Divergent Roles of CNGC2 and CNGC4 in the Regulation of Disease Resistance, Plant Growth and Heat Tolerance in Arabidopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Pathogen Growth Assay
2.3. Histochemical Staining
2.4. Gene Expression Assay
2.5. Heat Tolerance Assay
2.6. Stomatal Aperture Assay
2.7. Statistical Analysis
3. Results
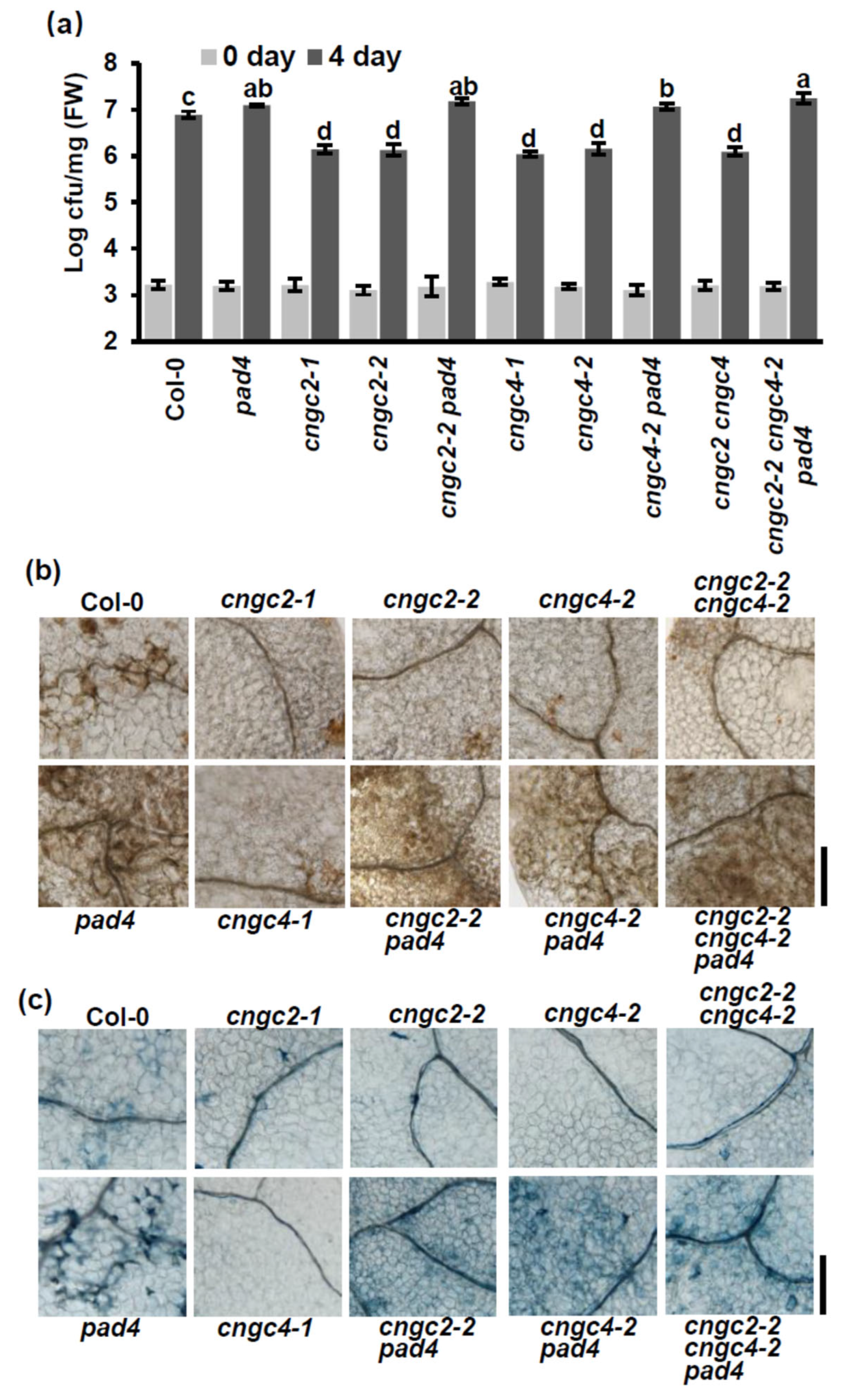
3.1. Enhanced Disease Resistance of the cngc2 and cngc4 Mutants Is Dependent on PAD4 at Normal Temperature
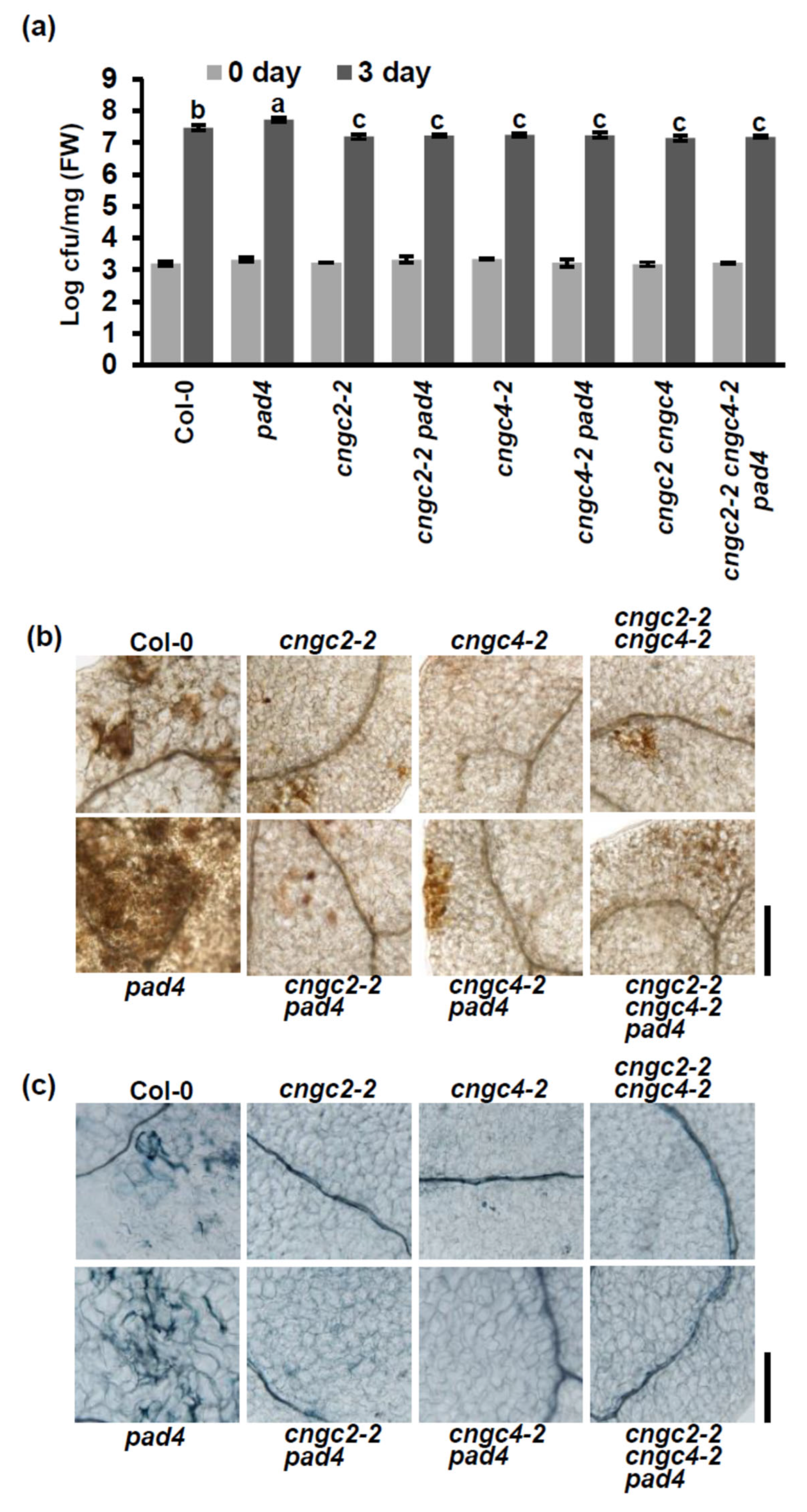
3.2. Enhanced Disease Resistance of the cngc2 and cngc4 Mutants Is Not Dependent on PAD4 at Moderately Elevated Temperature
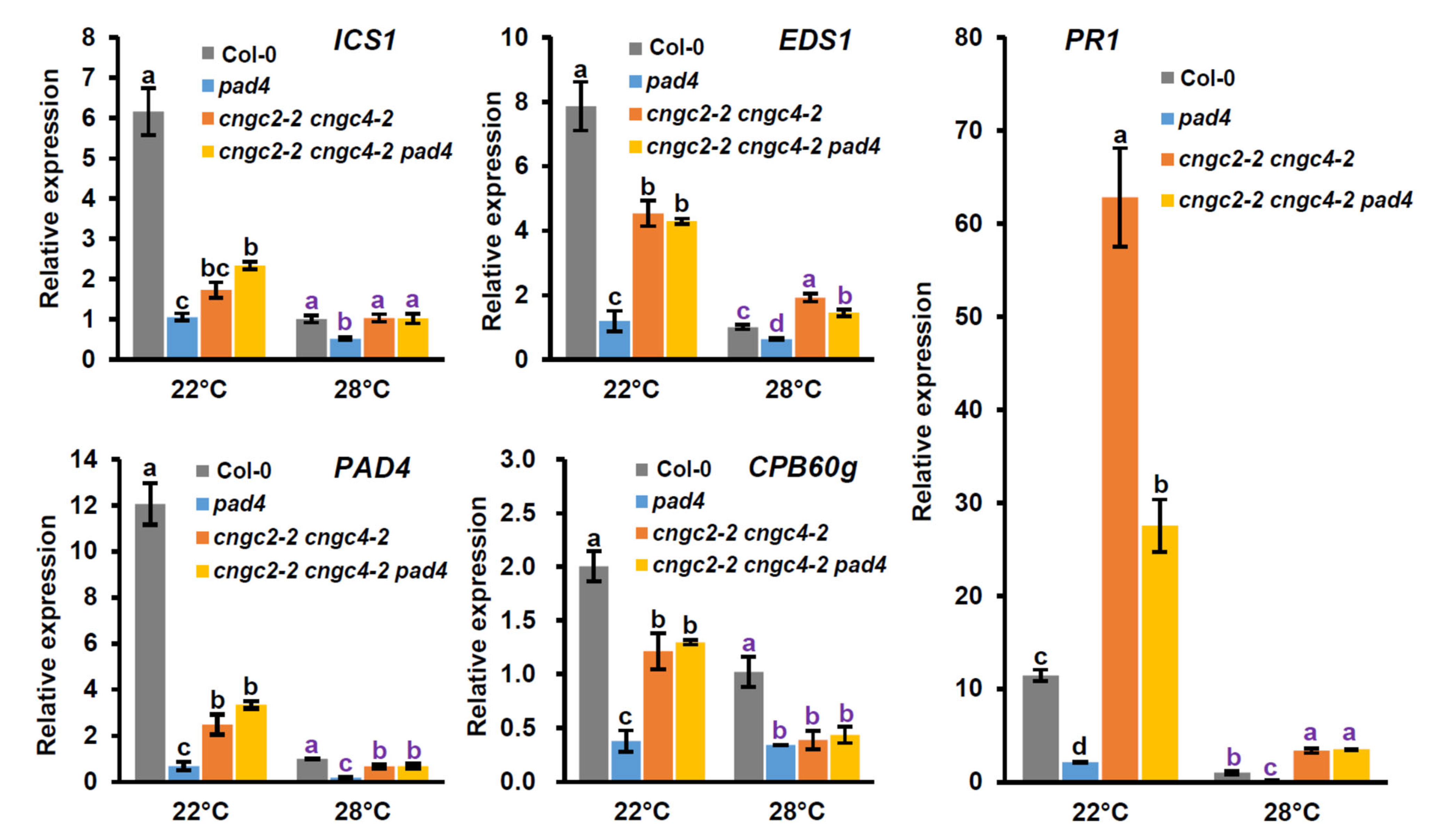
3.3. Expression of SA-Associated Genes at Normal and Moderately Elevated Temperatures after Infection
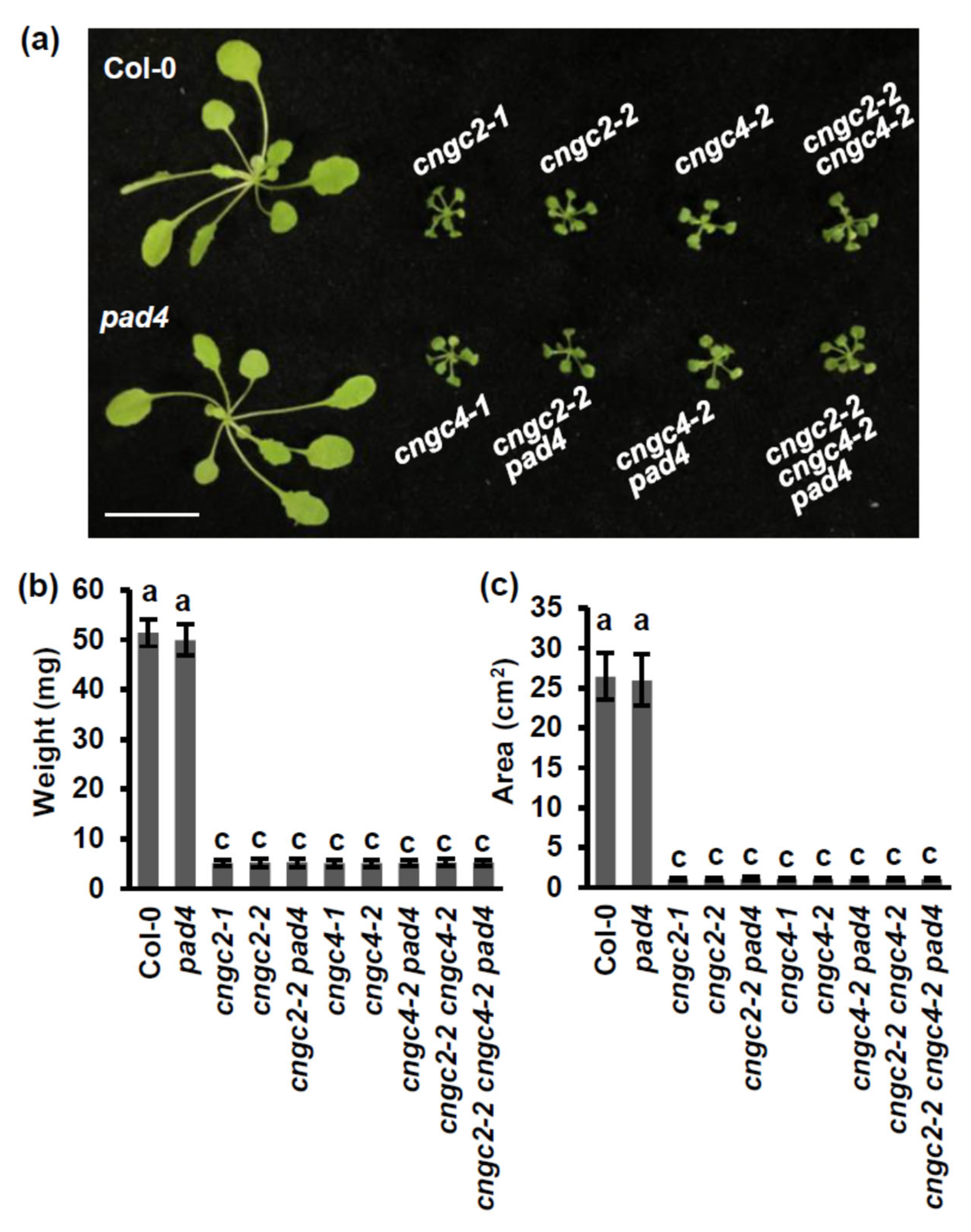
3.4. The Growth Defect of the cngc Mutants Is Partially Dependent on PAD4 at Normal Temperature
3.5. The Growth Defect of the cngc Mutants Is Not Dependent on PAD4 at Moderately Elevated Temperatures
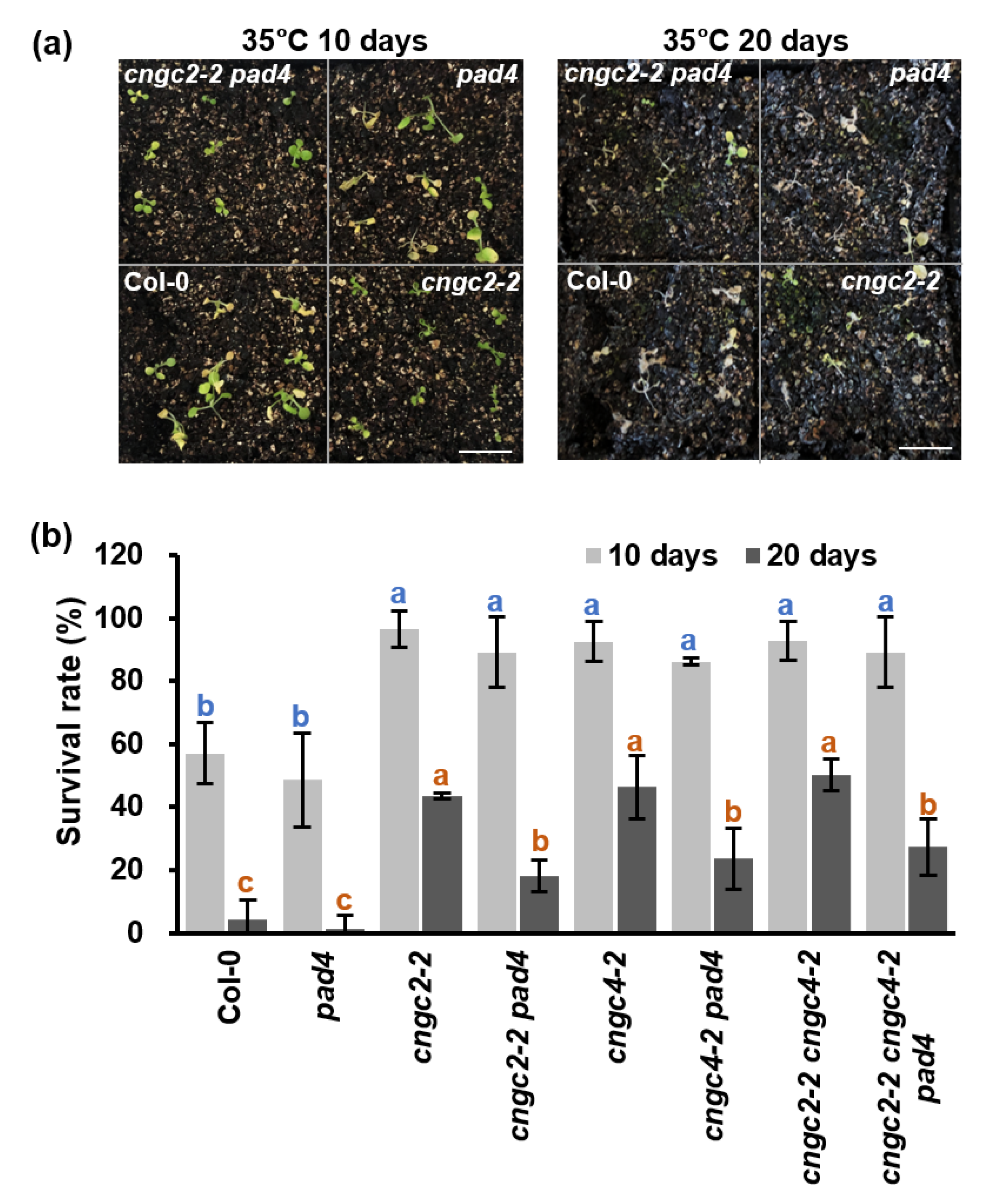
3.6. Enhanced Heat Tolerance of the cngc Mutants Is Partially Dependent on PAD4
3.7. The Stomatal Apertures of the cngc Mutants Are Partially Suppressed by the PAD4 Mutation under Heat
