Anticipating the Climate Change Impacts on Madeira’s Agriculture: The Characterization and Monitoring of a Vine Agrosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Study
2.2. Climate and Climatic Conditions
2.3. Climate Data Modeling
2.4. Soil Sampling and Characterization
2.5. Microbiologic Parameters
2.6. Floristic and Plant Diversity Indicators
2.7. Insect Survey and Diversity Indicators
2.8. Crop and Production Elements
2.9. Data Treatment
3. Results and Discussion
3.1. Climate and Climatic Conditions
3.1.1. Evolution of Temperature Variation in Winter and Summer
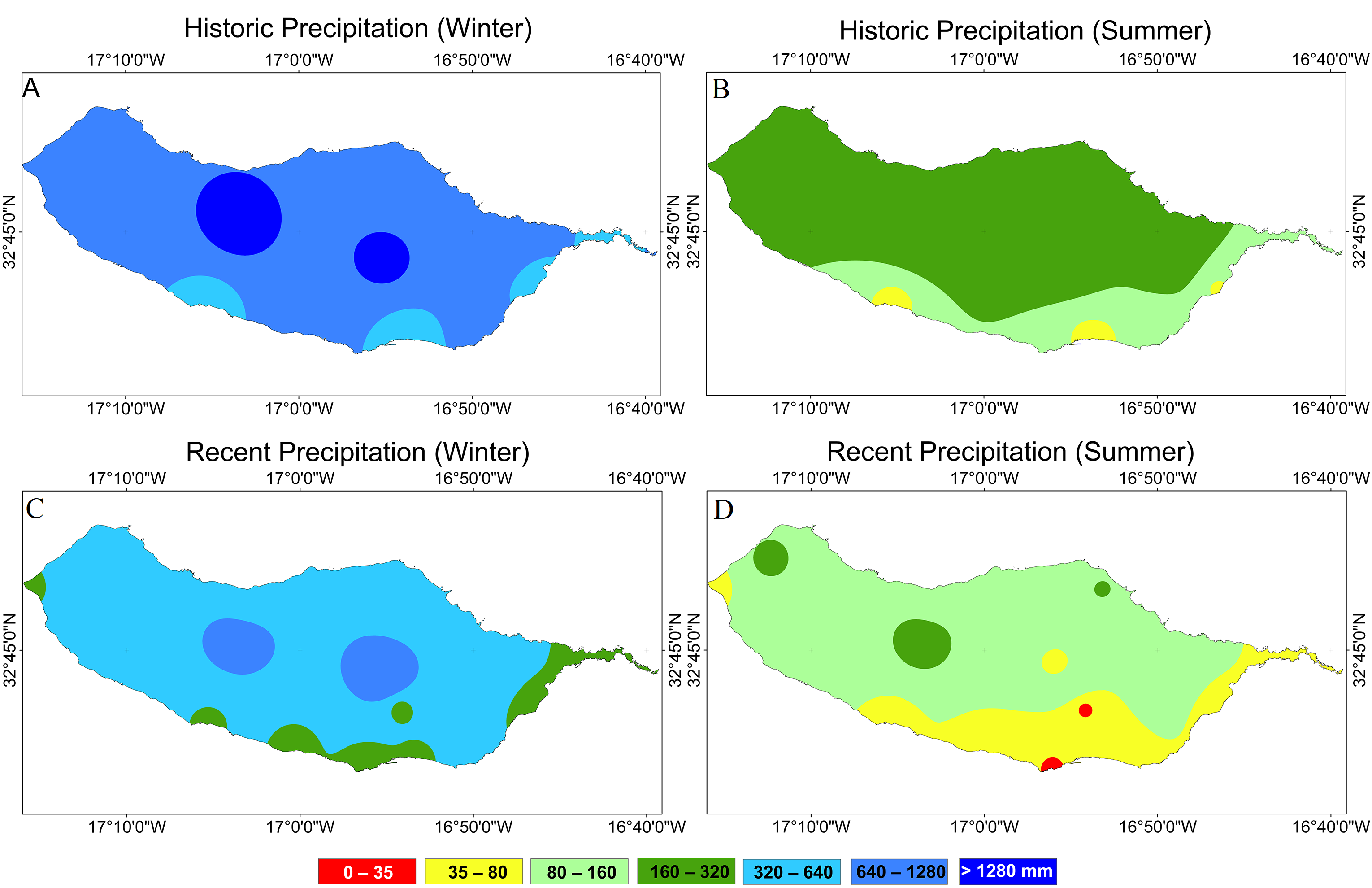
3.1.2. Evolution of Accumulated Precipitations in Winter and Summer
3.1.3. Comparison of Actual Climate Conditions with Future Climate Scenarios
3.2. Climate Water Balance
3.3. Soil Physicochemical Features or Properties
3.4. Soil Biological Properties
3.4.1. Microbiological Indicators
3.4.2. Microbiological Diversity Indices and Seasonal Variation
3.4.3. Relationship between Microbiological Diversity Indices, Climatic Data, and Edaphic Parameters
3.5. Floristic Survey and Plant Diversity Indicators
3.6. Insect Survey and Diversity Indicators
3.7. Crop and Production Elements
4. Final Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of emerging implications of climate change on food production systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; 976p, ISBN 978-0521-70597-4. [Google Scholar]
- Lennon, J.J. Potential impacts of climate change on agriculture and food safety within the island of Ireland. Trends Food Sci. Technol. 2015, 44, 1–10. [Google Scholar] [CrossRef]
- Lincoln Lenderking, H.; Robinson, S.-A.; Carlson, G. Climate change and food security in Caribbean small island developing states: Challenges and strategies. Int. J. Sustain. Dev. World Ecol. 2021, 28, 238–245. [Google Scholar] [CrossRef]
- Gomes, A.; Avelar, D.; Duarte Santos, F.; Costa, H.; Garrett, P. Estratégia CLIMA-Madeira. Estratégia de Adaptação às Altterações Climáticas da Região Autónoma da Madeira; Secretaria Regional do Ambiente e Recursos Naturais: Funchal, Portugal, 2015; ISBN 978-989-95709-6-2. [Google Scholar]
- Macedo, F.L.; de Carvalho, M.Â.A.P. The Expected Impact of Climate in the Grapevine Culture, in Madeira Region, Portugal. J. Agric. Environ. Sci. 2022, 11, 20–29. [Google Scholar] [CrossRef]
- Tonietto, J.; Carbonneau, A. Análise Mundial Do Clima Das Regiões Vitícolas E De Sua Influência Sobre a Tipicidade Dos Vinhos: A posição da viticultura brasileira comparada a 100 regiões em 30 países. In Proceedings of the IX Congresso Brasileiro de Viticultura e Enologia, Bento Gonçalves, RS, Brazil, 7–10 December 1999; pp. 75–90. [Google Scholar]
