qNR5.1 + BPH3 + qEUE11.1 |
| PAU 7180-5-14-0-0-0 | 8 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | xa13 + Gm4 + qGY10.1 + qGY1.1 + qDTY3.1 + BPH3 + qEUE11.1 |
| PAU 7180-9-15-0-0-0 | 9 | PR 121/IR 96321//PR 121///PR 121/IR 71033-121-15-B//PR 121 | Xa21 + xa13 + Gm4 + qGY10.1 + qDTY3.1 + BPH3 |
| PAU 5187-RIL1649-F8 | 10 | PR115/CRR 615-PR 27699-D-808-4-4 | Xa4 + qGY10.1 + qRHD5.1 + qRHD1.1 + BPH3 + qLDG3.1 |
| PAU 5567-32-3-1-5 | 11 | PR 120//PAU 201/UPR 1561-6-3 | Xa4 + Gm4 + qGY10.1 |
| PAU 5729-60-5-4-1 | 12 | IRBB 60/PAU 3699-13-2-2-4 | Gm4 + qGY10.1 + qAG9.1 + BPH3 + qEUE11.1 |
| RP 6273-HHZ4-DT3-LI1-LI1 | 13 | Huang-Hua-Zhan*2/IR 64 | Xa4 + qGY10.1 + qNR5.1 + qAG9.1 + qRHD1.1 + qEUE11.1 |
| RP 6314-GSR IR 1-DQ 150-R5-Y1 | 14 | IRRI 209/IRRI 192 | Xa4 + qNR5.1 + qAG9.1 |
| NVSR 2107 | 15 | Gurjari/PAU 201 | Xa4 + Gm4 + qGY10.1 + qNR5.1 + qAG9.1 + qRHD8.1 + qRHD1.1 + qRHD5.1 + BPH3 + qLDG3.1 + qEUE11.1 |
| PAU 6778-12-1-4-1-1 | 16 | CSR2720-2-IR82590-B-B-32-2-150/CR2702-185-16-1-1-1//IR71033-121-15-B | Xa4 + qGY10.1 + qDTY2.1 + qRHD1.1 + BPH3 + qLDG3.1 |
| PAU 6456-8-1-1-1-3 | 17 | PAU3699-13-2-2-4/IR78908-81-B-4-8//HKR07-95 | xa13 + Gm4 + qGY10.1 + qAG9.1 + qEUE11.1 |
| PAU 6456-8-2-1-1-1 | 18 | PAU3699-13-2-2-4/IR78908-81-B-4-8//HKR07-95 | Gm4 + qGY10.1 + qEUE11.1 + qRHD5.1 + qDTY1.1 |
| PAU 6456-8-2-1-1-2 | 19 | PAU3699-13-2-2-4/IR78908-81-B-4-8//HKR07-95 | xa13 + Gm4 + qGY10.1 + qAG9.1 + qEUE11.1 |
| PAU 5533-56-3-1-2-3-1-2 | 20 | PR120/MASARB 868 | Xa4 + Gm4 + qGY10.1 |
| PAU 5533-56-3-1-3-1-1-1 | 21 | PR120/MASARB 868 | Xa4 + qGY10.1 |
| CR 4116-3-2-1-1-1 | 22 | CR 4043-3-1-1-1/CR Dhan 204 | xa5 + Xa4 + Gm4 + qGY10.1 + qDTY12.1 + qRHD8.1 + qRHD1.1 + BPH3 + qEUE11.1 |
| PR 121 | 23 | - | |
| PR 126 | 24 | - | |
| PAU 9562-1-1 | 25 | BC3F2 [PR 122/O. punctata IRGC105137(amphi)]//3*PR 122 | xa13 + Xa4 + Gm4 + qGY10.1 + qAG9.1 + BPH3 + qLDG4.1 + qEUE11.1 + qEUE1.1 |
| PAU 9562-2-1 | 26 | BC3F2 PR 122/O. punctata IRGC105137(amphi)]//3*PR 122 | Xa21 + Xa4 + Gm4 + qGY10.1 + BPH3 + qLDG4.1 + qEUE11.1 + qEUE1.1 |
| PAU 9562-3-1 | 27 | BC3F2 [PR 122/O. punctata IRGC105137(amphi)]//3*PR 122 | xa13 + Xa4 + Gm4 + qGY10.1 + qRHD8.1 + qEUE11.1 |
| PAU 9563-1-1 | 28 | BC3F2 (PR 121/O. longistaminata IR104151)//2*PR 121 | Xa21 + xa13 + Gm4 + qGY10.1 + BPH3 + qLDG4.1 + qEUE11.1 |
