Assessing the Impacts of Fertilization Regimes on Soil Aggregate Dynamics in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Study Design
2.2.1. Soil Sampling and Processing
2.2.2. Soil Analyses
2.2.3. Calculation
3. Results and Analysis
3.1. Effects of Long-Term Fertilization Treatments on the Size Distribution and Stability of Soil Aggregates
3.1.1. Aggregate-Size Distribution
3.1.2. Aggregate Stability
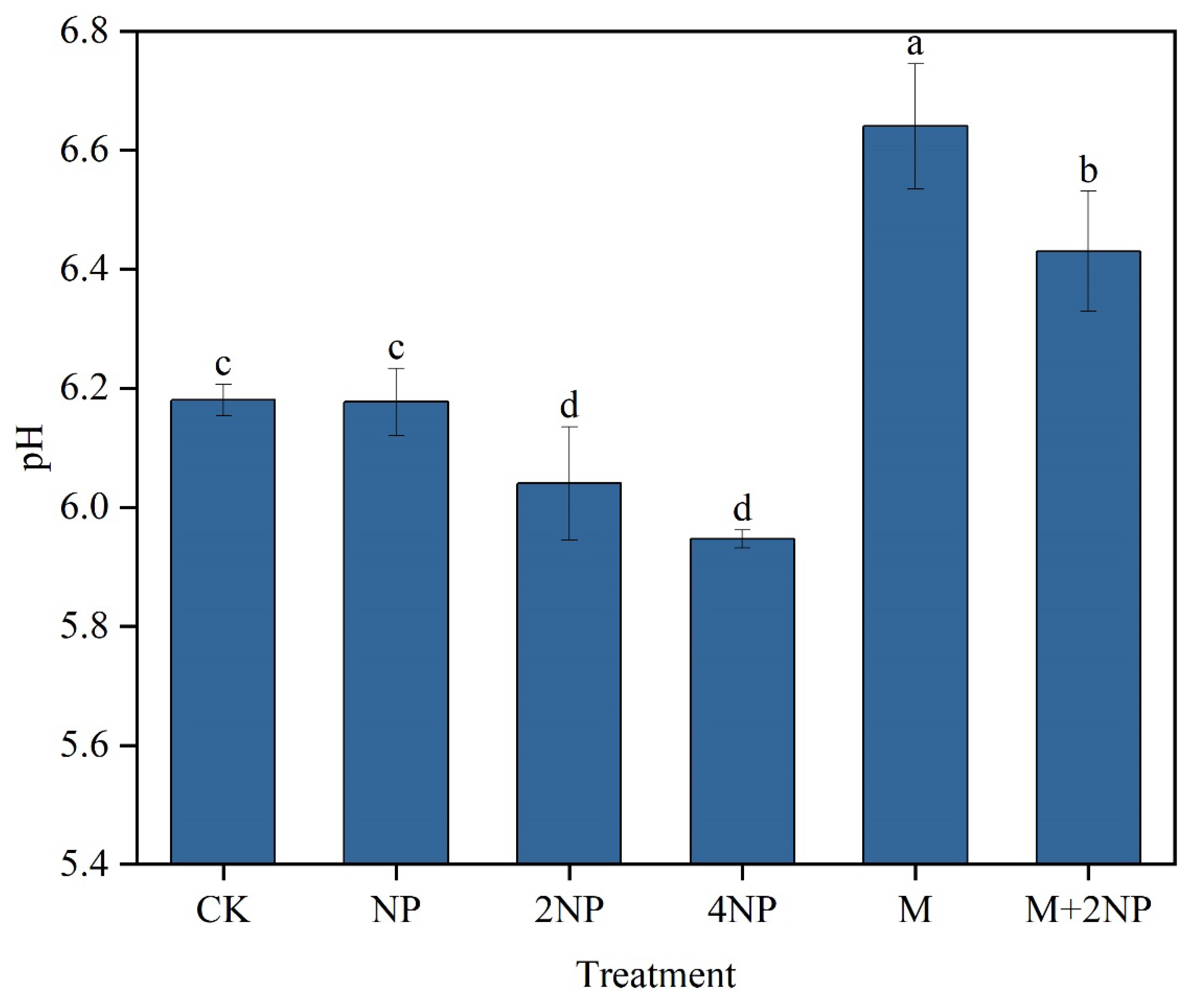
3.2. Effects of Long-Term Fertilization Treatments on Soil pH
3.3. Relationships between Soil pH and the Proportion of Soil MacroAggregates and Aggregate Stability
3.4. Effects of Long-Term Fertilization Treatments on SOC Content and Stock in Bulk Soil and Water-Stable Aggregates
3.4.1. SOC Content and Stock in Bulk Soil
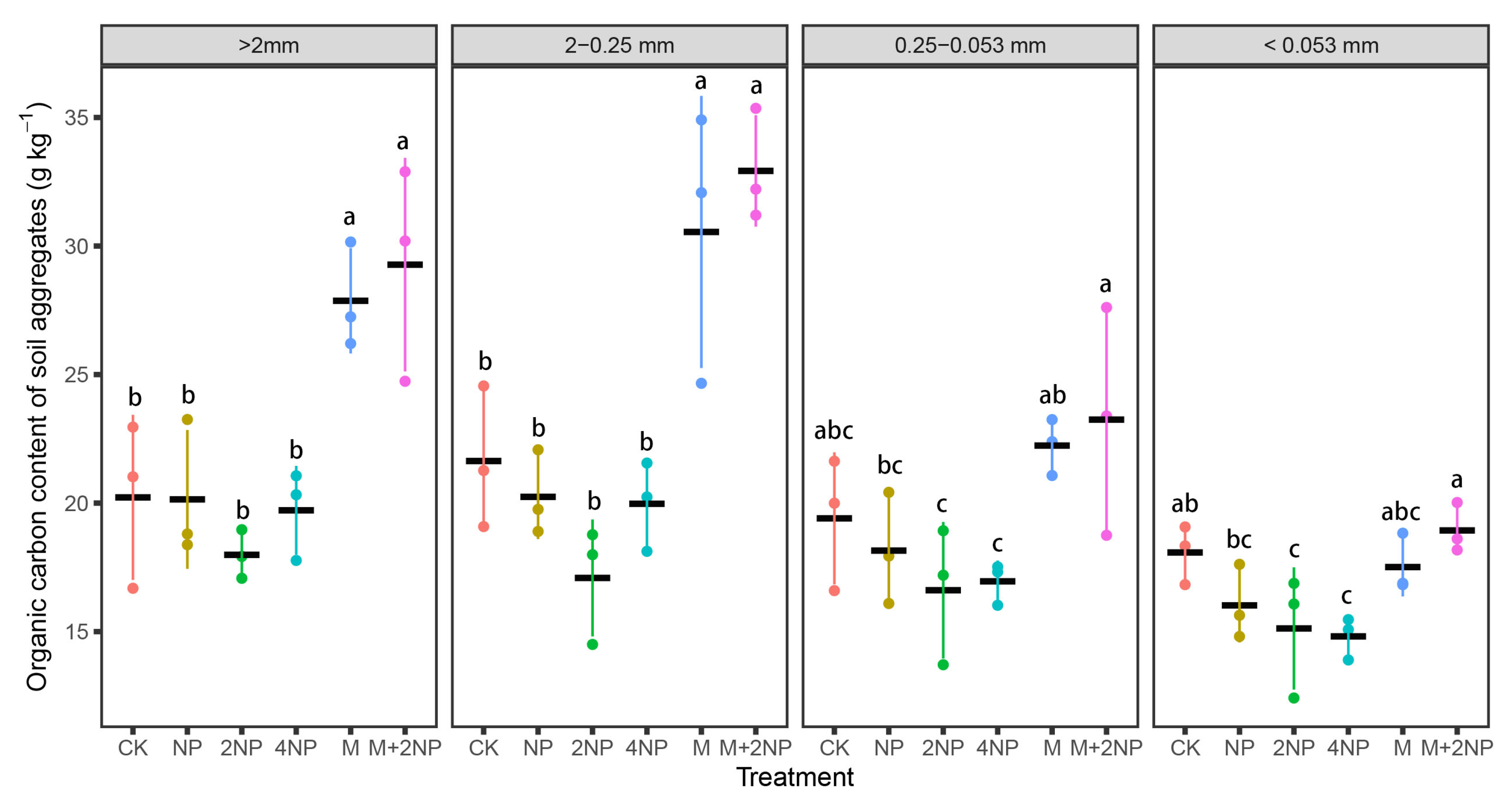
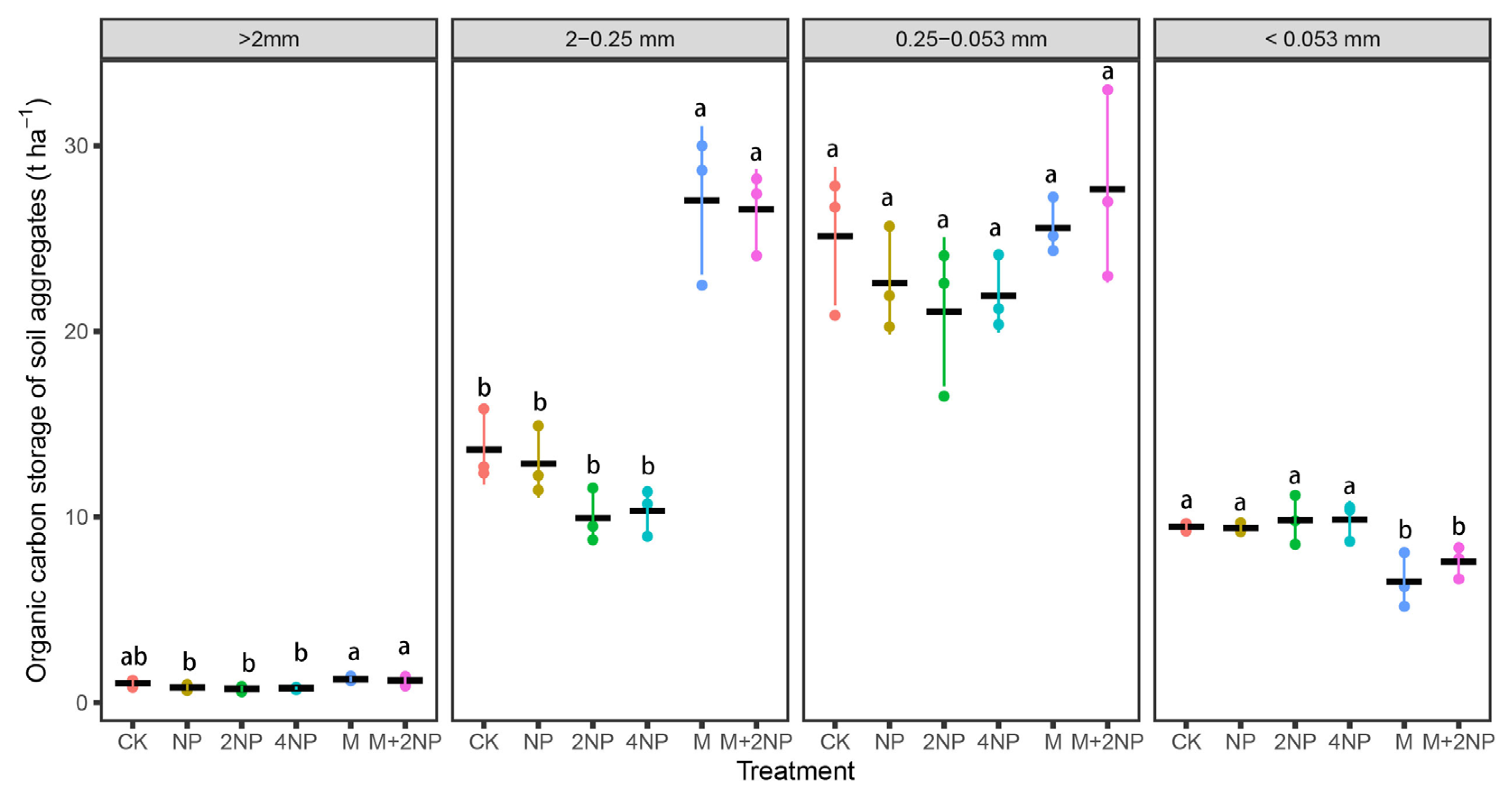
3.4.2. SOC Content and Stock in Water-Stable Soil Aggregates
3.5. Effects of Long-Term Fertilization Treatments on TN Content and Stock in Bulk Soil and Water-Stable Aggregates
3.5.1. Soil TN Content and Stock in Bulk Soil
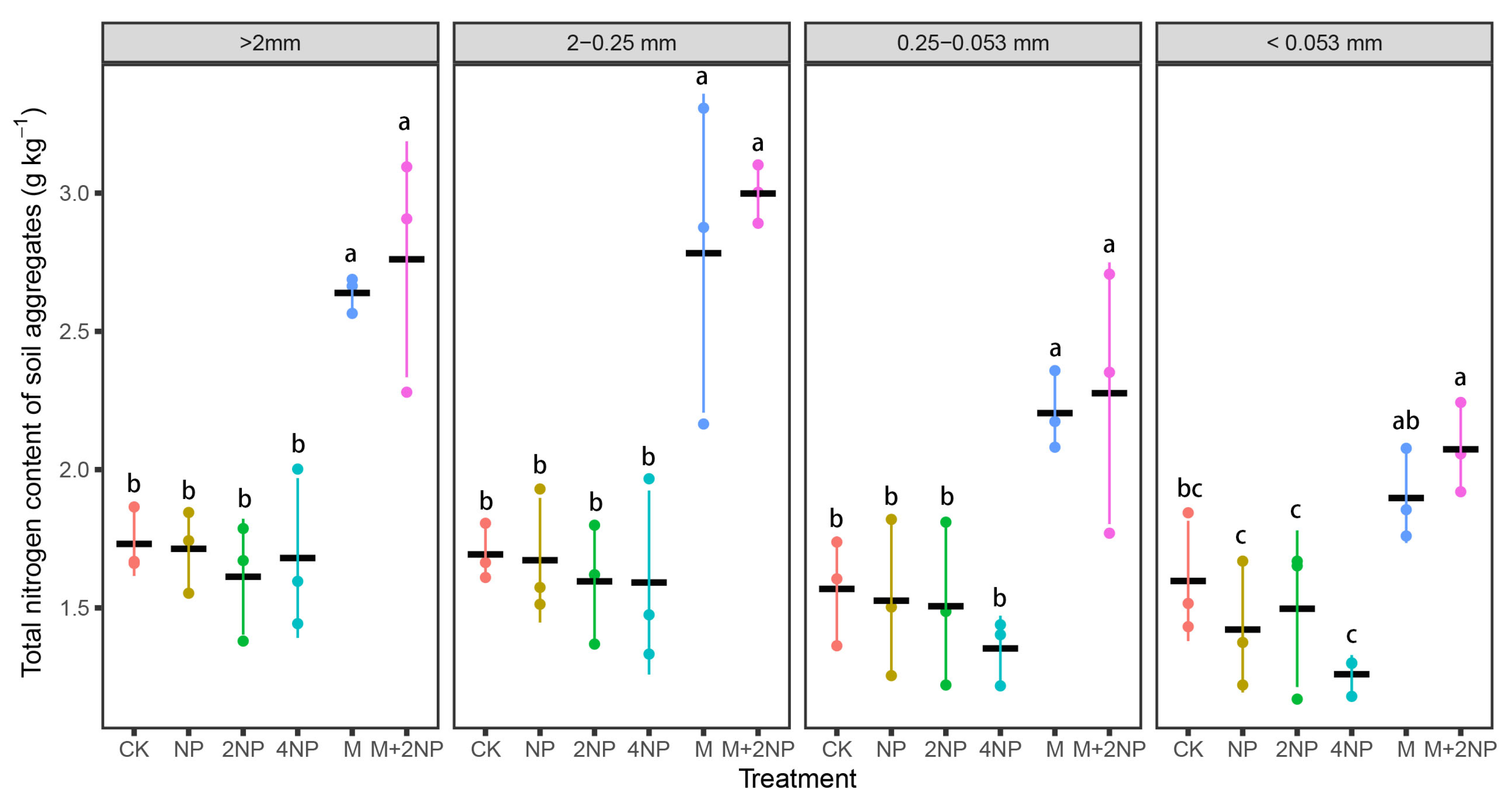
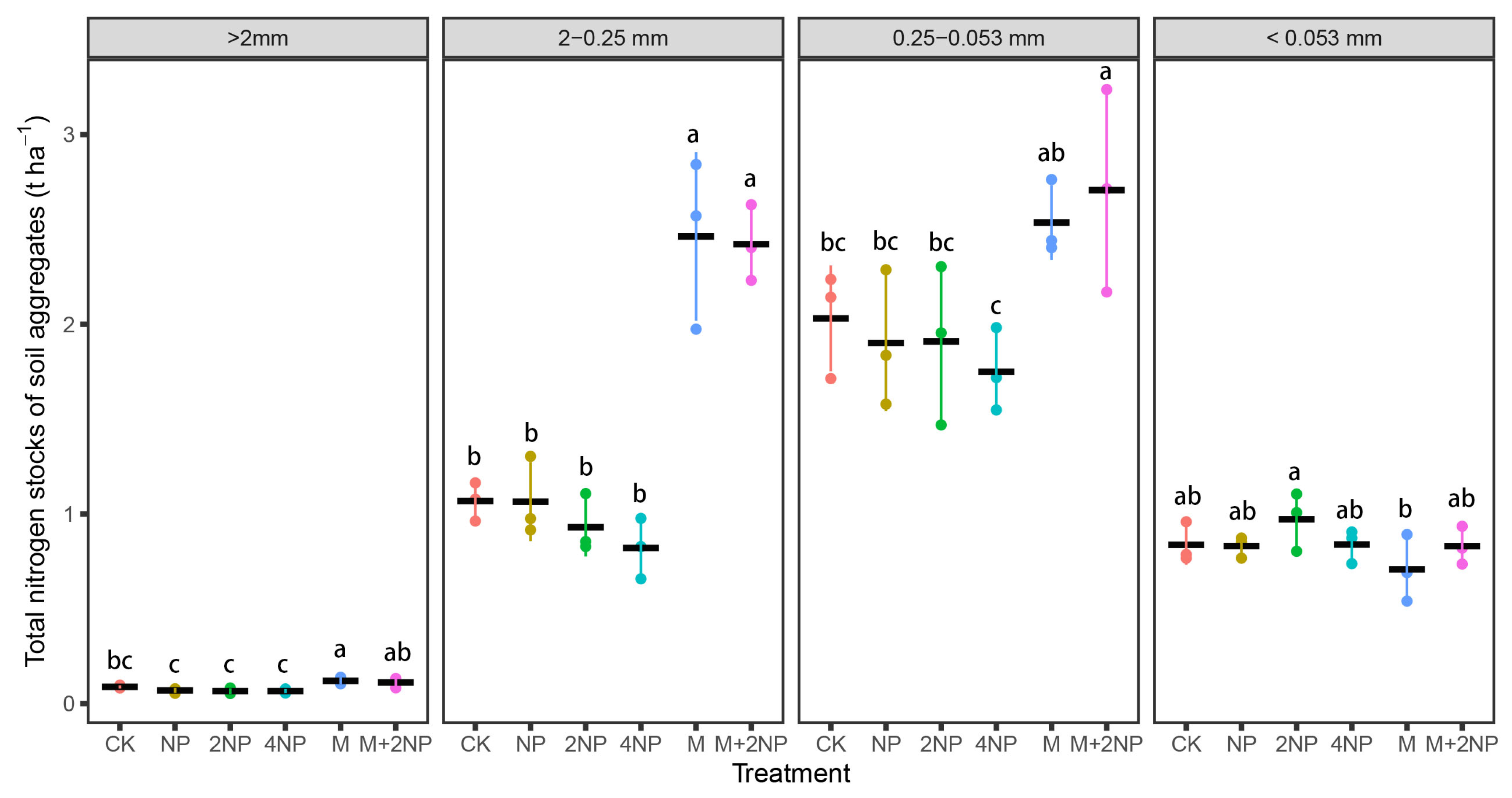
3.5.2. TN Content and Stock in Water-Stable Soil Aggregates
3.6. Contributions of SOC in Soil Aggregates to Bulk Soil SOC under Different Fertilization Treatments
4. Discussion
4.1. Effects of Long-Term Fertilization Practices on the Size Distribution and Stability of Aggregates in Dark Brown Soil
4.2. Effects of Soil pH on Aggregate Stability in Dark Brown Soil
4.3. Effects of Long-Term Fertilization Treatments on SOC and TN Content and Stock in Bulk Soil and Water-Stable Aggregates in Dark Brown Soil
4.4. Contributions of SOC and TN in Soil Aggregates to Bulk Soil SOC and TN under Different Fertilization Treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Ding, F.; Flury, M.; Schaeffer, S.M.; Xu, Y.; Wang, J. Does long-term use of biodegradable plastic mulch affect soil carbon stock? Resour. Conserv. Recycl. 2021, 175, 105895. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Liang, B.; Liu, J.; Zong, H.; Guo, X.; Wang, X.; Song, N. Variations in soil aggregate distribution and associated organic carbon and nitrogen fractions in long-term continuous vegetable rotation soil by nitrogen fertilization and plastic film mulching. Sci. Total Environ. 2022, 835, 155420. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Shangguan, Z.P.; Deng, L. Soil aggregate stability and aggregate-associated carbon and nitrogen in natural restoration grassland and Chinese red pine plantation on the Loess Plateau. Catena 2017, 149, 253–260. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Singh, B.R.; Sitaula, B.K.; Lal, R.; Bajracharya, R.M. Soil aggregate- and particle-associated organic carbon under different land uses in Nepal. Soil Sci. Soc. Am. J. 2007, 71, 1194–1203. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Liang, G.; Zheng, F.; Zhang, M.; Li, S. A global meta-analysis of the impacts of no-tillage on soil aggregation and aggregate-associated organic carbon. Land Degrad. Dev. 2021, 32, 5292–5305. [Google Scholar] [CrossRef]
- Kamran, M.; Huang, L.; Nie, J.; Geng, M.; Lu, Y.; Liao, Y.; Zhou, F.; Xu, Y. Effect of reduced mineral fertilization (NPK) combined with green manure on aggregate stability and soil organic carbon fractions in a fluvo-aquic paddy soil. Soil Tillage Res. 2021, 211, 105005. [Google Scholar] [CrossRef]
- Huang, R.; Tian, D.; Liu, J.; Lv, S.; He, X.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Babur, E.; Kara, O.; Fathi, R.A.; Susam, Y.E.; Riaz, M.; Arif, M.; Akhtar, K. Wattle fencing improved soil aggregate stability, organic carbon stocks and biochemical quality by restoring highly eroded mountain region soil. J. Environ. Manag. 2021, 288, 112489. [Google Scholar] [CrossRef]
- Sui, Y.Y.; Jiao, X.G.; Liu, X.B.; Zhang, X.Y.; Ding, G.W. Water-stable aggregates and their organic carbon distribution after five years of chemical fertilizer and manure treatments on eroded farmland of Chinese Mollisols. Can. J. Soil Sci. 2012, 92, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.I.; Bonilla, C.A. Predicting soil aggregate stability using readily available soil properties and machine learning techniques. Catena 2020, 187, 104408. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Dimoyiannis, D. Wet aggregate stability as affected by excess carbonate and other soil properties. Land Degrad. Dev. 2012, 23, 450–455. [Google Scholar] [CrossRef]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.; Lair, G.J.; Kram, P.; Nikolaidis, N.P.; Kercheva, M.; Banwart, S.; Comans, R.N.J. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247–248, 24–37. [Google Scholar] [CrossRef]
- Pernes-Debuyser, A.; Tessier, D. Soil physical properties affected by long-term fertilization. Eur. J. Soil Sci. 2004, 55, 505–512. [Google Scholar] [CrossRef]
- Gong, H.; Meng, D.; Li, X.; Zhu, F. Soil degradation and food security coupled with global climate change in northeastern China. Chin. Geogr. Sci. 2013, 23, 562–573. [Google Scholar] [CrossRef]