- Ju, M.; van der Velde, M.; Lin, E.; Xiong, W.; Li, Y. The impacts of climate changes on agricultural production systems in China. Clim. Chang. 2013, 120, 313–324. [Google Scholar] [CrossRef]
- Falloon, P.; Jones, C.D.; Cerri, C.E.; Al-Adamat, R.; Kamoni, P.; Bhattacharyya, T.; Easter, M.; Paustian, K.; Killian, K.; Coleman, K.; et al. Climate change and its impact on soil and vegetation carbon storage in Kenya, Jordan, India and Brazil. Agric. Ecosyst. Environ. 2007, 122, 114–124. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, C.I. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef]
- Supit, I.; van Diepen, C.A.; de Wit, A.J.W.; Kabat, P.; Baruth, B.; Ludwig, F. Recent changes in the climatic yield potential of various crops in Europe. Agric. Syst. 2010, 103, 683–694. [Google Scholar] [CrossRef]
- Macedo, F.L.; Ragonezi, C.; de Carvalho, M.Â.A.P. Zoneamento agroclimático da cultura da videira para a ilha da Madeira—Portugal. Rev. Caminhos Geogr. 2020, 21, 296–306. [Google Scholar]
- Esri, Inc. ArcGIS Desktop, Release 10.6.1; Environmental Systems Research Institute (Esri), Inc.: Redlands, CA, USA, 2018. [Google Scholar]
- Cavalcanti, E.P.; Silva, V.D.P.R.; de Sousa, F.D.A.S. Programa computacional para a estimativa da temperatura do ar para a região Nordeste do Brasil. Rev. Bras. Eng. Agrícola Ambient. 2006, 10, 140–147. [Google Scholar] [CrossRef]
- Santos, A.R.; Ribeiro, C.A.A.S.; Sediyama, G.C.; Peluzio, J.B.E.; Pezzodane, J.; Bragança, R. Espacialização de Dados Meteorológicos No ArcGIS 10.3 Passo a Passo; CAUFES: Alegre, ES, Brazil, 2015; ISBN 978-85-61890-62-9. [Google Scholar]
- Paetz, A.; Wilke, B.-M. Soil Sampling and Storage. In Monitoring and Assessing Soil Bioremediation; Springer: Berlin, Germany, 2005; Volume 5, pp. 1–45. [Google Scholar] [CrossRef]
- Yeates, C.; Gillings, M.R.; Davison, A.D.; Altavilla, N.; Veal, D.A. Methods for microbial DNA extraction from soil for PCR amplification. Biol. Proced. Online 1998, 1, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Culman, S.W.; Bukowski, R.; Gauch, H.G.; Cadillo-Quiroz, H.; Buckley, D.H. T-REX: Software for the processing and analysis of T-RFLP data. BMC Bioinform. 2009, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Drozd, P. ComEcoPaC—Community Ecology Parameter Calculator, Version 1; 2010. Available online: http://prf.osu.cz/kbe/dokumenty/sw/ComEcoPaC/ComEcoPaC.xls (accessed on 20 August 2020).
