Identification of Phytochemical Compounds, Functional Properties and Antioxidant Activity of Germinated Purple Corn Protein Concentrate and Its Gastrointestinal Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material
2.3. Preparation of Germinated Purple Corn Protein Concentrate (PCPC)
2.4. Gastrointestinal Hydrolysis of PCPC25 and PCPC35
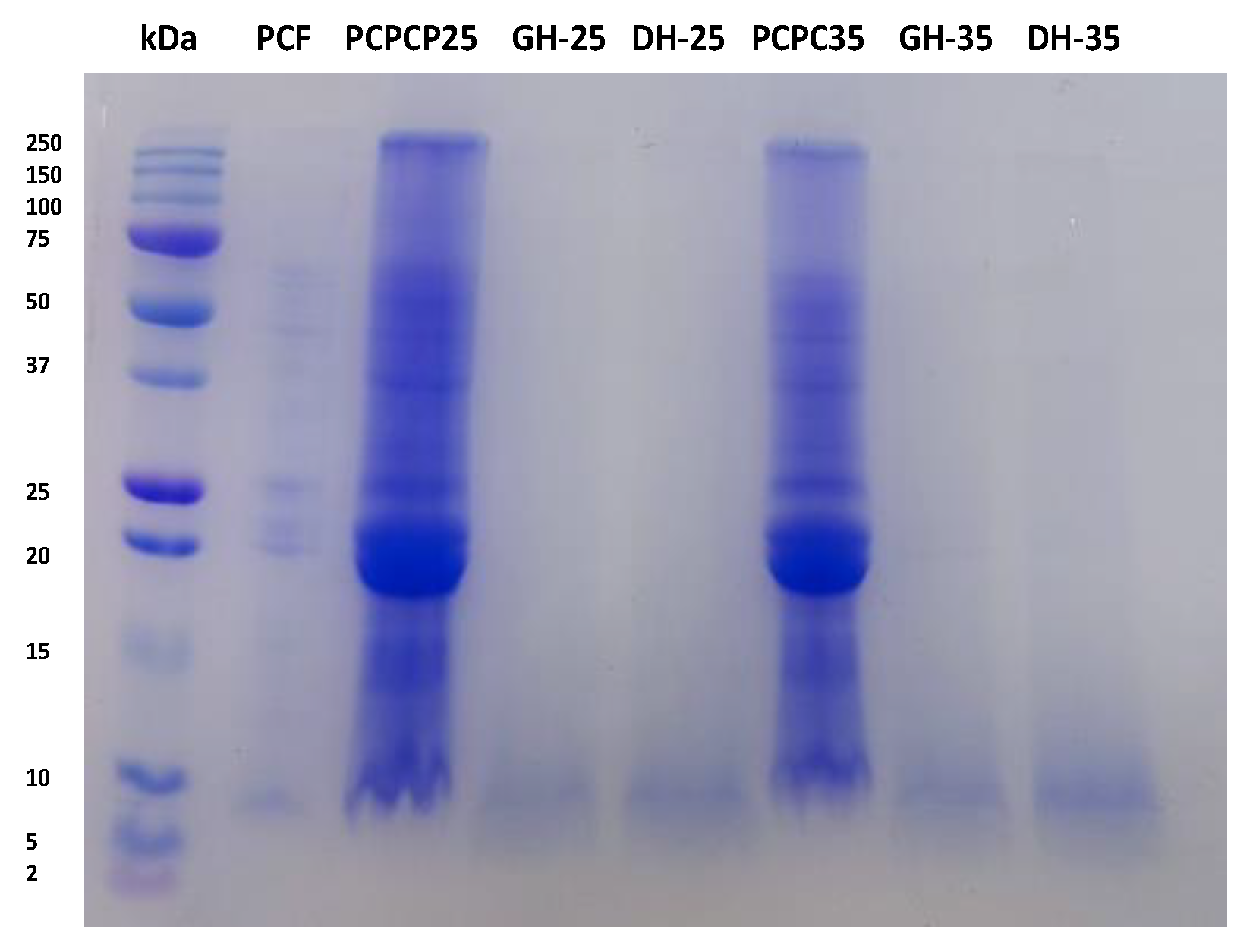
2.5. Analysis of the Protein Profile of PCPCs and Hydrolysates (GH25, DH25, GH35 and DH35) Using the Electrophoresis Technique
2.6. Elemental Composition of PCPC25, PCPC35 and Hydrolysates (GH25, DH25, GH35 and DH35) and Quantification of Protein Content
2.7. Total Anthocyanin Content (TAC)
2.8. Total Proanthocyanin Content (TPAC)
2.9. Total Flavonoids Content (TFC)
2.10. Total Flavonol Content (TFLC)
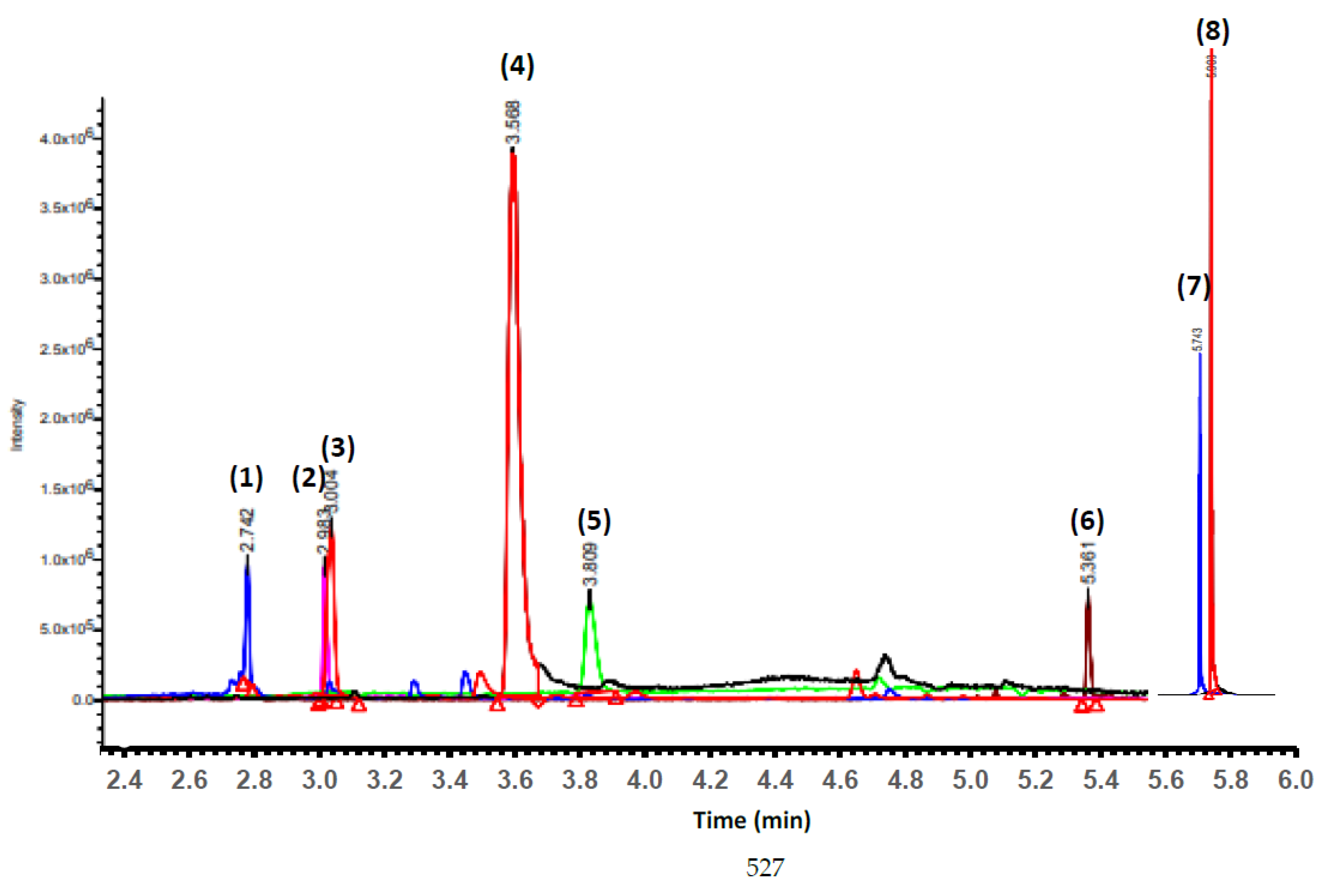
2.11. Analysis of Biocompounds of PCPC and Hydrolysates (GH25, DH25, GH35 and DH35) by Reversed-Phase Ultra-Performance Liquid Chromatography–Mass Spectrometry (RP-UPLC-MS) Technique
2.12. Functional Properties of PCPCs and Gastrointestinal Hydrolysates
2.12.1. Protein Solubility (PS)
2.12.2. Oil Absorption Capacity (OAC)
2.12.3. Water Absorption Capacity (WAC)
2.13. Evaluation of Antioxidant Activity of PCPC and Hydrolysates (GH25, DH25, GH35 and DH35)
2.13.1. Ferric-Reducing Antioxidant Power (FRAP) Method
2.13.2. 2.2′-Azinobis (3-Ethyl-benzothiazoline-6-sulfonic acid) Cation Bleaching ABTS Method
2.13.3. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.14. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Profile Protein of PCPC and Hydrolysates (GH25, DH25, GH35 and DH35)
3.2. Elemental Composition of PCPCs and Hydrolysates and Quantification of % Hydrolysis of Hydrolysates (GH25, DH25, GH35 and DH35)
3.3. Content of Phenol Compounds of PCPCs and Gastrointestinal Hydrolysates (GH25, DH25, GH35 and DH35)
3.4. Identification of Phenolic Acid and Flavonol Compounds of PCPC and hydrolysates
3.5. Evaluation of Functional Properties
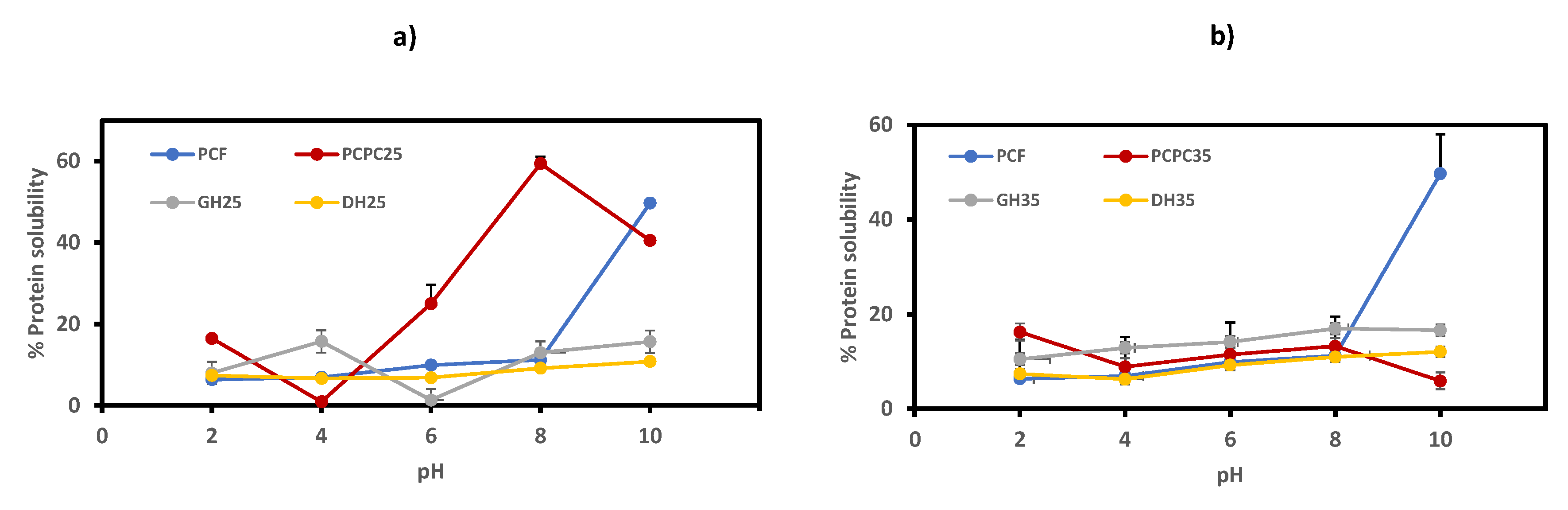
3.5.1. Protein Solubility (PS) of Purple Protein Corn Concentrate (PCPC) and Hydrolysates (GH25, DH25, GH35 and DH35)