| IR 11L101 | 29 | qGY10.1 + qDTY3.1 + qRHD8.1 + qRHD1.1 + BPH3 + qLDG4.1 + qEUE11.1 | |
| IR 91648-B-32-B | 30 | qGY10.1 + qRHD8.1 + qRHD1.1 + qLDG3.1 + qLDG4.1 + qEUE11.1 + qEUE1.1 | |
| IR 13L500 | 31 | qGY10.1 + qGY1.1 + qDTY12.1 + qDTY3.1 + qDTY2.1 + qNR5.1 + qRHD8.1 + qRHD1.1 + BPH3 + qLDG4.1 + qEUE11.1 | |
| IR 87707-446-B-B-B | 32 | Xa4 + qGY10.1 + qDTY2.1 + qRHD1.1 + BPH3 + qLDG3.1 + qLDG4.1 + qEUE11.1 | |
| Vandana | 33 | qGY10.1 + qAG9.1 + qRHD8.1 + qRHD1.1 + BPH3 + BPH17 + qLDG4.1 + qEUE11.1 | |
| Kali aus | 34 | Xa4 + qGY10.1 + qDTY12.1 + qDTY2.1 + qNR5.1 + qRHD8.1 + qRHD1.1 + BPH3 + qLDG3.1 + qLDG4.1 + qEUE11.1 | |
| MTU 1010 | 35 | xa13 + xa5 + Xa4 + qGY10.1 + qRHD1.1 + BPH3 + qLDG4.1 | |
| Abhaya | 36 | Gm4 + Xa4 + qGY10.1 + qNR5.1 + qRHD5.1 + BPH3 + qLDG3.1 + qLDG4.1 | |
| Tadukan | 37 | Gm4 + qLDG4.1 + qEUE11.1 | |
| IRBB60 | 38 | Xa21 + xa13 + xa5 + Xa4 + Gm4 + qGY10.1 + qLDG4.1 + qEUE11.1 | |
| IR 93312-30-101-2013-30-66-6 | 39 | Xa4 + qGY10.1 + qAG9.1 + qNR5.1 + qRHD5.1 + qRHD1.1 + BPH3 + qLDG3.1 + qLDG4.1 + qEUE11.1 | |
| IR 94226-B-177-B | 40 | Xa4 + qGY10.1 + qGY1.1 + qNR5.1 + qRHD1.1 + qLDG4.1 + qNR4.1 | |
| IR 96322-34-223 | 41 | xa5 + Xa4 + qGY10.1 + qDTY2.1 + qDTY3.1 + qLDG4.1 | |
| IR 94225-D-82-B | 42 | qGY10.1 + qGY1.1 + qRHD5.1 + qRHD8.1 + qRHD1.1 + qLDG4.1 + qEUE11.1 + qNR4.1 |
| Plant morphological and quality traits | ||||||||||
| Source of Variation | DTF | TN | PH | YLD | SPAD | TGW | FER % | TRR | MRR | HRR |
| Genotype | 40.256 *** | 13.984 *** | 101.731 *** | 233.795 *** | 4.519 *** | 54.578 *** | 35.208 *** | 22.684 *** | 6.355 *** | 624.661 *** |
| Replication | 2.376 | 4.815 ** | 1.645 | 2.423 | 0.132 | 0.79 | 0.653 | 1.784 | 1.502 | 0.646 |
| Treatment | 45.134 *** | 9.009 ** | 317.063 *** | 5072.625 *** | 42.008 *** | 334.761 *** | 124.275 *** | 76.696 *** | 11.534 *** | 3673.681 *** |
| Season | 228.49 *** | 0.399 | 39.835 *** | 284.334 *** | 0.65 | 177.04 *** | 6.892 *** | 2.459 | 0.009 | 2362.003 *** |
| Genotype × Replication | 1.049 | 0.756 | 1.173 | 1.112 | 1 | 1.146 | 0.766 | 2.274 *** | 1.21 | 0.745 |
| Genotype × Treatment | 6.365 *** | 4.475 *** | 12.889 *** | 37.763 *** | 1.234 | 12.637 *** | 14.427 *** | 1.123 | 4.378 *** | 143.564 *** |