- Nóbrega, H.; Freitas, G.; Zavattieri, M.A.; Ragonezi, C.; Frese, L.; de Carvalho, M.A.A.P. Monitoring system and in situ conservation of endemic and threatened Beta patula Aiton populations in Madeira Region. Genet. Resour. Crop Evol. 2021, 68, 939–956. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Benjamin Cummings an Imprint of Addison Wesley Longman: Menlo Park, CA, USA, 1999. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science Ltd., Osney Mead: Oxford, UK, 2009; ISBN 0632054778. [Google Scholar]
- Tischler, W. Die synökologischen Grundbegriffe. In Grundzüge der Terrestrischen Tierökologie; Vieweg + Teubner Verlag: Wiesbaden, Germany, 1949. [Google Scholar] [CrossRef]
- Guarino, R.; Willner, W.; Pignatti, S.; Attorre, F.; Loidi, J.J. Spatio-temporal variations in the application of the Braun-Blanquet approach in Europe. Phytocoenologia 2018, 48, 239–250. [Google Scholar] [CrossRef]
- Cruz, M.J.; Aguiar, R.; Correia, A.; Tavares, T.; Pereira, J.S.; Santos, F.D. Impacts of climate change on the terrestrial ecosystems of Madeira. Int. J. Des. Nat. Ecodynamics 2009, 4, 413–422. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, A.P., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; 2391p. [Google Scholar] [CrossRef]
- Netto, J.Á.; Azevedo, P.V.D.; Da Silva, B.B.; Soares, J.M.; De Castro Teixeira, A.H. Exigências Hídricas da Videira na Região do Submedio São Francisco. Pesq. Agropec. Bras. Brasília 2000, 35, 1559–1566. [Google Scholar] [CrossRef]
- Pinto, R.; da Câmara, E.S.; Ferreira, M.A.M. Carta dos Solos da Ilha da Madeira; Secretaria Regional da Economia: Madeira, Portugal, 1992. [Google Scholar]
- ISOPlexis Germplasm Resources Information Network [Internet]. ISOPlexis Centre of Sustainable Agriculture and Food Technology—University of Madeira: Madeira, Portugal. Available online: https://isoplexis.uma.pt/gringlobal/search.aspx (accessed on 13 June 2022).
- Nielsen, M.N.; Winding, A. Microorganisms as Indicators of Soil Health; Technical Report No. 388; National Environmental Research Institute: Roskilde, Denmark, 2002; ISBN 87-7772-658-8. [Google Scholar]
- Filip, Z. International approach to assessing soil quality by ecologically-related biological parameters. Agric. Ecosyst. Environ. 2002, 88, 169–174. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [Green Version]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Lawlor, K.; Knight, B.P.; Barbosa-Jefferson, V.L.; Lane, P.W.; Lilley, A.K.; Paton, G.I.; McGrath, S.P.; O’Flaherty, S.M.; Hirsch, P.R. Comparison of methods to investigate microbial populations in soils under different agricultural management. FEMS Microbiol. Ecol. 2000, 33, 129–137. [Google Scholar] [CrossRef]
- Molina-Guzmán, L.P.; Henao-Jaramillo, P.A.; Gutiérrez-Builes, L.A.; Ríos-Osorio, L.A. Microorganisms in Soils of Bovine Production Systems in Tropical Lowlands and Tropical Highlands in the Department of Antioquia, Colombia. Int. J. Agron. 2018, 2018, 5379047. [Google Scholar] [CrossRef]
- Higashida, S.; Takao, K. Seasonal Fluctuation Patterns of Microbial Numbers in the Surface Soil of a Grassland. Soil Sci. Plant Nutr. 1985, 31, 113–121. [Google Scholar] [CrossRef]
- Luo, X.; Wang, M.K.; Hu, G.; Weng, B. Seasonal Change in Microbial Diversity and Its Relationship with Soil Chemical Properties in an Orchard. PLoS ONE 2019, 14, e0215556. [Google Scholar] [CrossRef] [PubMed]
- Yokobe, T.; Hyodo, F.; Tokuchi, N. Seasonal Effects on Microbial Community Structure and Nitrogen Dynamics in Temperate Forest Soil. Forests 2018, 9, 153. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Lin, W.-X.; Li, J.J.; Liu, J.F. Effects of seasonal variations on soil microbial community composition of two typical zonal vegetation types in the e Wuyi Mountains. J. Mt. Sci. 2016, 13, 1056–1065. [Google Scholar] [CrossRef]
- Zulfarina, Z.; Rusmana, I.; Mubarik, N.R.; Santosa, D.A. The Abundance of Nitrogen Fixing, Nitrifying, Denitrifying and Ammonifying Bacteria in the Soil of Tropical Rainforests and Oil Palm Plantations in Jambi. Makara J. Sci. 2017, 21, 187–194. [Google Scholar] [CrossRef]