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.Y.; Niu, Y.F.; Zhang, J.J.; Zhou, Y.Q.; Ma, Z.; Huang, X. Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: Recent advances. Plant Cell Tiss. Org. 2018, 132, 413–424. [Google Scholar] [CrossRef]
- Maser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.M.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef] [PubMed]
- Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davies, J.M. The complex story of plant cyclic nucleotide-gated channels. Int. J. Mol. Sci. 2021, 22, 874. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.C.; Parker, J.; Bent, A.F. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc. Natl. Acad. Sci. USA 1998, 95, 7819–7824. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Fengler, K.A.; Yu, I.C.; Lippok, B.; Smith, R.K.; Bent, A.F. The Arabidopsis dnd1 "defense, no death" gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 2000, 97, 9323–9328. [Google Scholar] [CrossRef] [PubMed]
- Jurkowski, G.I.; Smith, R.K., Jr.; Yu, I.C.; Ham, J.H.; Sharma, S.B.; Klessig, D.F.; Fengler, K.A.; Bent, A.F. Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the "defense, no death" phenotype. Mol. Plant Microbe Interact. 2004, 17, 511–520. [Google Scholar] [CrossRef]
- Chin, K.; DeFalco, T.A.; Moeder, W.; Yoshioka, K. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol. 2013, 163, 611–624. [Google Scholar] [CrossRef]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef]