| Genotype × Season | 7.727 *** | 4.961 *** | 11.523 *** | 64.499 *** | 3.693 *** | 6.601 *** | 1.877 ** | 18.804 *** | 2.732 *** | 47.87 *** |
| Treatment × Season | 96.572 *** | 6.136 * | 23.503 *** | 301.025 *** | 33.363 *** | 13.259 *** | 6.116 * | 66.403 *** | 5.851 * | 1437.944 *** |
| Replication × Treatment | 0.192 | 0.319 | 0.428 | 1.178 | 1.015 | 0.751 | 0.925 | 0.366 | 1.116 | 2.157 |
| Replication × Season | 0.65 | 1.421 | 1.691 | 1.123 | 0.297 | 0.687 | 0.692 | 0.039 | 0.016 | 1.487 |
| Genotype × Treatment × Season | 5.637 *** | 4.121 *** | 4.577 *** | 33.465 *** | 1.708 * | 5.628 *** | 1.911 ** | 1.477 | 2.34 *** | 49.228 *** |
| Genotype × Replication × Treatment | 1.076 | 0.992 | 1.186 | 0.947 | 1.057 | 1.094 | 0.819 | 0.363 | 0.947 | 1.284 |
| Replication × Treatment × Season | 0.077 | 0.231 | 2.607 | 1.093 | 1.178 | 0.102 | 0.417 | 0.185 | 0.411 | 1.406 |
| Root architecture traits | ||||||||||
| Source of Variation | RL | AD | RV | SA | Tips | Forks | Crossing | RSRL | RSRB | |
| Genotype | 19.35 *** | 3.202 *** | 10.462 *** | 14.083 *** | 33.941 *** | 20.311 *** | 22.389 *** | 1.002 | 3.036 *** | |
| Replication | 0.365 | 0.959 | 0.925 | 0.883 | 0.227 | 0.2 | 33.478 *** | 1.006 | 1.016 | |
| Treatment | 18.279 *** | 188.855 *** | 788.187 *** | 206.714 *** | 1.983 | 4.105 * | 673.809 *** | 1.704 | 4.568 * | |
| Season | 3137.355 *** | 11,092.418 *** | 1009.157 *** | 88.271 *** | 4633.28 *** | 4319.686 *** | 4542.341 *** | 1.689 | 4.022 * | |
| Genotype × Replication | 1.085 | 1.05 | 1.082 | 1.069 | 1.01 | 1.055 | 1.006 | 0.998 | 0.995 | |
| Genotype × Treatment | 2.222 *** | 3.343 *** | 3.726 *** | 2.201 *** | 0.056 | 0.081 | 1.464 * | 0.99 | 3.049 **** | |
| Genotype × Season | 16.189 *** | 5.778 *** | 5.259 *** | 10.239 *** | 33.984 *** | 19.761 *** | 22.37 *** | 1.018 | 2.979 *** | |
| Treatment × Season | 2.961 | 8.16 ** | 13.522 *** | 44.525 *** | 1.886 | 0.576 | 679.464 *** | 0.323 | 2.963 | |
| Replication × Treatment | 0.336 | 0.67 | 0.527 | 0.308 | 0.27 | 0.242 | 38.689 *** | 1.092 | 1.065 | |
| Replication × Season | 0.448 | 0.947 | 1.183 | 1.352 | 0.244 | 0.253 | 35.021 *** | 1.086 | 1.088 | |