- Mergel, A.; Schmitz, O.; Mallmann, T.; Bothe, H. Relative abundance of denitrifying and dinitrogen-fixing bacteria in layers of a forest soil. FEMS Microbiol. Ecol. 2001, 36, 33–42. [Google Scholar] [CrossRef]
- Charles Munch, J.; Velthof, G.L. Denitrification and Agriculture. In Biology of the Nitrogen Cycle; Bothe, H., Ferguson, S.J., Newton, W.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Chapter 21; pp. 331–341. ISBN 978-0-444-52857-5. [Google Scholar]
- Martin, K.; Parsons, L.L.; Murray, R.E.; Smith, M.S. Dynamics of Soil Denitrifier Populations: Relationships between Enzyme Activity, Most-Probable-Number Counts, and Actual N Gas Loss. Appl. Environ. Microbiol. 1988, 54, 2711–2716. [Google Scholar] [CrossRef] [Green Version]
- Rilling, J.I.; Acuña, J.J.; Sadowsky, M.J.; Jorquera, M.A. Putative Nitrogen-Fixing Bacteria Associated With the Rhizosphere and Root Endosphere of Wheat Plants Grown in an Andisol From Southern Chile. Front. Microbiol. 2018, 9, 2710. [Google Scholar] [CrossRef]
- Andrea, M.-M.E.; Carolina, T.-E.A.; Anderson, V.-G.; Laura, R.-G. Relationship between soil physicochemical characteristics and nitrogen-fixing bacteria in agricultural soils of the Atlántico department, Colombia. Soil Environ. 2017, 36, 174–181. [Google Scholar] [CrossRef]
- Braker, G.; Schwarz, J.; Conrad, R. Influence of temperature on the composition and activity of denitrifying soil communities. FEMS Microbiol. Ecol. 2010, 73, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Keil, D.; Niklaus, P.A.; von Riedmatten, L.R.; Boeddinghaus, R.S.; Dormann, C.F.; Scherer-Lorenzen, M.; Kandeler, E.; Marhan, S. Effects of warming and drought on potential N2O emissions and denitrifying bacteria abundance in grasslands with different land-use. FEMS Microbiol. Ecol. 2015, 91, fiv066. [Google Scholar] [CrossRef] [PubMed]
- Corneo, P.E.; Pellegrini, A.; Cappellin, L.; Roncador, M.; Chierici, M.; Gessler, C.; Pertot, I. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. FEMS Microbiol. Ecol. 2013, 84, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Bouamri, R.; Dalpé, Y.; Serrhini, M.M. Effect of seasonal variation on arbuscular mycorrhizal fungi associated with date palm. Emir. J. Food Agric. 2014, 26, 977–986. [Google Scholar] [CrossRef]
- Santos-González, J.C.; Finlay, R.D.; Tehler, A. Seasonal Dynamics of Arbuscular Mycorrhizal Fungal Communities in Roots in a Seminatural Grassland. Appl. Environ. Microbiol. 2007, 73, 5613–5623. [Google Scholar] [CrossRef]
- Gelybó, G.; Tóth, E.; Farkas, C.; Horel, Á.; Kása, I.; Bakacsi, Z. Potential impacts of climate change on soil properties. Agrokem. Talajt. 2018, 67, 121–141. [Google Scholar] [CrossRef]
- Brouder, S.M.; Volenec, J.J. Impact of climate change on crop nutrient and water use efficiencies. Physiol. Plant. 2008, 133, 705–724. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Hirao, T. Seasonal Dynamics of Soil Fungal and Bacterial Communities in Cool-Temperate Montane Forests. Front. Microbiol. 2019, 10, 1944. [Google Scholar] [CrossRef] [Green Version]
- Mendes, W.D.C.; Alves, J.; da Cunha, P.C.R.; da Silva, A.R.; Evangelista, A.W.P.; Casaroli, D. Potassium leaching in different soils as a function of irrigation depths. Rev. Bras. Eng. Agric. Ambient. 2016, 20, 972–977. [Google Scholar] [CrossRef]
- Lu, D.; Dong, Y.; Chen, X.; Wang, H.; Zhou, J. Comparison of potential potassium leaching associated with organic and inorganic potassium sources in different arable soils in China. Pedosphere 2022, 32, 330–338. [Google Scholar] [CrossRef]
- Smith, W.G. Raunkiaer’s “Life-Forms” and Statistical Methods. J. Ecol. 1913, 1, 16–26. [Google Scholar] [CrossRef]
- Gillison, A.N. Plant functional indicators of vegetation response to climate change, past present and future: I. Trends, emerging hypotheses and plant functional modality. Flora Morphol. Distrib. Funct. Ecol. Plants 2019, 254, 12–30. [Google Scholar] [CrossRef]
