Novel Allele of FAD2-1A from an EMS-Induced Mutant Soybean Line (PE529) Produces Elevated Levels of Oleic Acid in Soybean Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. EMS-Induced Mutation Population
2.2. Fatty Acid Composition Determination
2.3. FAD2-1A and FAD2-1B Sequencing
2.4. The Development of FAD2-1A W293STOP Genotyping Assay
2.5. Development, Phenotyping, and Genotyping of Segregating Populations
2.6. Statistical Analysis
3. Results
3.1. Phenotypic Distribution of a Mutation Population
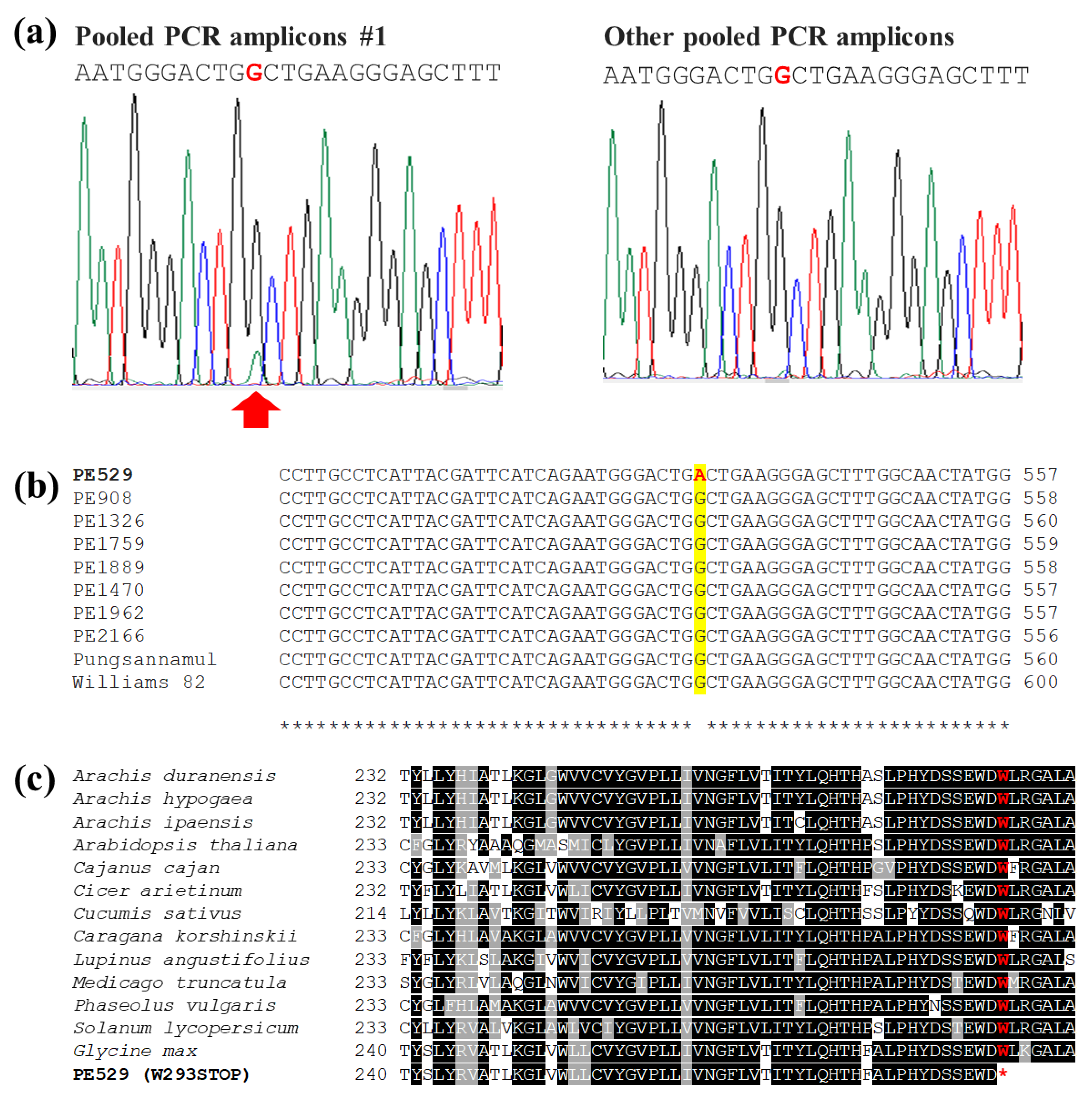
3.2. Pooled-DNA Sequencing of FAD2-1A and FAD2-1B Genes
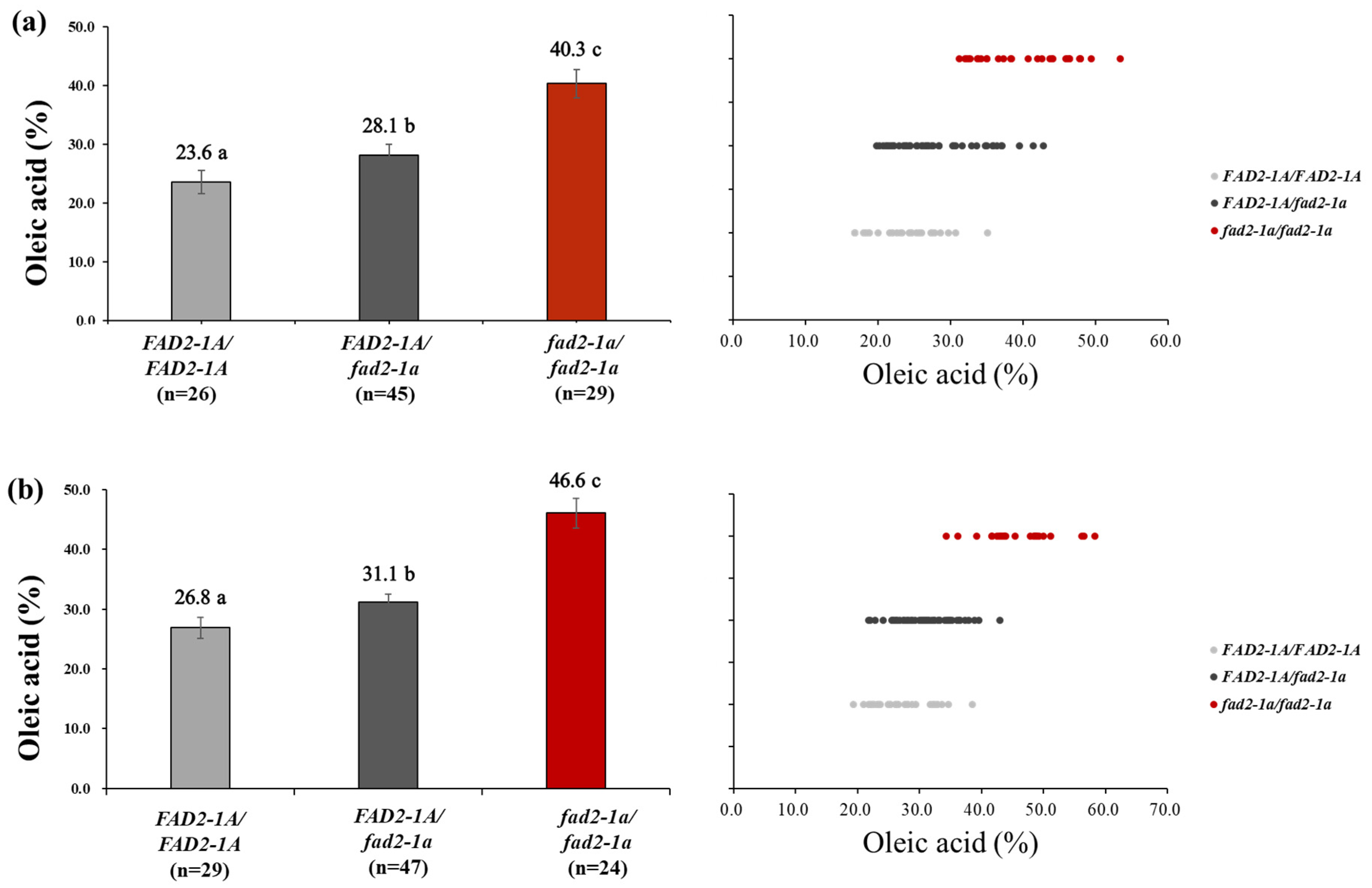