| Genotype × Treatment × Season | 2.038 *** | 4.817 *** | 4.701 *** | 1.535 * | 0.032 | 0.089 | 1.754 ** | 0.985 | 3.005 *** | |
| Genotype × Replication × Treatment | 1.012 | 0.994 | 1.033 | 1.12 | 0.99 | 1.007 | 0.999 | 1.004 | 1.002 | |
| Replication × Treatment × Season | 0.364 | 0.471 | 0.534 | 0.679 | 0.27 | 0.183 | 39.105 *** | 0.916 | 0.991 | |
| Traits | Season | Mean | Max | Min | Std. Dev. | S.E. | F Value |
|---|---|---|---|---|---|---|---|
| DTF | DSR 2020 | 97 | 109 | 82 | 7.289 | 1.220 | 70.728 *** |
| TPR 2020 | 102 | 109 | 93 | 4.393 | 0.964 | 40.164 *** | |
| DSR 2021 | 104 | 113 | 91 | 7.092 | 3.143 | 8.321 *** | |
| TPR 2021 | 103 | 112 | 89 | 6.290 | 2.739 | 8.694 *** | |
| TN | DSR 2020 | 285 | 357 | 204 | 40.410 | 14.997 | 12.741 *** |
| TPR 2020 | 287 | 353 | 209 | 43.407 | 11.636 | 26.243 *** | |
| DSR 2021 | 281 | 343 | 216 | 41.760 | 21.677 | 5.429 *** | |
| TPR 2021 | 293 | 332 | 242 | 32.144 | 22.936 | 1.90 * | |
| PH | DSR 2020 | 101 | 128 | 84 | 10.432 | 2.365 | 37.534 *** |
| TPR 2020 | 105 | 124 | 93 | 9.094 | 2.224 | 31.939 *** | |
| DSR 2021 | 98 | 136 | 81 | 12.162 | 3.686 | 20.131 *** | |
| TPR 2021 | 104 | 128 | 93 | 10.771 | 2.189 | 47.305 *** | |
| YLD | DSR 2020 | 4971 | 7310.05 | 1365.71 | 1824.31 | 217.51 | 141.316 *** |
| TPR 2020 | 6090 | 7521.00 | 3236.00 | 969.05 | 122.73 | 124.880 *** | |
| DSR 2021 | 4260 | 6214.00 | 2075.43 | 846.86 | 141.72 | 70.653 *** | |
| TPR 2021 | 6100 | 7469.00 | 4136.00 | 882.19 | 208.89 | 34.294 *** | |
| SPAD | DSR 2020 | 37 | 44 | 33 | 3.589 | 2.086 | 3.975 *** |
| TPR 2020 | 37 | 41 | 34 | 2.526 | 1.716 | 2.358 *** | |
| DSR 2021 | 36 | 41 | 23 | 4.722 | 3.222 | 2.320 *** | |
| TPR 2021 | 39 | 44 | 34 | 3.254 | 2.076 | 2.951 *** | |
| TGW | DSR 2020 | 24.08 | 32.07 | 19.03 | 2.93 | 1.32 | 7.997 *** |
| TPR 2020 | 25.84 | 34.77 | 18.10 | 3.60 | 1.38 | 11.729 *** | |
| DSR 2021 | 22.06 | 30.23 | 13.20 | 3.34 | 0.55 | 72.020 *** | |
| TPR 2021 | 24.68 | 34.63 | 18.69 | 3.44 | 0.57 | 73.212 *** | |
| FER % | DSR 2020 | 86 | 94 | 70 | 6.716 | 2.308 | 23.980 *** |
| TPR 2020 | 89 | 95 | 80 | 4.053 | 2.308 | 4.228 *** | |
| DSR 2021 | 86 | 95 | 70 | 6.791 | 1.230 | 60.088 *** | |
| TPR 2021 | 88 | 95 | 78 | 4.410 | 2.172 | 6.347 *** | |
| TRR | DSR 2020 | 80.42 | 82.36 | 78.50 | 1.07 | 0.42 | 10.767 *** |