- Irl, S.D.H.; Obermeier, A.; Beierkuhnlein, C.; Steinbauer, M.J. Climate controls plant life-form patterns on a high-elevation oceanic island. J. Biogeogr. 2020, 47, 2261–2273. [Google Scholar] [CrossRef]
- Gillison, A.N. Plant functional indicators of vegetation response to climate change, past present and future: II. Modal plant functional types as response indicators for present and future climates. Flora Morphol. Distrib. Funct. Ecol. Plants 2019, 254, 31–58. [Google Scholar] [CrossRef]
- Fand, B.B.; Kamble, A.L.; Kumar, M. Will climate change pose serious threat to crop pest management: A critical review? Int. J. Sci. Res. Publ. 2012, 2, 1–14. [Google Scholar]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Yamamura, K.; Kiritani, K. A simple method to estimate the potential increase in the number of generations under global warming in temperate zones. Appl. Entomol. Zool. 1998, 33, 289–298. [Google Scholar] [CrossRef]
- Savopoulou-Soultani, M.; Papadopoulos, N.T.; Milonas, P.; Moyal, P. Abiotic factors and insect abundance. Psyche 2012, 2012, 167420. [Google Scholar] [CrossRef]
- Mellanby, K. Low Temperature and Insect Activity. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1939, 127, 473–487. [Google Scholar]
- Kocmánková, E.; Trnka, M.; Juroch, J.; Dubrovský, M.; Semerádová, D.; Možný, M.; Žalud, Z. Impact of climate change on the occurrence and activity of harmful organisms. Plant Prot. Sci. 2009, 45, S48–S52. [Google Scholar] [CrossRef] [Green Version]
- McColloch, J.W.; Hayes, W.P. The Reciprocal Relation of Soil and Insects. Ecol. Soc. Am. 2020, 3, 288–301. [Google Scholar] [CrossRef]
- Lepage, M.P.; Bourgeois, G.; Brodeur, J.; Boivin, G. Effect of soil temperature and moisture on survival of eggs and first-instar larvae of Delia radicum. Environ. Entomol. 2012, 41, 159–165. [Google Scholar] [CrossRef]
- Pimentel, R.; Mexia, A.M.M.; Mumford, J.; Lopes, D.H. A mosca-do-Mediterrâneo (Ceratitis capitata Wiedemann) (Diptera: Tephritidae) na ilha Terceira—Monitorização das populações e infestação dos frutos. In A mosca-do-Mediterrâneo nas ilhas Terceira e de S. Jorge; Pimentel, R., Lopes, D.H., Cabrera Perez, R., Dantas, L., Eds.; Grupo da Biodiversidade dos Açores, Universidade dos Açores: Angra do Heroísmo, Portugal, 2016; pp. 6–13. [Google Scholar]
- Díaz-Fleischer, F.; Piñero, J.C.; Shelly, T.E. Interactions between tephritid fruit fly physiological state and stimuli from baits and traps: Looking for the Pied Piper of Hamelin to lure pestiferous fruit flies. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Shelly, T., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 145–172. ISBN 978-94-017-9192-2. [Google Scholar]
- Pimentel, R.M.S. Contributo Para o Conhecimento da Mosca-do- Mediterrâneo (Ceratitis capitata Wiedemann) (Diptera: Tephritidae) na Ilha Terceira. Master’s Thesis, Departamento de Ciências Agrárias, Universidade dos Açores, Angra do Heroísmo, Portugal, 2010. Available online: http://hdl.handle.net/10400.3/1154 (accessed on 15 June 2022).
- Nolasco, N.; Iannacone, J. Fluctuación estacional de moscas de la fruta Anastrepha spp. y Ceratitis capitata (Wiedemann, 1824) (Diptera: Tephritidae) en trampas Mcphail en Piura y en Ica, Perú. Acta Zoológica Mex. 2008, 24, 33–44. [Google Scholar] [CrossRef]
- Habibe, T.C.; Viana, R.E.; Nascimento, A.S.; Paranhos, B.A.J.; Haji, F.N.P.; Carvalho, R.S.; Damasceno, Í.C.; Malavasi, A. Infestation of Grape Vitis vinifera by Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) in Sub-Medium Sao Francisco Valley, Brazil. In Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance, Salvador, Brazil, 10–15 September 2006; pp. 183–185. [Google Scholar]