- Xu, X.Z.; Xu, Y.; Chen, S.C.; Xu, S.G.; Zhang, H.W. Soil loss and conservation in the black soil region of Northeast China: A retrospective study. Environ. Sci. Policy 2010, 13, 793–800. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; He, G.; Wang, Z. Benefits of Yield, Environment and economy from substituting fertilizer by manure for wheat production of China. Sci. Agric. Sin. 2020, 53, 4879–4890. [Google Scholar]
- Zhang, X.; Li, Q.; Gao, H.; Peng, C.; Zhu, P.; Gao, Q. Effects of long-term fertilization on the stability of black soil water stable aggregates and the distribution of organic carbon. Sci. Agric. Sin. 2020, 53, 1214–1223. [Google Scholar]
- Su, H.Q.; Han, X.R.; Yang, J.F.; Luo, P.Y.; Dai, J.; Yang, M.C.; He, R. Effect of long-term fertilization on distribution of aggregates and organic carbon and total nitrogen contents in a brown soil. J. Plant Nutr. Fertil. 2017, 23, 924–932. [Google Scholar]
- Geng, R.L.; Yu, H.Y.; Ding, W.X.; Cai, Z.C. Effects of long-term application of organic manure and chemical fertilizers on organic carbon in aggregates of a sandy loam. Soils 2010, 42, 908–914. [Google Scholar]
- He, R.Q.; Wang, B.Q.; Zhang, Y.; Wang, R. Distribution of organic carbon and nutrients in water stable aggregates of Lou soil under long-term application of chemical fertilizer. Bull. Soil Water Conserv. 2016, 36, 347–351. [Google Scholar]
- Miao, S.; Zhou, L.; Qiao, Y.; Qu, J.; Xu, W. Organic carbon mineralization and carbon contribution in aggregates as affected by long-term fertilization. Acta Pedol. Sin. 2009, 46, 1068–1075. [Google Scholar]
- Zhang, J.M.; Chi, F.Q.; Han, J.Z.; Zhou, B.K.; Kuang, E.J. SOC distribution characteristics of mollisols aggregates in different long-term fertilization systems. Soils Crops 2017, 6, 49–54. [Google Scholar]
- Liang, Y.; Yuan, Y.; Han, X.Z.; Li, L.; Zou, W.X.; Ren, J. Distribution of organic carbon and humic acids in aggregates of mollisol as affected by amendments with different rates of organic manure plus mineral fertilizer. J. Plant Nutr. Fertil. 2016, 22, 1586–1594. [Google Scholar]
- Shi, Z.X.; Wu, J.G. Composition and stability of the aggregates in black soil applied with different cattle manures. Soil Fertil. Sci. China 2013, 4, 10–15. [Google Scholar]
- Liu, X.Y.; Wang, Z.Q.; Zhang, X.Y.; Xu, L.L.; Shi, Y.; Sun, X.M.; Yang, F.T. Effects of long-term fertilization on aggregate dynamics and organic carbon and total nitrogen contents in a reddish paddy soil. Acta Ecol. Sin. 2013, 33, 4949–4955. [Google Scholar]
- Xu, J.; Li, C.; He, Y.; Wang, Y.; Liu, X. Effect of fertilization on organic carbon content and fractionation of aggregates in upland red soil. Acta Pedol. Sin. 2007, 44, 675–682. [Google Scholar]