| TPR 2020 | 79.20 | 81.01 | 75.88 | 1.22 | 0.40 | 16.417 *** | |
| DSR 2021 | 79.72 | 82.97 | 76.03 | 1.89 | 0.77 | 10.217 *** | |
| TPR 2021 | 79.68 | 82.97 | 76.03 | 1.89 | 0.87 | 7.555 *** | |
| MRR | DSR 2020 | 67.94 | 73.34 | 35.42 | 7.97 | 4.88 | 3.389 *** |
| TPR 2020 | 69.83 | 72.50 | 64.89 | 2.14 | 1.14 | 5.078 *** | |
| DSR 2021 | 68.69 | 72.57 | 51.98 | 4.29 | 2.32 | 4.903 *** | |
| TPR 2021 | 69.01 | 73.74 | 65.25 | 2.01 | 0.92 | 7.622 *** | |
| HRR | DSR 2020 | 51.62 | 67.71 | 26.26 | 12.21 | 1.11 | 245.971 *** |
| TPR 2020 | 62.12 | 68.47 | 43.16 | 6.34 | 1.17 | 57.322 *** | |
| DSR 2021 | 50.49 | 65.31 | 27.73 | 10.68 | 0.59 | 674.510 *** | |
| TPR 2021 | 52.90 | 64.25 | 33.28 | 6.84 | 0.55 | 317.877 *** |
| Traits | Season | Mean | Max | Min | Std. Dev. | S.E | F Value |
|---|---|---|---|---|---|---|---|
| RL | DSR 2020 | 2322.77 | 4755.69 | 862.33 | 954.53 | 408.84 | 6.018 *** |
| TPR 2020 | 2107.15 | 4019.78 | 677.43 | 647.03 | 26.13 | 1250.837 *** | |
| DSR 2021 | 763.03 | 1172.16 | 467.99 | 230.25 | 115.48 | 2.846 *** | |
| TPR 2021 | 666.79 | 866.82 | 478.45 | 202.13 | 111.36 | 1.603 * | |
| RV | DSR 2020 | 0.54 | 0.85 | 0.18 | 0.23 | 0.10 | 6.210 *** |
| TPR 2020 | 1.02 | 1.62 | 0.46 | 0.32 | 0.01 | 1331.454 *** | |
| DSR 2021 | 1.10 | 1.62 | 0.78 | 0.41 | 0.21 | 2.482 *** | |
| TPR 2021 | 1.73 | 2.66 | 1.03 | 0.56 | 0.25 | 4.886 *** | |
| AD | DSR 2020 | 0.18 | 0.22 | 0.14 | 0.03 | 0.01 | 7.268 *** |
| TPR 2020 | 0.22 | 0.34 | 0.15 | 0.04 | 0.00 | 419.597 *** | |
| DSR 2021 | 0.50 | 0.59 | 0.43 | 0.07 | 0.04 | 1.637 * | |
| TPR 2021 | 0.55 | 0.62 | 0.47 | 0.07 | 0.03 | 4.599 *** | |
| SA | DSR 2020 | 116.98 | 193.31 | 33.84 | 48.32 | 20.47 | 6.268 *** |
| TPR 2020 | 130.74 | 200.47 | 54.72 | 34.66 | 1.42 | 1214.425 *** | |
| DSR 2021 | 88.28 | 131.27 | 56.95 | 30.36 | 15.16 | 2.954 *** | |
| TPR 2021 | 125.88 | 176.12 | 83.39 | 33.04 | 17.33 | 2.301 *** | |
| Tips | DSR 2020 | 27,519.87 | 53,375.83 | 9634.50 | 16,897.24 | 6494.08 | 8.719 *** |
| TPR 2020 | 28,600.38 | 54,046.56 | 9760.27 | 13,461.89 | 325.14 | 3505.043 *** | |
| DSR 2021 | 1612.40 | 2544.17 | 949.33 | 740.36 | 385.00 | 2.429 *** | |
| TPR 2021 | 1626.04 | 2350.00 | 974.00 | 656.83 | 344.06 | 2.263 *** | |