- Gómez, M.; Paranhos, B.A.J.; Silva, J.G.; De Lima, M.A.C.; Silva, M.A.; Macedo, A.T.; Virginio, J.F.; Walder, J.M.M. Oviposition preference of Ceratitis capitata (Diptera: Tephritidae) at different times after pruning “Italia” table grapes grown in Brazil. J. Insect Sci. 2019, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.; Montoya, P.; Ruiz-Montoya, L.; Villaseñor, A.; Valle, A.; Enkerlin, W.; Liedo, P. Population Fluctuation of Ceratitis capitata (Diptera: Tephritidae) as a Function of Altitude in Eastern Guatemala. Environ. Entomol. 2016, 45, 802–811. [Google Scholar] [CrossRef]
- De Meyer, M.; Copeland, R.S.; Wharton, R.A.; McPheron, B.A. On the geographic origin of the Medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). In Proceedings of the 6th International Fruit Fly Symposium, Stellenbosch, South Africa, 6–10 May 2002; pp. 45–53. [Google Scholar]
- Bento, F.D.M.M.; Marques, R.N.; Costa, M.L.Z.; Walder, J.M.M.; Silva, A.P.; Parra, J.R.P. Pupal development of Ceratitis capitata (Diptera: Tephritidae) and Diachasmimorpha longicaudata (Hymenoptera: Braconidae) at different moisture values in four soil types. Environ. Entomol. 2010, 39, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.N.; Zhang, X.; Crawford, J.W.; Gregory, P.J.; Young, I.M. Egg hatching and survival time of soil-dwelling insect larvae: A partial differential equation model and experimental validation. Ecol. Modell. 2007, 202, 493–502. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Valverde-García, P.; Garrido-Jurado, I. The effect of temperature and soil moisture on the development of the preimaginal mediterranean fruit fly (Diptera: Tephritidae). Environ. Entomol. 2012, 41, 966–970. [Google Scholar] [CrossRef]
- Rohde, C.; Moino, A.; Da Silva, M.A.T.; Carvalho, F.D.; Ferreira, C.S. Influence of soil temperature and moisture on the infectivity of entomopathogenic nematodes (Rhabditida: Heterorhabditidae, Steinernematidae) against Larvae of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Neotrop. Entomol. 2010, 39, 608–611. [Google Scholar] [CrossRef]
- Fraga, H.; García de Cortázar Atauri, I.; Santos, J.A. Viticultural irrigation demands under climate change scenarios in Portugal. Agric. Water Manag. 2018, 196, 66–74. [Google Scholar] [CrossRef]
- Goldammer, T. Grape Grower’s Handbook: A Guide to Viticulture for Wine Production; Apex Publishers: Centreville, Virginia, 2018; ISBN 9780967521275. [Google Scholar]
- Bai, X.; Zhao, W.; Wang, J.; Ferreira, C.S.S. Precipitation drives the floristic composition and diversity of temperate grasslands in China. Glob. Ecol. Conserv. 2021, 32, e01933. [Google Scholar] [CrossRef]
- Trejo, I.; Dirzo, R. Floristic diversity of Mexican seasonally dry tropical forests. Biodivers. Conserv. 2002, 11, 2063–2084. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, R.; Jiang, Y.; Li, C. Contributions of climate change and human activities to vegetation dynamics in Qilian Mountain National Park, northwest China. Glob. Ecol. Conserv. 2021, 32, e01947. [Google Scholar] [CrossRef]
- Pyke, C.R.; Condit, R.; Aguilar, S.; Lao, S. Floristic composition across a climatic gradient in a neotropical lowland forest. J. Veg. Sci. 2001, 12, 553–566. [Google Scholar] [CrossRef]








| Stations | Owner | Latitude (DD) | Longitude (DD) | Altitude (m) |
|---|---|---|---|---|
| Funchal/Observatório | IPMA | 32.65 | −16.89 | 58 |
| Sanatório do Monte | IPMA | 32.65 | −16.90 | 380 |
| Santa Catarina/Airport | IPMA | 32.69 | −16.77 | 58 |
| Lugar de Baixo/P. do Sol | IPMA | 32.68 | −17.09 | 40 |
| Camacha | IPMA | 32.66 | −16.83 | 680 |
| Bom Sucesso | IPMA | 32.65 | −16.90 | 290 |
| Chão do Areeiro | IPMA | 32.72 | −16.92 | 1590 |
| Ponta Delgada | IPMA | 32.75 | −16.71 | 133 |
| Santana | IPMA | 32.81 | −16.89 | 380 |
| Bica da Cana | IPMA | 32.76 | −17.06 | 1560 |
| Santo da Serra | IPMA | 32.73 | −16.82 | 660 |
| Quinta das Vinhas | ISOPlexis | 32.73 | −17.19 | 337 |
| Month | Temperature Increases in ΔA2 (°C) | Temperature Increases in ΔB2 (°C) | Precipitation Decreases in ΔA2 (%) | Precipitation Decreases in ΔB2 (%) |
|---|---|---|---|---|
| January | 2.4 | 1.5 | −34 | −40 |
| February | 2.8 | 1.8 | −0.5 | −34 |
| March | 2.5 | 1.6 | −33 | −32 |
| April | 2.7 | 1.6 | −39 | −30 |