3.3. Inheritance of FAD2-1A W293STOP Allele in PE529
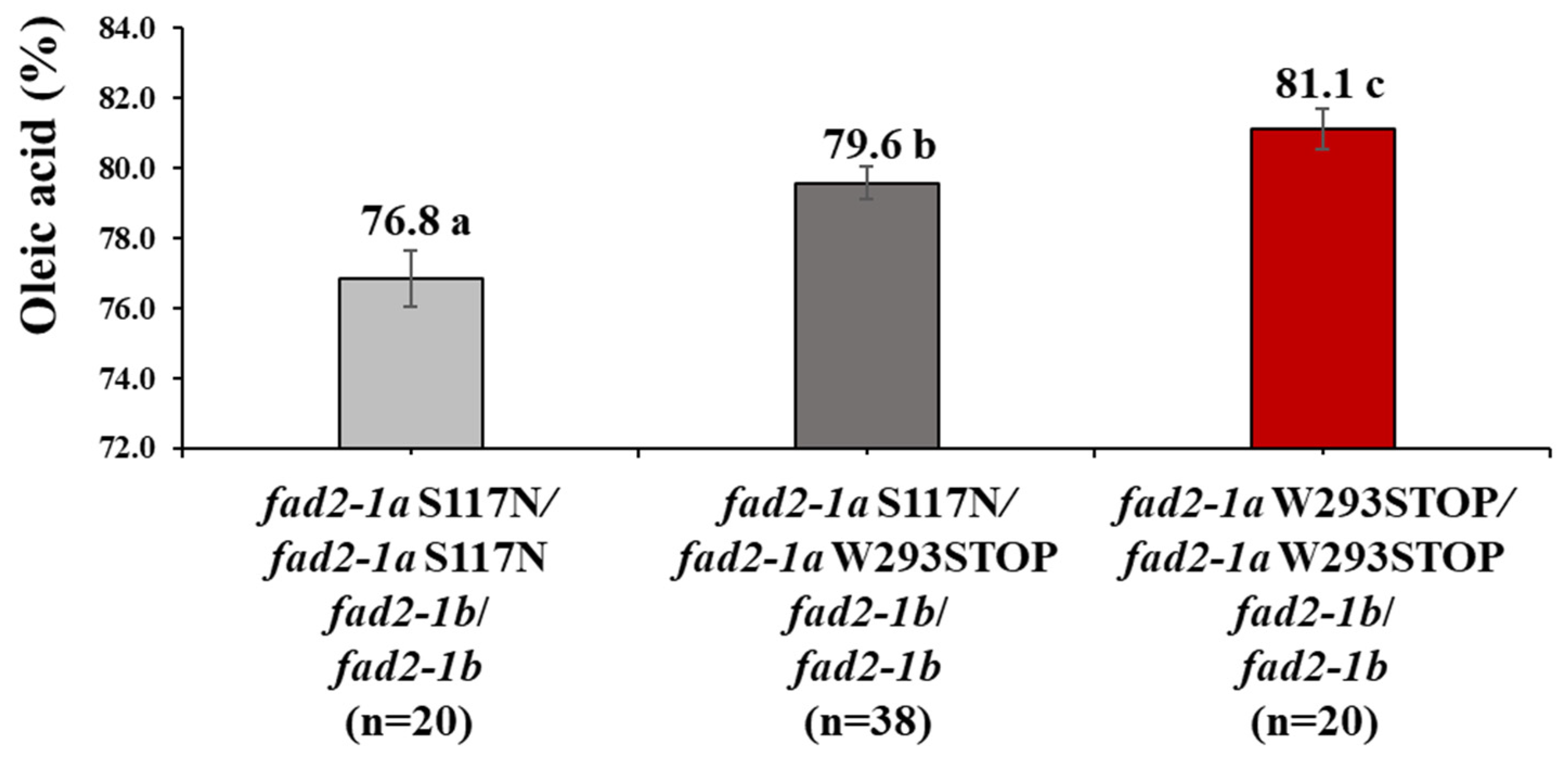
3.4. Production of 80% Oleic Acid Concentration with a FAD2-1A W293STOP Allele in PE529
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SoyStats. 2021. Available online: http://www.soystats.com (accessed on 1 August 2022).