- Adesodun, J.K.; Mbagwu, J.S.C.; Oti, N. Distribution of carbon, nitrogen and phosphorus in water-stable aggregates of an organic waste amended Ultisol in southern Nigeria. Bioresour. Technol. 2005, 96, 509–516. [Google Scholar] [CrossRef]
- Yi, L.; Shiqing, L.; Shengxiu, L. Distribution of nitrogen pools in different sizes of Loess Plateau soil aggregates. Sci. Agric. Sin. 2007, 40, 304–313. [Google Scholar]
- Xu, Z.J.; Liu, G.S.; Yu, J.D.; Liu, W.P. Effects of acid rain on aggregate and lts cemedins of red soil by simulated experiments. J. Soil Water Conserv. 2002, 16, 9–11. [Google Scholar]
- Hu, G.C.; Zhang, M.K. Mineralogical evidence for strong cementation of soil particles by lron oxides. Chin. J. Soil Sci. 2002, 33, 25–27. [Google Scholar]
- Cui, X.; Qiu, J.; Dou, S. Effects of long-time fertilization on crop yield and soil nutrients of dark brown earth. J. Jilin Agric. Univ. 2011, 33, 545–550. [Google Scholar]
- Cambardella, C.A.; Elliott, E.T. Carbon and Nitrogen Distribution in Aggregates from Cultivated and Native Grassland Soils. Soil Sci. Soc. Am. J. 1993, 57, 1071–1076. [Google Scholar] [CrossRef]
- Bi, Y.; Zou, H.; Zhu, C. Dynamic monitoring of soil bulk density and infiltration rate during coal mining in sandy land with different vegetation. Int. J. Coal Sci. Technol. 2014, 1, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.J.; Wang, B.R.; Xu, M.G.; Peng, C.; Liu, H. Changes of soil organic carbon storage under long-term fertilization in black and grey-desert soils. J. Plant Nutr. Fertil. 2012, 18, 98–105. [Google Scholar]
- Pinheiro, E.F.M.; Pereira, M.G.; Anjos, L.H.C. Aggregate distribution and soil organic matter under different tillage systems for vegetable crops in a Red Latosol from Brazil. Soil Till Res. 2004, 77, 79–84. [Google Scholar] [CrossRef]
- Rasool, R.; Kukal, S.S.; Hira, G.S. Soil organic carbon and physical properties as affected by long-term application of FYM and inorganic fertilizers in maize-wheat system. Soil Till Res. 2008, 101, 31–36. [Google Scholar] [CrossRef]
- Aoyama, M.; Angers, D.A.; N’Dayegamiye, A. Particulate and mineral-associated organic matter in water-stable aggregates as affected by mineral fertilizer and manure applications. Can. J. Soil Sci. 1999, 79, 295–302. [Google Scholar] [CrossRef]