3.5.2. Quantification of %WAC and %OAC of PCPC25, PCPC35 and Hydrolysates (GH25, DH25, GH35 and DH35)
3.6. Antioxidant Activity of PCPC and Gastrointestinal Hydrolysates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salvador-Reyes, R.; Clerici, M.T.P.S. Peruvian Andean maize: General characteristics, nutritional properties, bioactive compounds, and culinary uses. Food Res. Int. 2020, 130, 108934. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Chenlo, F.; Arufe, S.; Rubinos, S.N. Physicochemical characterization of white, yellow and purple maize flours and rheological characterization of their doughs. J. Food Sci. Technol. 2015, 52, 7954–7963. [Google Scholar] [CrossRef]
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize-A potential source of human nutrition and health: A review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization Corporate Statistical Database. 2022. Available online: http://faostat.fao.org (accessed on 20 May 2022).
- Lao, F.; Giusti, M.M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- FAO. Traditional High Andean Cuisine. 2013. Available online: http://www.fao.org/docrep/018/i1466e/i1466e.pdf (accessed on 21 May 2022).
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tools in pigmented maize. LWT Food Sci. Technol. 2021, 144, 111257. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.-J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic compounds and biological rhythms: Who takes the lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.S. Amino acid composition of an organic brown rice protein concentrate and isolate compared to soy and whey concentrates and isolates. Foods 2014, 3, 394–402. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. Effects of high hydrostatic pressure on the quality and functionality of protein isolates, concentrates, and hydrolysates derived from pulse legumes: A review. Trends Food Sci. Technol. 2021, 107, 466–479. [Google Scholar] [CrossRef]
- Jia, W.; Rodriguez-Alonso, E.; Bianeis, M.; Keppler, J.K.; Van der Goot, A.J. Assessing functional properties of rapeseed protein concentrate versus isolate for food applications. Innov. Food Sci. Emerg. Technol. 2021, 68, 102636. [Google Scholar] [CrossRef]