- Butzen, S.; Schnebly, S. High oleic soybean. Crop Insights 2007, 17, 3. [Google Scholar]
- Fehr, W.R. Breeding for modified fatty acid composition in soybean. Crop Sci. 2007, 47, S-72–S-87. [Google Scholar] [CrossRef]
- Okuley, J.; Lightner, J.; Feldmann, K.; Yadav, N.; Lark, E.; Browse, J. Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid-synthesis. Plant Cell 1994, 6, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Beló, A.; Zheng, P.; Luck, S.; Shen, B.; Meyer, D.; Li, B.; Tingey, S.; Rafalski, A. Whole genome scan detects an allelic variant of fad2 associated with increased oleic acid levels in maize. Mol. Genet. Genom. 2008, 279, 1–10. [Google Scholar] [CrossRef]
- De Souza, R.; Mente, A.; Maroleanu, A.; Cozma, A.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ-Br. Med. J. 2015, 351, 3978. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, J.; Vasylenko-Sanders, I.; Deshpande, S.; Yi, J.; Siegfried, M.; Roe, B.; Schlueter, S.; Scheffler, B.; Shoemaker, R. The FAD2 Gene Family of Soybean: Insights into the Structural and Functional Divergence of a Paleopolyploid Genome. Crop Sci. 2007, 47, S-14–S-26. [Google Scholar] [CrossRef]
- Tang, G.; Novitzky, W.; Griffin, H.; Huber, S.; Dewey, R. Oleate desaturase enzymes of soybean: Evidence of regulation through differential stability and phosphorylation. Plant J. 2005, 44, 433–446. [Google Scholar] [CrossRef]
- Combs, R.; Bilyeu, K. Novel alleles of FAD2-1A induce high levels of oleic acid in soybean oil. Mol. Breed. 2019, 39, 79. [Google Scholar] [CrossRef]
- Bilyeu, K.; Škrabišová, M.; Allen, D.; Rajcan, I.; Palmquist, D.E.; Gillen, A.; Mian, R.; Jo, H. The interaction of the soybean seed high oleic acid oil trait with other fatty acid modifications. JAOCS 2018, 95, 39–49. [Google Scholar] [CrossRef]
- Hagely, K.; Konda, A.R.; Kim, J.H.; Cahoon, E.B.; Bilyeu, K. Molecular-assisted breeding for soybean with high oleic/low linolenic acid and elevated vitamin E in the seed oil. Mol. Breed. 2021, 41, 3. [Google Scholar] [CrossRef]
- Pham, A.T.; Shannon, J.; Bilyeu, K. Combinations of mutant FAD2 and FAD3 genes to produce high oleic acid and low linolenic acid soybean oil. Theor. Appl. Genet. 2012, 125, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Kinoshita, T.; Anai, T.; Takagi, Y. Combining ability in loci for high oleic and low linolenic acids in soybean. Crop Sci. 2001, 41, 26–29. [Google Scholar] [CrossRef]
- Willette, A.; Fallen, B.; Bhandari, H.; Sams, C.; Chen, F.; Sykes, V.; Smallwood, C.; Bilyeu, K.; Li, Z.; Pantalone, V. Agronomic performance of high oleic, low linolenic soybean in Tennessee. JAOCS 2021, 98, 861–869. [Google Scholar] [CrossRef]
- Knowles, P.; Hill, A. Inheritance of fatty acid content in the seed oil of a safflower introduction from Iran. Crop Sci. 1964, 4, 406–409. [Google Scholar] [CrossRef]
- Liu, Q.; Singh, S.; Green, A. High-oleic and high-stearic cottonseed oils: Nutritionally improved cooking oils developed using gene silencing. J. Alloys Compd. Nutr. 2002, 21, 205S–211S. [Google Scholar] [CrossRef] [PubMed]
- Bruner, A.; Jung, S.; Abbott, A.; Powell, G.L. The naturally occurring high oleate oil character in some peanut varieties results from reduced oleoyl-PC desaturase activity from mutation of Aspartate 150 to Asparagine. Crop Sci. 2001, 41, 522–526. [Google Scholar] [CrossRef]