- Yang, C.M.; Yang, L.Z.; Zhu, O.Y. Organic carbon and its fractions in paddy soil as affected by different nutrient and water regimes. Geoderma 2005, 124, 133–142. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Majumder, B.; Ruehlmann, J.; Kuzyakov, Y. Effects of aggregation processes on distribution of aggregate size fractions and organic C content of a long-term fertilized soil. Eur. J. Soil Biol. 2010, 46, 365–370. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, X.; Wei, X.; Kai, Z.; Xu, Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021, 11, 23015. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Timney, B. Alcohol slows interhemispheric transmission, increases the flash-lag effect, and prolongs masking: Evidence for a slowing of neural processing and transmission. Vision Res. 2007, 47, 1821–1832. [Google Scholar] [CrossRef]
- Sradnick, A.; Murugan, R.; Oltmanns, M.; Raupp, J.; Joergensen, R.G. Changes in functional diversity of the soil microbial community in a heterogeneous sandy soil after long-term fertilization with cattle manure and mineral fertilizer. Appl. Soil Ecol. 2013, 63, 23–28. [Google Scholar] [CrossRef]
- Sleutel, S.; De Neve, S.; Németh, T.; Tóth, T.; Hofman, G. Effect of manure and fertilizer application on the distribution of organic carbon in different soil fractions in long-term field experiments. Eur. J. Agron. 2006, 25, 280–288. [Google Scholar] [CrossRef]
- Sekiguchi, R.; Saito, H.; Tanaka, H.; Kohgo, Y. Temperature dependence of volcanic ash soil aggregate stability: Effects of fertilizer application. Soil Tillage Res. 2021, 207, 104870. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Li, J.; Zhang, X.H.; Yang, P.; Wang, J.X.; Zhou, N.Q. Fractal characteristics and stability of soil aggregates in karst rocky desertification areas. Nat. Hazards 2013, 65, 563–579. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle manure amendments can increase the pH of acid soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, W.; Zhang, X. The dispersion mechanism of dispersive seasonally frozen soil in western Jilin Province. Bull. Eng. Geol. Environ. 2021, 80, 5493–5503. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Prakash, V.; Kundu, S.; Srivastva, A.K.; Gupta, H.S.; Mitra, S. Long term effects of fertilization on carbon and nitrogen sequestration and aggregate associated carbon and nitrogen in the Indian sub-Himalayas. Nutr. Cycl. Agroecosystems 2010, 86, 1–16. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.D.; He, H.B.; Xie, H.T.; Yan, Y.; Zhu, P.; Ren, J.; Wang, L.C. Carbon and nitrogen pools in different aggregates of a Chinese Mollisol as influenced by long-term fertilization. J. Soil Sediment 2010, 10, 1018–1026. [Google Scholar] [CrossRef]