| Forks | DSR 2020 | 55,875.60 | 94,177.50 | 15,593.50 | 29,069.93 | 12,849.73 | 5.336 *** |
| TPR 2020 | 58,027.58 | 95,830.71 | 18,184.42 | 20,273.16 | 638.83 | 2057.025 *** | |
| DSR 2021 | 5679.29 | 11,114.33 | 3028.67 | 3276.95 | 1722.86 | 2.193 *** | |
| TPR 2021 | 6658.14 | 9295.83 | 4293.00 | 2644.53 | 1506.90 | 1.163 | |
| Crossing | DSR 2020 | 21,126.42 | 47,983.83 | 5360.50 | 16,071.81 | 7560.85 | 4.109 *** |
| TPR 2020 | 46,367.65 | 79,948.34 | 13,979.57 | 21,785.48 | 2907.52 | 68.252 *** | |
| DSR 2021 | 1073.71 | 3434.17 | 365.33 | 1423.28 | 788.29 | 1.532 * | |
| TPR 2021 | 1020.98 | 2264.00 | 648.00 | 955.85 | 553.53 | 0.963 | |
| RSR L | DSR 2020 | 0.68 | 0.57 | 0.27 | 3.24 | 2.65 | 0.995 |
| TPR 2020 | 0.39 | 0.55 | 0.22 | 0.10 | 0.05 | 6.375 *** | |
| DSR 2021 | 0.39 | 0.48 | 0.29 | 0.08 | 0.06 | 1.575 | |
| TPR 2021 | 0.27 | 0.35 | 0.20 | 0.05 | 0.04 | 1.756 * | |
| RSR B | DSR 2020 | 0.36 | 0.79 | 0.19 | 0.10 | 0.03 | 27.746 *** |
| TPR 2020 | 0.27 | 0.39 | 0.15 | 0.08 | 0.04 | 5.978 *** | |
| DSR 2021 | 1.18 | 0.61 | 0.30 | 0.85 | 3.71 | 3.014 *** | |
| TPR 2021 | 0.33 | 0.56 | 0.18 | 0.10 | 0.06 | 3.264 *** |
| DSR | |||||||||||||||||||||
| Advanced Breeding Line | QTL/Gene Combination | DTF | TN | PH | YLD | SPD | TGW | TRR | MRR | HRR | FER % | RL | AD | RV | SA | Tips | Forks | Crossings | RSR L | RSR B | SV |
| PAU 6456-8-2-1-1-1 | Gm4 + qGY10.1 + qEUE11.1 + qRHD5.1 + qDTY1.1 | 98 | 260 | 100 | 6503.83 | 37 | 25.78 | 79.90 | 69.98 | 53.93 | 93 | 1763.64 | 0.32 | 0.95 | 122.14 | 21,608 | 37,783 | 13,468 | 0.42 | 0.42 | 3 |
| PAU 5187-RIL1649-F8 | Xa4 + qGY10.1 + qRHD5.1 + qRHD1.1 + BPH3 + qLDG3.1 | 99 | 228 | 97 | 6296.48 | 40 | 22.27 | 78.17 | 68.73 | 44.39 | 89 | 1889.21 | 0.33 | 0.78 | 133.21 | 18,822 | 32,339 | 15,620 | 0.41 | 0.40 | 3 |
| PAU 6456-8-1-1-1-3 | xa13 + Gm4 + qGY10.1 + qAG9.1 + qEUE11.1 | 97 | 250 | 104 | 6077.37 | 38 | 25.18 | 80.86 | 69.46 | 51.05 | 92 | 1921.01 | 0.32 | 1.11 | 144.47 | 20,345 | 42,305 | 15,822 | 0.37 | 0.51 | 3 |
| PAU 6456-8-2-1-1-2 | xa13 + Gm4 + qGY10.1 + qAG9.1 + qEUE11.1 | 100 | 256 | 99 | 5758.21 | 40 | 26.50 | 80.62 | 69.14 | 45.66 | 93 | 1784.71 | 0.31 | 0.78 | 111.86 | 22,394 | 38,319 | 14,359 | 0.36 | 0.50 | 3 |