- Jung, S.; Powell, G.; Moore, K.; Abbott, A. The high oleate trait in the cultivated peanut [Arachis hypogaea L.]. II. Molecular basis and genetics of the trait. Mol. Genet. Genom. 2000, 263, 806–811. [Google Scholar] [CrossRef]
- Patel, M.; Jung, S.; Moore, K.; Powell, G.; Ainsworth, C.; Abbott, A. High-oleate peanut mutants result from a MITE insertion into the FAD2 gene. Theor. Appl. Genet. 2004, 108, 1492–1502. [Google Scholar] [CrossRef]
- Hu, X.; Sullivan-Gilbert, M.; Gupta, M.; Thompson, S. Mapping of the loci controlling oleic and linolenic acid contents and development of fad2 and fad3 allele-specific markers in canola (Brassica napus L.). Theor. Appl. Genet. 2006, 113, 497–507. [Google Scholar] [CrossRef]
- Stoutjesdijk, P.; Hurlestone, C.; Singh, S.P.; Green, A. High-oleic acid Australian Brassica napus and B. juncea varieties produced by co-suppression of endogenous Delta12 desaturases. Biochem. Soc. Trans. 2000, 28, 938–940. [Google Scholar] [CrossRef]
- Haun, W.; Coffman, A.; Clasen, B.; Demorest, Z.; Lowy, A.; Ray, E.; Rettrath, A.; Stoddard, T.; Juillerat, A.; Cedrone, F.; et al. Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 2014, 12, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Takagi, Y.; Anai, T. Novel GmFAD2-1b mutant alleles created by reverse genetics induce marked elevation of oleic acid content in soybean seed in combination with GmFAD2-1a mutant alleles. Breed. Sci. 2010, 60, 419–425. [Google Scholar] [CrossRef]
- Al Amin, N.; Ahmad, N.; Wu, N.; Pu, X.; Ma, T.; Du, Y.; Bo, X.; Wang, N.; Sharif, R.; Wang, P. CRISPR-Cas9 mediated targeted disruption of FAD2-2 microsomal omega-6 desaturase in soybean (Glycine max L.). BMC Biotechnol. 2019, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.G.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.T.; Lee, J.D.; Shannon, J.G.; Bilyeu, K.D. Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol. 2010, 10, 195. [Google Scholar] [CrossRef]
- Alt, J.L.; Fehr, W.R.; Welke, G.A.; Sandhu, D. Phenotypic and molecular analysis of oleate content in the mutant soybean line M23. Crop Sci. 2005, 45, 1997–2000. [Google Scholar] [CrossRef]
- Anai, T.; Yamada, T.; Hideshima, R.; Kinoshita, T.; Rahman, S.M.; Takagi, Y. Two high-oleic-acid soybean mutants, M23 and KK21, have disrupted microsomal omega-6 fatty acid desaturase, encoded by GmFAD2-1A. Breed. Sci. 2008, 58, 447–452. [Google Scholar] [CrossRef]
- Dierking, E.C.; Bilyeu, K.D. New sources of soybean seed meal and oil composition traits identified through TILLING. BMC Plant Biol. 2009, 9, 89. [Google Scholar] [CrossRef]
- Burton, J.W.; Wilson, R.F.; Rebetzke, G.J.; Pantalone, V.R. Registration of N98-4445A mid-oleic soybean germplasm line. Crop Sci. 2006, 46, 1010–1012. [Google Scholar] [CrossRef]
- Oliva, M.L.; Shannon, J.G.; Sleper, D.A.; Ellersieck, M.R.; Cardinal, A.J.; Paris, R.L.; Lee, J.D. Stability of fatty acid profile in soybean genotypes with modified seed oil composition. Crop Sci. 2006, 46, 2069–2075. [Google Scholar] [CrossRef]
- Pham, A.T.; Lee, J.D.; Shannon, J.G.; Bilyeu, K.D. A novel FAD2-1 A allele in a soybean plant introduction offers an alternate means to produce soybean seed oil with 85% oleic acid content. Theor. Appl. Genet. 2011, 123, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.W.; Carrero-Colón, M.; Hudson, K.A. Characterization of new allelic combinations for high-oleic soybeans. Crop Sci. 2017, 57, 611–616. [Google Scholar] [CrossRef]