- Tripathi, R.; Nayak, A.K.; Bhattacharyya, P.; Shukla, A.K.; Shahid, M.; Raja, R.; Panda, B.B.; Mohanty, S.; Kumar, A.; Thilagam, V.K. Soil aggregation and distribution of carbon and nitrogen in different fractions after 41 years long-term fertilizer experiment in tropical rice-rice system. Geoderma 2014, 213, 280–286. [Google Scholar] [CrossRef]
- Song, Z.W.; Zhu, P.; Gao, H.J.; Peng, C.; Deng, A.X.; Zheng, C.Y.; Mannaf, M.A.; Islam, M.N.; Zhang, W.J. Effects of long-term fertilization on soil organic carbon content and aggregate composition under continuous maize cropping in Northeast China. J. Agr. Sci. Camb. 2015, 153, 236–244. [Google Scholar] [CrossRef]
- Xie, H.T.; Li, J.W.; Zhu, P.; Peng, C.; Wang, J.K.; He, H.B.; Zhang, X.D. Long-term manure amendments enhance neutral sugar accumulation in bulk soil and particulate organic matter in a Mollisol. Soil Biol. Biochem. 2014, 78, 45–53. [Google Scholar] [CrossRef]
- Wright, A.L.; Hons, F.M. Soil aggregation and Carbon and Nitrogen storage under soybean cropping sequences. Soil Sci. Soc. Am. J. 2004, 68, 507–513. [Google Scholar] [CrossRef]
- Green, V.S.; Cavigelli, M.A.; Dao, T.H.; Flanagan, D.C. Soil physical properties and aggregate-associated C, N, and P distributions in organic and conventional cropping systems. Soil Sci. 2005, 170, 822–831. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Manuring and rotation effects on soil organic carbon concentration for different aggregate size fractions on two soils in northeastern Ohio, USA. Soil Till Res. 2005, 81, 239–252. [Google Scholar] [CrossRef]
- Wang, X.; Qi, J.Y.; Jing, Z.H.; Li, C.; Zhang, H.L. Effects of long-term conservation tillage on soil aggregate stability and carbon and nitrogen in paddy field. Trans. Chin. Soc. Agric. Eng. 2019, 35, 121–128. [Google Scholar]
- Huang, X.L.; Jiang, H.; Li, Y.; Ma, Y.C.; Tang, H.Y.; Ran, W.; Shen, Q.R. The role of poorly crystalline iron oxides in the stability of soil aggregate-associated organic carbon in a rice-wheat cropping system. Geoderma 2016, 279, 1–10. [Google Scholar] [CrossRef]
- Han, Z.Q.; Ha, Z.C.; Zhang, D.X.; Wang, Q.B.; Chen, H.B.; Chang, L.S.; Yu, Y.Q.; Liu, D.Q. Distributions of organic carbon and nitrogen in cinnamon soil micro-aggregates and effect on soil fertility under the different fertilization regimes. Acta Agric. Boreali-Sin. 2008, 23, 190–195. [Google Scholar]
- Li, W.J.; Yang, J.F.; Peng, B.F.; Cui, J.Z. Effects of fertilization on aggregate characteristics and organic carbon distribution in a Paddy soil in Dongting lake Plain of China. Sci. Agric. Sin. 2014, 47, 4007–4015. [Google Scholar]