- Jirage, D.; Tootle, T.L.; Reuber, T.L.; Frost, L.N.; Feys, B.J.; Parker, J.E.; Ausubel, F.M.; Glazebrook, J. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 13583–13588. [Google Scholar] [CrossRef]
- Jirage, D.; Zhou, N.; Cooper, B.; Clarke, J.D.; Dong, X.; Glazebrook, J. Constitutive salicylic acid-dependent signaling in cpr1 and cpr6 mutants requires PAD4. Plant J. 2001, 26, 395–407. [Google Scholar] [CrossRef]
- Zhou, N.; Tootle, T.L.; Tsui, F.; Klessig, D.F.; Glazebrook, J. PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 1998, 10, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Urquhart, W.; Ung, H.; Yoshioka, K. The role of cyclic nucleotide-gated ion channels in plant immunity. Mol. Plant 2011, 4, 442–452. [Google Scholar] [CrossRef]
- Jogawat, A.; Meena, M.K.; Kundu, A.; Varma, M.; Vadassery, J. Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J. Exp. Bot. 2020, 71, 2752–2768. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, G.Y.; Li, B.; Vespoli, L.D.; Liu, H.; Moeder, W.; Chen, S.X.; de Oliveira, M.V.V.; de Souza, S.A.; Shao, W.Y.; et al. The receptor kinases BAK1/SERK4 regulate Ca2+ channel-mediated cellular homeostasis for cell death containment. Curr. Biol. 2019, 29, 3778–3790. [Google Scholar] [CrossRef] [PubMed]
- Finka, A.; Cuendet, A.F.; Maathuis, F.J.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef]