| NVSR 2107 | Xa4 + Gm4 + qGY10.1 + qNR5.1 + qAG9.1 + qRHD8.1 + qRHD5.1 + qRHD1.1 + BPH3 + qLDG3.1 + qEUE11.1 | 95 | 220 | 115 | 5498.57 | 38 | 31.15 | 80.85 | 71.13 | 34.45 | 90 | 2090.10 | 0.33 | 0.86 | 124.54 | 27,465 | 49,785 | 22,970 | 0.59 | 0.55 | 3 |
| PAU 6778-12-1-4-1-1 | Xa4 + qGY10.1 + qDTY2.1 + qRHD1.1 + BPH3 + qLDG3.1 | 94 | 270 | 111 | 5422.08 | 35 | 19.43 | 80.95 | 68.17 | 48.94 | 88 | 1821.69 | 0.32 | 0.94 | 127.63 | 22,086 | 42,351 | 16,854 | 0.36 | 0.39 | 3 |
| PR 126 | - | 88 | 286 | 97 | 6312.15 | 33 | 21.03 | 79.67 | 69.47 | 56.71 | 92 | 1903.20 | 0.32 | 0.78 | 112.43 | 22,168 | 39,862 | 15,974 | 0.38 | 0.35 | 3 |
| PR 121 | - | 107 | 308 | 85 | 5638.23 | 40 | 22.37 | 81.81 | 71.38 | 64.25 | 91 | 1656.70 | 0.34 | 0.82 | 110.52 | 17,015 | 33,187 | 10,950 | 0.36 | 0.38 | 3 |
| Trial mean | 100 | 210 | 99 | 4610.00 | 36 | 23.07 | 80.00 | 68.31 | 43.48 | 86 | 1542.90 | 0.34 | 0.78 | 102.63 | 14,566 | 30,777 | 11,100 | 0.23 | 0.37 | 3 | |
| LSD | 2.184 | 18 | 3 | 299.8 | 1.99 | 1.22 | 0.73 | 0.86 | 0.85 | 1.82 | 200.11 | 0.22 | 0.26 | 10.11 | 2998 | 5442 | 3345 | 0.11 | 0.15 | 0.12 | |
| TPR | |||||||||||||||||||||
| Advanced Breeding Line | QTL/Gene Combination | DTF | TN | PH | YLD | SPD | TGW | TRR | MRR | HRR | FER % | RL | AD | RV | S A | Tips | Forks | Crossings | RSR L | RSR B | SV |
| PAU 6456-8-2-1-1-1 | Gm4 + qGY10.1 + qEUE11.1 + qRHD5.1 + qDTY1.1 | 101 | 293 | 106 | 7070.07 | 40 | 27.85 | 79.08 | 53.47 | 58.37 | 92 | 1611.31 | 0.38 | 1.66 | 128.62 | 22,048 | 38,719 | 28,197 | 0.40 | 0.42 | 3 |
| PAU 5187-RIL1649-F8 | Xa4 + qGY10.1 + qRHD5.1 + qRHD1.1 + BPH3 + qLDG3.1 | 105 | 277 | 100 | 6718.90 | 41 | 25.13 | 77.46 | 51.30 | 62.63 | 87 | 1556.65 | 0.35 | 1.45 | 133.2 | 15,279 | 29,982 | 22,312 | 0.37 | 0.30 | 3 |
| PAU 6456-8-1-1-1-3 | xa13 + Gm4 + qGY10.1 + qAG9.1 + qEUE11.1 | 102 | 256 | 106 | 7047.72 | 40 | 28.60 | 81.45 | 55.02 | 58.48 | 90 | 1714.64 | 0.37 | 1.89 | 145.37 | 20,450 | 41,369 | 30,733 | 0.39 | 0.32 | 3 |
| PAU 6456-8-2-1-1-2 | Xa13 + Gm4 + qGY10.1 + qAG9.1 + qEUE11.1 | 101 | 264 | 109 | 7003.56 | 38 | 25.72 | 80.03 | 52.87 | 56.70 | 91 | 1605.19 | 0.34 | 1.45 | 130.57 | 22,527 | 38,111 | 28,346 | 0.29 | 0.34 | 1 |