- Kamilaris, A.; Prenafeta-Boldú, F.X. Examining the perspectives of using manure from livestock farms as fertilizer to crop fields based on a realistic simulation. Comput. Electron. Agric. 2021, 191, 106486. [Google Scholar] [CrossRef]
- Vu, T.K.V.; Tran, M.T.; Dang, T.T.S. A survey of manure management on pig farms in Northern Vietnam. Livest. Sci. 2007, 112, 288–297. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Tan, L.; Li, Q.; Zhang, C.; Wei, X.; Wang, Q.; Zheng, X.; Xu, Y. Coconut shell and its biochar as fertilizer amendment applied with organic fertilizer: Efficacy and course of actions on eliminating antibiotic resistance genes in agricultural soil. J. Hazard Mater. 2022, 437, 129322. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.-C.; Gao, P.-D.; Wang, B.-Q.; Lin, W.-P.; Jiang, N.-H.; Cai, K.-Z. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef] [Green Version]

| Treatment | Chemical Fertilizer | Organic Fertilizer | The Total dose Per Year | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | N | P2O5 | K2O | N | P2O5 | K2O | Total | |
| CK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NP | 37.5 | 37.5 | 0 | 0 | 0 | 0 | 37.5 | 37.5 | 0 | 75 |
| 2NP | 75 | 75 | 0 | 0 | 0 | 0 | 75 | 75 | 0 | 150 |
| 4NP | 150 | 150 | 0 | 0 | 0 | 0 | 150 | 150 | 0 | 300 |
| M | 0 | 0 | 0 | 29 | 15 | 12 | 29 | 15 | 12 | 56 |
| M+2NP | 75 | 75 | 0 | 29 | 15 | 12 | 104 | 90 | 12 | 206 |
| Treatment | MWD (mm) | GMD (mm) | D | R > 0.25 mm (%) |
|---|---|---|---|---|
| CK | 0.49 ± 0.01 b | 0.19 ± 0.00 b | 2.62 ± 0.01 b | 0.27 ± 0.01 c |
| NP | 0.46 ± 0.03 bc | 0.18 ± 0.01 bc | 2.65 ± 0.02 ab | 0.27 ± 0.02 c |
| 2NP | 0.44 ± 0.04 cd | 0.16 ± 0.01 c | 2.67 ± 0.01 a | 0.25 ± 0.02 cd |
| 4NP | 0.41 ± 0.02 d | 0.15 ± 0.01 c | 2.68 ± 0.01 a | 0.22 ± 0.02 d |
| M | 0.59 ± 0.03 a | 0.26 ± 0.02 a | 2.53 ± 0.04 c | 0.38 ± 0.01 a |
| M+2NP | 0.55 ± 0.03 a | 0.24 ± 0.02 a | 2.55 ± 0.02 c | 0.35 ± 0.03 b |
| R > 0.25 mm | MWD | GMD | D | pH | |
|---|---|---|---|---|---|
| R > 0.25 mm | 1 | ||||
| MWD | 0.986 ** | 1 | |||
| GMD | 0.988 ** | 0.984 ** | 1 | ||
| D | −0.955 ** | −0.963 ** | −0.988 ** | 1 | |
| pH | 0.974 ** | 0.965 ** | 0.972 ** | −0.947 ** | 1 |
| SOC Content g kg−1 | TN Content g kg−1 | C/N | SOC Stock t C ha−1 | TN Stock t N ha−1 | |
|---|---|---|---|---|---|
| CK | 20.25 ± 0.68 b | 1.54 ± 0.04 b | 13.18 ± 0.21 a | 50.60 ± 1.52 b | 3.84 ± 0.08 b |
| NP | 19.34 ± 0.97 bc | 1.44 ± 0.07 bc | 13.46 ± 0.39 a | 48.54 ± 2.19 bc | 3.61 ± 0.16 bc |
| 2NP | 16.85 ± 0.32 c | 1.21 ± 0.05 d | 13.96 ± 0.89 a | 42.80 ± 0.75 c | 3.07 ± 0.14 d |
| 4NP | 19.16 ± 1.85 bc | 1.30 ± 0.08 cd | 14.77 ± 1.78 a | 48.10 ± 4.23 bc | 3.27 ± 0.21 cd |