- Morr, C.V.; Ha, E.Y.W. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nut. 1993, 33, 431–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Johnson, L.A.; Wang, T. Preparation of soy protein concentrate and isolate from extruded-expelled soybean meals. J. Ame. Oil Chem. Soc. 2004, 81, 713–717. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhan, S.K.; Shinde, G.; Subramanian, V.; Nadanasabapathi, S. Whey Proteins: A potential ingredient for food industry—A review. Asian J. Dairy Food Res. 2018, 37, 283–290. [Google Scholar]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Malav, O.P.; Talukder, S.; Gokulakrishnan, P.; Chand, S. Meat analog: A review. Crit. Rev. Food Sci. Nut. 2015, 55, 1241–1245. [Google Scholar] [CrossRef]
- O′ Flynn, T.D.; Hogan, S.A.; Daly, D.F.; O′ Mahony, J.A.; McCarthy, N.A. Rheological and solubility properties of soy protein isolate. Molecules 2021, 26, 3015. [Google Scholar] [CrossRef]
- Momen, S.; Alavi, F.; Aider, M. Alkali-mediated treatments for extraction and functional modification of proteins: Critical and application review. Trends Food Sci. Technol. 2021, 110, 778–797. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocol. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Wu, W.; Wang, L.; Qin, F.; Xie, W. Effect of extraction pH on functional properties, structural properties, and in vitro gastrointestinal digestion of tartary buckwheat protein isolates. J. Cereal Sci. 2021, 101, 103314. [Google Scholar] [CrossRef]
- Khalesi, M.; FitzGerald, R.J. In vitro digestibility and antioxidant activity of plant protein isolate and milk protein concentrate Blends. Catalysts 2021, 11, 787. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef]
- Aimutis, W.R. Plant-based proteins: The good, bad, and ugly. Ann. Rev. Food Sci. Technol. 2022, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, E.; García, A.; Vilcacundo, M.; Morán, R.; Samaniego, I.; Carrillo, W. Antioxidant purple corn protein concentrate from germinated Andean purple corn seeds. Agronomy 2020, 10, 1282. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Aboubacar, A.; Axtell, J.D.; Huang, C.P.; Hamaker, B.R. A rapid protein digestibility assay for identifying highly digestible sorghum lines. Cereal Chem. 2001, 78, 160–165. [Google Scholar] [CrossRef]