- Katano, K.; Kataoka, R.; Fujii, M.; Suzuki, N. Differences between seedlings and flowers in anti-ROS based heat responses of Arabidopsis plants deficient in cyclic nucleotide gated channel 2. Plant Physiol. Biochem. 2018, 123, 288–296. [Google Scholar] [CrossRef]
- Cui, Y.M.; Lu, S.; Li, Z.; Cheng, J.W.; Hu, P.; Zhu, T.Q.; Wang, X.; Jin, M.; Wang, X.X.; Li, L.Q.; et al. Cyclic nucleotide-gated ion channels 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Feller, U. Stomatal opening at elevated temperature: An underestimated regulatory mechanism. Gen. Appl. Plant Physiol. 2006, 32, 19–31. [Google Scholar]
- Wang, P.; Song, C.P. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 2008, 178, 703–718. [Google Scholar] [CrossRef]
- Song, P.; Jia, Q.; Chen, L.; Jin, X.; Xiao, X.; Li, L.; Chen, H.; Qu, Y.; Su, Y.; Zhang, W.; et al. Involvement of Arabidopsis phospholipase D delta in regulation of ROS-mediated microtubule organization and stomatal movement upon heat shock. J. Exp. Bot. 2020, 71, 6555–6570. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Wei, C.; Liu, X.; Wang, M.; Yu, F.; Xie, Q.; Tu, J. The ring finger ubiquitin E3 ligase OsHTAS enhances heat tolerance by promoting H2O2-induced stomatal closure in rice. Plant Physiol. 2016, 170, 429–443. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Xu, F.; Zhang, Y.; Zhang, Q.; Miao, R.; Zhang, J.; Liang, J.; Xu, W. Suppression of OsMDHAR4 enhances heat tolerance by mediating H2O2-induced stomatal closure in rice plants. Rice 2018, 11, 38. [Google Scholar] [CrossRef]
- Tsuda, K.; Sato, M.; Stoddard, T.; Glazebrook, J.; Katagiri, F. Network properties of robust immunity in plants. PLoS Genet. 2009, 5, e1000772. [Google Scholar] [CrossRef]
- Gou, M.; Zhang, Z.; Zhang, N.; Huang, Q.; Monaghan, J.; Yang, H.; Shi, Z.; Zipfel, C.; Hua, J. Opposing effects on two phases of defense responses from concerted actions of heat shock cognate70 and bonzai1 in arabidopsis. Plant Physiol. 2015, 169, 2304–2323. [Google Scholar]
- Bach-Pages, M.; Preston, G.M. Methods to quantify biotic-induced stress in plants. Methods Mol. Biol. 2018, 1734, 241–255. [Google Scholar]
- Wu, S.; Zhao, B. Using clear nail polish to make Arabidopsis epidermal impressions for measuring the change of stomatal aperture size in immune response. Methods Mol. Biol. 2017, 1578, 243–248. [Google Scholar]
- Wu, S.; Shan, L.; He, P. Microbial signature-triggered plant defense responses and early signaling mechanisms. Plant Sci. 2014, 228, 118–126. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Z.; Zhu, Y.; Hua, J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol. Plant Microbe Interact. 2009, 22, 498–506. [Google Scholar] [CrossRef]