- Chae, J.H.; Dhakal, K.H.; Asekova, S.; Song, J.T.; Lee, J.D. Variation of fatty acid composition in soybean ‘Pungsannamul’ mutation population from EMS treatment. Curr. Res. Agric. Life Sci. 2013, 31, 45–50. [Google Scholar]
- Lee, C.; Choi, M.S.; Kim, H.T.; Yun, H.T.; Lee, B.; Chung, Y.S.; Kim, R.W.; Choi, H.K. Soybean [Glycine max (L.) Merrill]: Importance as a crop and pedigree reconstruction of Korean varieties. Plant Breed. Biotechnol. 2015, 3, 179–196. [Google Scholar] [CrossRef]
- Lee, J.D.; Kim, M.; Kulkarni, K.P.; Song, J.T. Agronomic traits and fatty acid composition of high–oleic acid cultivar Hosim. Plant Breed. Biotechnol. 2018, 6, 44–50. [Google Scholar] [CrossRef]
- Thapa, R.; Carrero-Colon, M.; Crowe, M.; Gaskin, E.; Hudson, K. Novel FAD2–1A alleles confer an elevated oleic acid phenotype in soybean seeds. Crop Sci. 2016, 56, 226–231. [Google Scholar] [CrossRef]
- Nan, H.; Lu, S.; Fang, C.; Hou, Z.; Yang, C.; Zhang, Q.; Liu, B.; Kong, F. Molecular breeding of a high oleic acid soybean line by integrating natural variations. Mol. Breed. 2020, 40, 87. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Solanki, S.; Hussain, S.M. Influence of growing environment on the biochemical composition and physical characteristics of soybean seed. J. Food Compost Anal. 2006, 19, 188–195. [Google Scholar] [CrossRef]
- Lee, J.D.; Bilyeu, K.D.; Pantalone, V.R.; Gillen, A.M.; So, Y.S.; Shannon, J.G. Environmental stability of oleic acid concentration in seed oil for soybean lines with FAD2-1A and FAD2-1B mutant genes. Crop Sci. 2012, 52, 1290–1297. [Google Scholar] [CrossRef]
- Kim, H.J.; Ha, B.K.; Ha, K.S.; Chae, J.H.; Park, J.H.; Kim, M.S.; Asekova, S.; Shannon, J.G.; Son, C.K.; Lee, J.D. Comparison of a high oleic acid soybean line to cultivated cultivars for seed yield, protein and oil concentrations. Euphytica 2015, 201, 285–292. [Google Scholar] [CrossRef]

| Population | Progeny | Observed for Genotypic Ratio | Expected for Genotypic Ratio | Chi-Square (1:2:1) | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| FAD2-1A/ FAD2-1A | FAD2-1A/ fad2-1a | fad2-1a/ fad2-1a | FAD2-1A/ FAD2-1A | FAD2-1A/ fad2-1a | fad2-1a/ fad2-1a | ||||
| Pungsannamul × PE529 | F2 (n = 100) | 26 | 45 | 29 | 25 | 50 | 25 | 1.18 | 0.554 |
| Sunpung × PE529 | F2 (n = 100) | 29 | 47 | 24 | 25 | 50 | 25 | 0.86 | 0.651 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Woo, C.; Norah, N.; Song, J.T.; Lee, J.-D. Novel Allele of FAD2-1A from an EMS-Induced Mutant Soybean Line (PE529) Produces Elevated Levels of Oleic Acid in Soybean Oil. Agronomy 2022, 12, 2115. https://doi.org/10.3390/agronomy12092115
Jo H, Woo C, Norah N, Song JT, Lee J-D. Novel Allele of FAD2-1A from an EMS-Induced Mutant Soybean Line (PE529) Produces Elevated Levels of Oleic Acid in Soybean Oil. Agronomy. 2022; 12(9):2115. https://doi.org/10.3390/agronomy12092115
Chicago/Turabian StyleJo, Hyun, Changwan Woo, Nabachwa Norah, Jong Tae Song, and Jeong-Dong Lee. 2022. "Novel Allele of FAD2-1A from an EMS-Induced Mutant Soybean Line (PE529) Produces Elevated Levels of Oleic Acid in Soybean Oil" Agronomy 12, no. 9: 2115. https://doi.org/10.3390/agronomy12092115