- Quinteros, M.F.; Martínez, J.; Barrionuevo, A.; Rojas, M.; Carrillo, W. Functional, antioxidant, and anti-Inflammatory properties of cricket protein concentrate (Gryllus assimilis). Biology 2022, 11, 776. [Google Scholar] [CrossRef]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of analytical methods for determining anthocyanins in blood orange juices. J. Agric. Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Content of proanthocyanidins in selected plant extracts as determined via n-butanol/HCl hydrolysis and a colorimetric assay or by HPLC-a short report. Polish J. Food Nutr. Sci. 2006, 15, 319. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, A.; Yáñez, J.A. Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Racine, K.C.; Lee, A.H.; Wiersema, B.D.; Huang, H.; Lambert, J.D.; Stewart, A.C.; Neilson, A.P. Development and characterization of a pilot-scale model cocoa fermentation system suitable for studying the impact of fermentation on putative bioactive compounds and bioactivity of cocoa. Foods 2019, 8, 102. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Screening of phenolic compounds in Australian grown berries by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Antioxidants 2020, 10, 26. [Google Scholar] [CrossRef]
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Bario, D.A.; Carrillo, W. Argentine Patagonia barberry chemical composition and evaluation of its antioxidant capacity. J. Food Biochem. 2020, 44, e13254. [Google Scholar] [CrossRef]
- Samaniego, I.; Brito, B.; Viera, W.; Cabrera, A.; Llerena, W.; Kannangara, T.; Vilcacundo, R.; Angós, I.; Carrillo, W. Influence of the maturity stage on the phytochemical composition and the antioxidant activity of four Andean blackberry cultivars (Rubus glaucus Benth) from Ecuador. Plants 2020, 9, 1027. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Castorena-Torres, F.; Preciado-Ortiz, R.E.; García-Lara, S. Anti-Cancer Activity of Maize Bioactive Peptides. Front. Chem. 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, C.; Qu, M.; Zhang, J. Kernel Amino acid composition and protein content of introgression lines from Zea mays Ssp. Mexicana into cultivated maize. J. Cereal Sci. 2008, 48, 387–393. [Google Scholar] [CrossRef]