| May | 2.8 | 1.7 | −61 | −48 |
| June | 2.7 | 1.8 | 9 | 23 |
| July | 2.6 | 1.7 | 92 | 33 |
| August | 2.3 | 1.5 | 94 | −34 |
| September | 2.3 | 1.6 | −33 | −37 |
| October | 2.3 | 1.4 | −56 | −25 |
| November | 2.5 | 1.5 | −53 | −40 |
| December | 2.3 | 1.2 | −34 | −30 |
| Year | Season | Bacteria (CFU/g) | Fungi (CFU/g) | Nitrogen-Fixing Bacteria (CFU/g) | Denitrifying Bacteria (MPN/g) |
|---|---|---|---|---|---|
| 2018 | Winter | 1.19 × 107 | 1.60 × 107 | 1.35 × 107 | 5.09 × 103 |
| Spring | 1.31 × 107 | 1.18 × 107 | 1.31 × 107 | 4.06 × 103 | |
| Summer | 7.60 × 106 | 9.22 × 106 | 1.38 × 107 | 5.62 × 103 | |
| Autumn | 1.74 × 107 | 1.29 × 107 | 2.35 × 107 | 1.27 × 103 | |
| 2019 | Winter | 1.37 × 107 | 1.19 × 107 | 1.97 × 107 | 4.23 × 103 |
| Spring | 2.77 × 107 | 2.31 × 107 | 2.61 × 107 | 1.08 × 103 | |
| Summer | 1.19 × 107 | 7.77 × 106 | 1.03 × 107 | 5.34 × 103 | |
| Autumn | 1.30 × 107 | 1.07 × 107 | 1.13 × 107 | 4.33 × 103 |
| H′ | E′ | D | |||||
|---|---|---|---|---|---|---|---|
| Season | Enz 1 | Enz 2 | Enz 1 | Enz 2 | Enz 1 | Enz 2 | |
| Bacteria | Winter | 4.56 ± 0.64 a | 4.61 ± 0.54 | 0.77 ± 0.07 | 0.76 ± 0.05 | 0.07 ± 0.03 b | 0.06 ± 0.02 |
| Spring | 5.63 ± 0.27 b | 4.97 ± 0.10 | 0.84 ± 0.04 | 0.77 ± 0.02 | 0.03 ± 0.01 a | 0.04 ± 0.00 | |
| Summer | 5.39 ± 0.38 a,b | 4.72 ± 0.09 | 0.81 ± 0.03 | 0.74 ± 0.02 | 0.03 ± 0.01 a,b | 0.06 ± 0.01 | |
| Autumn | 5.58 ± 0.27 b | 4.90 ± 0.11 | 0.82 ± 0.03 | 0.76 ± 0.02 | 0.02 ± 0.01 a | 0.05 ± 0.01 | |
| Fungi | Winter | 4.69 ± 0.92 | 4.66 ± 0.48 a | 0.47 ± 0.29 a | 0.62 ± 0.17 a | 0.07 ± 0.04 | 0.06 ± 0.02 b |
| Spring | 5.81 ± 0.33 | 5.50 ± 0.26 b | 0.79 ± 0.06 a,b | 0.80 ± 0.04 b | 0.03 ± 0.01 | 0.03 ± 0.01 a | |
| Summer | 4.56 ± 1.94 | 5.29 ± 0.09 a,b | 0.60 ± 0.26 a,b | 0.74 ± 0.10 a,b | 0.13 ± 0.23 | 0.04 ± 0.01 a,b | |
| Autumn | 5.65 ± 0.42 | 5.36 ± 0.23 b | 0.81 ± 0.06 b | 0.81 ± 0.03 b | 0.03 ± 0.01 | 0.04 ± 0.01 a,b | |
| AMF | Winter | 3.55 ± 1.2 | 3.56 ± 0.9 | 0.55 ± 0.24 | 0.48 ± 0.20 | 0.2 ± 0.15 | 0.20 ± 0.11 |
| Spring | 3.97 ± 0.93 | 3.88 ± 0.25 | 0.58 ± 0.17 | 0.52 ± 0.15 | 0.14 ± 0.08 | 0.12 ± 0.02 | |
| Summer | 3.35 ± 0.67 | 3.85 ± 0.63 | 0.48 ± 0.13 | 0.58 ± 0.12 | 0.22 ± 0.09 | 0.14 ± 0.05 | |
| Autumn | 4.08 ± 0.54 | 3.95 ± 0.46 | 0.61 ± 0.12 | 0.61 ± 0.15 | 0.13 ± 0.04 | 0.13 ± 0.06 | |
| Archaea | Winter | 4.39 ± 0.50 | 4.87 ± 1.14 | 0.77 ± 0.16 | 0.67 ± 0.10 | 0.09 ± 0.05 | 0.07 ± 0.05 |
| Spring | 4.94 ± 0.56 | 5.29 ± 0.57 | 0.80 ± 0.07 | 0.75 ± 0.11 | 0.06 ± 0.04 | 0.04 ± 0.02 | |
| Summer | 4.34 ± 0.63 | 5.34 ± 0.52 | 0.81 ± 0.10 | 0.66 ± 0.12 | 0.11 ± 0.07 | 0.04 ± 0.03 | |
| Autumn | 5.15 ± 0.72 | 5.35 ± 0.81 | 0.80 ± 0.13 | 0.77 ± 0.10 | 0.05 ± 0.03 | 0.05 ± 0.04 | |
| Year | Season | Total Species | Total Individuals | Therophyte (Th) | Hemicryptophyte (H) | Geophyte (G) | Remaining Classes (R) |
|---|---|---|---|---|---|---|---|
| 2018 | Spring | 53 | 605 | 42 | 7 | 3 | 1 |
| Summer | 20 | 27 | 11 | 4 | 2 | 3 | |
| Autumn | 48 | 425 | 33 | 6 | 7 | 2 | |
| Winter | 68 | 713 | 44 | 11 | 7 | 6 | |
| 2019 | Spring | 43 | 164 | 22 | 10 | 6 | 5 |
| Summer | 56 | 289 | 29 | 13 | 6 | 8 | |
| Autumn | 66 | 1004 | 36 | 10 | 9 | 11 | |
| Winter | 55 | 238 | 35 | 9 | 5 | 6 |
| Species | Family | Status | Life Form |
|---|---|---|---|
| Bidens pilosa L. | Compositae | Introduced | Therophyte |
| Sonchus oleraceus L. | Compositae | Native probable | Therophyte |
| Conyza sumatrensis (Retz.) E. Walker | Compositae | Introduced | Therophyte |
| Convolvulus arvensis L. | Convolvulaceae | Native | Geophyte |
| Fumaria muralis W.D.J.Koch | Papaveraceae | Native | Therophyte |
| Malva parviflora L. | Malvaceae | Native | Therophyte |
| Solanum nigrum L. | Solanaceae | Native probable | Therophyte |
| Helminthotheca echioides (L.) Holub | Compositae | Native probable | Therophyte |
| Lactuca serriola L. | Compositae | Intr. Probable | Hemicryptophyte |
| Avena barbata Link | Poaceae | Native probable | Therophyte |
| Calendula arvensis L. | Compositae | Native | Hemicryptophyte |