| M | 24.08 ± 2.56 a | 2.36 ± 0.12 a | 10.19 ± 0.60 b | 59.03 ± 5.56 a | 5.79 ± 0.22 a |
| M+2NP | 25.18 ± 1.54 a | 2.46 ± 0.19 a | 10.25 ± 0.22 b | 61.44 ± 3.33 a | 6.00 ± 0.43 a |
| Treatment | Contribution of OC in Aggregate to SOC (%) | |||||
|---|---|---|---|---|---|---|
| >2 mm | 2–0.25 mm | Sum | 0.25–0.053 mm | <0.053 mm | Sum | |
| CK | 2.03 ± 0.32 a | 26.89 ± 2.91 b | 28.92 ± 3.17 b | 49.60 ± 6.59 a | 18.73 ± 0.38 b | 68.34 ± 6.72 ab |
| NP | 1.68 ± 0.27 a | 26.43 ± 2.50 b | 28.10 ± 2.74 b | 46.47 ± 3.58 a | 19.41 ± 1.31 b | 65.88 ± 2.28 ab |
| 2NP | 1.73 ± 0.39 a | 23.25 ± 3.71 b | 24.99 ± 3.97 b | 49.24 ± 9.64 a | 22.96 ± 2.89 a | 72.20 ± 11.99 a |
| 4NP | 1.63 ± 0.26 a | 21.64 ± 3.55 b | 23.27 ± 3.80 b | 46.04 ± 8.51 a | 20.48 ± 0.38 ab | 66.52 ± 8.18 ab |
| M | 2.14 ± 0.15 a | 45.74 ± 4.15 a | 47.87 ± 4.01 a | 43.43 ± 2.01 a | 11.13 ± 2.81 c | 54.57 ± 3.69 b |
| M+2NP | 1.92 ± 0.33 a | 43.20 ± 1.39 a | 45.12 ± 1.72 a | 44.86 ± 6.36 a | 12.38 ± 1.62 c | 57.25 ± 6.88 b |
| Treatment | Contribution of TN in Aggregate to TN (%) | |||||
|---|---|---|---|---|---|---|
| >2 mm | 2–0.25 mm | Sum | 0.25–0.053 mm | <0.053 mm | Sum | |
| CK | 2.29 ± 0.17 a | 27.79 ± 2.04 b | 30.09 ± 2.15 b | 52.90 ± 7.12 ab | 21.86 ± 3.21 b | 74.76 ± 8.82 bc |
| NP | 1.92 ± 0.29 a | 29.44 ± 4.77 b | 31.36 ± 4.90 b | 52.52 ± 7.89 ab | 23.07 ± 1.97 b | 75.59 ± 7.90 bc |
| 2NP | 2.14 ± 0.38 a | 30.20 ± 3.85 b | 32.34 ± 4.17 b | 62.09 ± 12.58 a | 31.74 ± 5.80 a | 93.83 ± 17.19 a |
| 4NP | 2.02 ± 0.27 a | 25.03 ± 3.97 b | 27.05 ± 4.24 b | 53.52 ± 5.40 ab | 25.78 ± 3.82 ab | 79.30 ± 3.65 ab |
| M | 2.07 ± 0.29 a | 42.41 ± 6.33 a | 44.49 ± 6.19 a | 43.81 ± 2.56 b | 12.24 ± 3.03 c | 56.05 ± 0.48 d |
| M+2NP | 1.85 ± 0.31 a | 40.35 ± 1.28 a | 42.21 ± 1.37 a | 44.93 ± 6.84 b | 13.89 ± 1.96 c | 58.82 ± 7.71 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; He, H.; Zheng, F.; Liu, X.; Wu, X.; Jiang, Y.; Zhang, J. Assessing the Impacts of Fertilization Regimes on Soil Aggregate Dynamics in Northeast China. Agronomy 2022, 12, 2101. https://doi.org/10.3390/agronomy12092101
Ding W, He H, Zheng F, Liu X, Wu X, Jiang Y, Zhang J. Assessing the Impacts of Fertilization Regimes on Soil Aggregate Dynamics in Northeast China. Agronomy. 2022; 12(9):2101. https://doi.org/10.3390/agronomy12092101
Chicago/Turabian StyleDing, Weiting, Hailong He, Fengjun Zheng, Xiaotong Liu, Xueping Wu, Yu Jiang, and Jizong Zhang. 2022. "Assessing the Impacts of Fertilization Regimes on Soil Aggregate Dynamics in Northeast China" Agronomy 12, no. 9: 2101. https://doi.org/10.3390/agronomy12092101