- Wu, Y.; Messing, J. Proteome balancing of the maize seed for higher nutritional value. Front. Plant Sci. 2014, 5, 240. [Google Scholar] [CrossRef]
- Esen, A. A proposed nomenclature for the alcohol-soluble proteins (zeins) of maize (Zea mays L.). J. Cereal Sci. 1987, 5, 117–128. [Google Scholar] [CrossRef]
- Coleman, C.E.; Larkins, B.A. The prolamins of maize. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 109–139. [Google Scholar]
- Wu, Y.; Yin, Z.; Qie, X.; Chen, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Interaction of soy protein isolate hydrolysates with cyanidin-3-O-glucoside and its effect on the in vitro antioxidant capacity of the complexes under neutral condition. Molecules 2021, 26, 1721. [Google Scholar] [CrossRef] [PubMed]
- Vivas, N.E.; Waniska, R.D.; Rooney, L.W. Effect of tortilla production on proteins in sorghum and maize. Cereal Chem. 1987, 64, 384–389. [Google Scholar]
- Kiers, J.L.; Nout, R.M.J.; Rombouts, F.M. In vitro digestibility of processed and fermented soya bean, cowpea and maize. J. Sci. Food Agric. 2000, 80, 1325–1331. [Google Scholar] [CrossRef]
- Galvez Ranilla, L.; Christopher, A.; Sarkar, D.; Shetty, K.; Chirinos, R.; Campos, D. Phenolic composition and evaluation of the antimicrobial activity of free and bound phenolic fractions from a Peruvian purple corn (Zea mays L.) accession. J. Food Sci. 2017, 82, 2968–2976. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, C.G.; Mendoza-Castillo, M.; Delgado-Alvarado, A.; Sánchez-Ramírez, F.J.; Kato-Yamakake, T. Anthocyanins content in the kernel and corncob of Mexican purple corn populations. Maydica 2020, 65, 1–10. [Google Scholar]
- Zhang, Q.; de Mejia, E.G.; Luna-Vital, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750. [Google Scholar]