| Stachys arvensis L. | Lamiaceae | Native | Therophyte |
| Allium neapolitanum Cirillo | Amaryllidaceae | Intr. Probable | Geophyte |
| Erodium moschatum (L.) L’Hér. | Geraniaceae | Native | Therophyte |
| Bromus catharticus Vahl | Poaceae | Introduced | Hemicryptophyte |
| Polycarpon tetraphyllum L. | Caryophyllaceae | Native | Therophyte |
| Medicago polymorpha L. | Fabaceae | Native | Therophyte |
| Ipomoea indica (Burm.) Merr. | Convolvulaceae | Introduced | Geophyte |
| Bituminaria bituminosa (L.) C.H.Stirt. | Fabaceae | Native | Hemicryptophyte |
| Senecio vulgaris L. | Compositae | Native probable | Therophyte |
| Year | Season | S | N | H′ | E′ | N2 |
|---|---|---|---|---|---|---|
| 2018 | Winter | 6.17 ± 2.03 b | 118.83 ± 64.62 b | 1.62 ± 0.43 a,b | 0.56 ± 0.11 b | 2.51 ± 0.60 a,b |
| Spring | 8.33 ± 3.20 b | 100.83 ± 72.29 b | 2.13 ± 0.79 b | 0.58 ± 0.28 b | 3.96 ± 1.90 b | |
| Summer | 1.80 ± 0.40 a | 5.40 ± 3.50 a | 0.66 ± 0.38 a | 0.00 ± 0.00 a | 1.56 ± 0.39 a | |
| Autumn | 8.83 ± 2.11 b | 71.17 ± 28.61 a,b | 2.03 ± 0.47 b | 0.67 ± 0.08 b | 3.43 ± 0.94 a,b | |
| 2019 | Winter | 5.83 ± 3.31 a,b | 39.67 ± 19.54 a | 1.54 ± 1.16 | 0.53 ± 0.30 | 2.98 ± 1.82 |
| Spring | 3.33 ± 1.51 a | 27.33 ± 12.04 a | 1.09 ± 0.70 | 0.49 ± 0.29 | 2.01 ± 0.94 | |
| Summer | 4.67 ± 1.97 a | 48.17 ± 43.23 a | 1.51 ± 0.79 | 0.55 ± 0.21 | 2.74 ± 1.46 | |
| Autumn | 9.33 ± 3.01 b | 167.33 ± 72.07 b | 2.38 ± 0.78 | 0.70 ± 0.19 | 4.67 ± 2.45 |
| Insect Diversity | |||
|---|---|---|---|
| Year | Season | Total Weight (G) | Total N° of Specimens |
| 2018 | Winter | No data | No data |
| Spring | 0.26 | 123 | |
| Summer | 1.87 | 818 | |
| Autumn | 2.67 | 1123 | |
| 2019 | Winter | 0.06 | 25 |
| Spring | 0.18 | 83 | |
| Summer | 0.68 | 306 | |
| Autumn | 1.47 | 640 | |
| Ceratitis capitata | |||||
|---|---|---|---|---|---|
| Year | Season | Total N° of Specimens | Males | Females | Total Weight (g) |
| 2018 | Winter | No data | No data | No data | No data |
| Spring | 182 | 67 | 115 | 2.22 | |
| Summer | 351 | 175 | 176 | 5.06 | |
| Autumn | 982 | 475 | 507 | 13.35 | |
| 2019 | Winter | 8 | 3 | 5 | 0.10 |
| Spring | 330 | 110 | 220 | 4.51 | |
| Summer | 1177 | 507 | 670 | 17.08 | |
| Autumn | 408 | 105 | 303 | 5.90 | |
| Year | Average Temperature (°C) | Maximum Temperature (°C) | Minimum Temperature (°C) | Relative Humidity (%) | Precipitation (mm) | Thermic Days (°C) |
|---|---|---|---|---|---|---|
| 2018 | 19.6 | 22.6 | 16.6 | 72.5 | 74.7 | 2966.3 |
| 2019 | 20.3 | 23.5 | 17.0 | 73.2 | 67.5 | 3025.6 |
| Year | PC Length (days) | Water Deficit (mm) | Malvasia (kg·h−1) | Sercial (kg·h−1) | Terrantez (kg·h−1) | Verdelho (kg·h−1) | Bastardo (kg·h−1) | Syrah (kg·h−1) |
|---|---|---|---|---|---|---|---|---|
| 2018 | 159.0 | 625.4 | 1634.20 | 3989.43 | 1847.52 | 934.47 | 3205.51 | 10,204.08 |
| 2019 | 146.0 | 632.5 | 2695.35 | 4346.10 | 3320.86 | 641.30 | 7817.11 | 16,326.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro de Carvalho, M.Â.A.; Ragonezi, C.; Oliveira, M.C.O.; Reis, F.; Macedo, F.L.; de Freitas, J.G.R.; Nóbrega, H.; Ganança, J.F.T. Anticipating the Climate Change Impacts on Madeira’s Agriculture: The Characterization and Monitoring of a Vine Agrosystem. Agronomy 2022, 12, 2201. https://doi.org/10.3390/agronomy12092201
Pinheiro de Carvalho MÂA, Ragonezi C, Oliveira MCO, Reis F, Macedo FL, de Freitas JGR, Nóbrega H, Ganança JFT. Anticipating the Climate Change Impacts on Madeira’s Agriculture: The Characterization and Monitoring of a Vine Agrosystem. Agronomy. 2022; 12(9):2201. https://doi.org/10.3390/agronomy12092201
Chicago/Turabian StylePinheiro de Carvalho, Miguel Â. A., Carla Ragonezi, Maria Cristina O. Oliveira, Fábio Reis, Fabrício Lopes Macedo, José G. R. de Freitas, Humberto Nóbrega, and José Filipe T. Ganança. 2022. "Anticipating the Climate Change Impacts on Madeira’s Agriculture: The Characterization and Monitoring of a Vine Agrosystem" Agronomy 12, no. 9: 2201. https://doi.org/10.3390/agronomy12092201