- Kim, J.H.; Castroverde, C.D.M.; Huang, S.; Li, C.; Hilleary, R.; Seroka, A.; Sohrabi, R.; Medina-Yerena, D.; Huot, B.; Wang, J.; et al. Increasing the resilience of plant immunity to a warming climate. Nature 2022, 607, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.T.; Gobbato, E.; Kracher, B.; Qiu, J.D.; Bautor, J.; Parker, J.E. A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.N.; Locci, F.; Wanke, F.; Zhang, L.; Saile, S.C.; Joe, A.; Karelina, D.; Hua, C.; Frohlich, K.; Wan, W.L.; et al. The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature 2021, 598, 495–499. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Ding, Z.; Yan, J.; Yu, H.; Pan, R.; Hu, J.; Guan, Y.; Hua, J. Low temperature enhances plant immunity via salicylic acid pathway genes that are repressed by ethylene. Plant Physiol. 2020, 182, 626–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kang, Y.; Ma, C.L.; Miao, R.Y.; Wu, C.L.; Long, Y.; Ge, T.; Wu, Z.N.; Hou, X.Y.; Zhang, J.X.; et al. CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiol. 2017, 173, 1342–1354. [Google Scholar] [CrossRef]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Melotto, M.; Zhang, L.; Oblessuc, P.R.; He, S.Y. Stomatal defense a decade later. Plant Physiol. 2017, 174, 561–571. [Google Scholar] [CrossRef]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klusener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef]