| NVSR 2107 | Xa4 + Gm4 + qGY10.1 + qNR5.1 + qAG9.1 + qRHD8.1 + qRHD5.1 + qRHD1.1 + BPH3 + qLDG3.1 + qEUE11.1 | 94 | 272 | 121 | 7182.57 | 39 | 32.68 | 80.58 | 56.63 | 38.52 | 85 | 1922.61 | 0.40 | 2.04 | 151.48 | 27,829 | 51,423 | 40,003 | 0.34 | 0.41 | 3 |
| PAU 6778-12-1-4-1-1 | Xa4 + qGY10.1 + qDTY2.1 + qRHD1.1 + BPH3 + qLDG3.1 | 102 | 274 | 115 | 6622.74 | 39 | 26.38 | 80.91 | 53.65 | 59.81 | 90 | 1717.46 | 0.39 | 1.67 | 134.27 | 22,531 | 41,784 | 32,315 | 0.37 | 0.32 | 3 |
| PR 126 | - | 93 | 272 | 97 | 7333.84 | 39 | 22.22 | 79.29 | 50.75 | 62.03 | 89 | 1669.51 | 0.37 | 1.48 | 146.33 | 22,975 | 40,585 | 30,558 | 0.33 | 0.21 | 3 |
| PR 121 | - | 107 | 322 | 94 | 6890.17 | 40 | 25.98 | 81.11 | 53.55 | 65.24 | 89 | 1444.68 | 0.42 | 1.61 | 129.43 | 17,413 | 34,817 | 25,394 | 0.37 | 0.31 | 3 |
| Trial mean | 102 | 233 | 104.5 | 6095 | 38 | 23.33 | 74.44 | 50.44 | 57.42 | 88 | 1386.97 | 0.32 | 1.33 | 112.3 | 12,334 | 24,432 | 20,212 | 0.30 | 0.28 | 3 | |
| LSD | 1.85 | 18 | 2.21 | 219.0 | 1.89 | 1.07 | 2.11 | 1.11 | 1.01 | 1.2 | 168.8 | 0.17 | 0.11 | 8.8 | 566.7 | 2247 | 1887 | 0.08 | 0.10 | 0.12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, H.; Singh, J.; Ade, P.A.; Raigar, O.P.; Kaur, R.; Khanna, R.; Mangat, G.S.; Sandhu, N. Genetic Evaluation of a Diverse Rice Panel for Direct Seeded Adapted Traits Using Kompetitive Allele Specific Primer Assay. Agronomy 2022, 12, 2083. https://doi.org/10.3390/agronomy12092083
Singh H, Singh J, Ade PA, Raigar OP, Kaur R, Khanna R, Mangat GS, Sandhu N. Genetic Evaluation of a Diverse Rice Panel for Direct Seeded Adapted Traits Using Kompetitive Allele Specific Primer Assay. Agronomy. 2022; 12(9):2083. https://doi.org/10.3390/agronomy12092083
Chicago/Turabian StyleSingh, Harpreet, Jasneet Singh, Pooja Ankush Ade, Om Prakash Raigar, Rupinder Kaur, Renu Khanna, Gurjit Singh Mangat, and Nitika Sandhu. 2022. "Genetic Evaluation of a Diverse Rice Panel for Direct Seeded Adapted Traits Using Kompetitive Allele Specific Primer Assay" Agronomy 12, no. 9: 2083. https://doi.org/10.3390/agronomy12092083
APA StyleSingh, H., Singh, J., Ade, P. A., Raigar, O. P., Kaur, R., Khanna, R., Mangat, G. S., & Sandhu, N. (2022). Genetic Evaluation of a Diverse Rice Panel for Direct Seeded Adapted Traits Using Kompetitive Allele Specific Primer Assay. Agronomy, 12(9), 2083. https://doi.org/10.3390/agronomy12092083