- Muhlenbock, P.; Szechynska-Hebda, M.; Plaszczyca, M.; Baudo, M.; Mullineaux, P.M.; Parker, J.E.; Karpinska, B.; Karpinski, S. Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 2008, 20, 2339–2356. [Google Scholar] [CrossRef]
- Bernacki, M.J.; Czarnocka, W.; Szechynska-Hebda, M.; Mittler, R.; Karpinski, S. Biotechnological potential of LSD1, EDS1, and PAD4 in the improvement of crops and industrial plants. Plants 2019, 8, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Zhu, T.; Luo, L.; Ouyang, N.; Hua, J.; Zou, B. Divergent Roles of CNGC2 and CNGC4 in the Regulation of Disease Resistance, Plant Growth and Heat Tolerance in Arabidopsis. Agronomy 2022, 12, 2176. https://doi.org/10.3390/agronomy12092176
Lu S, Zhu T, Luo L, Ouyang N, Hua J, Zou B. Divergent Roles of CNGC2 and CNGC4 in the Regulation of Disease Resistance, Plant Growth and Heat Tolerance in Arabidopsis. Agronomy. 2022; 12(9):2176. https://doi.org/10.3390/agronomy12092176
Chicago/Turabian StyleLu, Shan, Tianquan Zhu, Lilin Luo, Nana Ouyang, Jian Hua, and Baohong Zou. 2022. "Divergent Roles of CNGC2 and CNGC4 in the Regulation of Disease Resistance, Plant Growth and Heat Tolerance in Arabidopsis" Agronomy 12, no. 9: 2176. https://doi.org/10.3390/agronomy12092176