- Cuevas Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Aprodu, I.; Milea, Ș.A.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Thermal degradation kinetics of anthocyanins extracted from purple maize flour extract and the effect of heating on selected biological functionality. Foods 2020, 9, 1593. [Google Scholar]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Optimisation of extraction procedure and development of LC–DAD–MS methodology for anthocyanin analysis in anthocyanin-pigmented corn kernels. Food Chem. 2020, 319, 126515. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Chen, B.H.; Stephen Inbaraj, B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Pedreschi, R.; Cisneros-Zevallos, L. Phenolic profiles of Andean purple corn (Zea mays L.). Food Chem. 2007, 100, 956–963. [Google Scholar] [CrossRef]
- Somavat, P.; Li, Q.; De Mejia, E.G.; Liu, W.; Singh, V. Coproduct yield comparisons of purple, blue and yellow dent corn for various milling processes. Ind. Crops Prod. 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabrala, F.A. Extraction of bioactive compounds from cob and pericarp of purple corn (Zea mays L.) by sequential extraction in fixed bed extractor using supercritical CO2, ethanol, and water as solvents. J. Superc. Fluids 2016, 107, 250–259. [Google Scholar] [CrossRef]
- Magaña-Cerino, J.M.; Peniche-Pavía, H.A.; Tiessen, A.; Gurrola-Díaz, C.M. Pigmented maize (Zea mays L.) contains anthocyanins with potential therapeutic action against oxidative stress—A Review. Polish J. Food Nutr. Sci. 2020, 70, 85–99. [Google Scholar] [CrossRef]
- Piyapanrungrueang, W.; Chantrapornchai, W.; Haruthaithanasan, V.; Sukatta, U.; Aekatasanawan, C. Comparison of anthocyanin extraction methods from high anthocyanin purple corn cob hybrid: KPSC 901, and quality of the extract powder. J. Food Process. Preserv. 2016, 40, 1125–1133. [Google Scholar] [CrossRef]
- Adebo, O.A.; Oyedeji, A.B.; Adebiyi, J.A.; Chinma, C.E.; Oyeyinka, S.A.; Olatunde, O.O.; Green, E.; Njobeh, P.B.; Kondiah, K. Kinetics of phenolic compounds modification during maize flour fermentation. Molecules 2021, 26, 6702. [Google Scholar] [CrossRef]
- Ren, S.C.; Liu, Z.L.; Ding, X.L. Isolation and identification of two novel flavone glycosides from corn silk (Stigma maydis). J. Med. Plant Res. 2009, 3, 1009–1015. [Google Scholar]
- Casas, M.I.; Duarte, S.; Doseff, A.I.; Grotewold, E. Flavone-rich maize: An opportunity to improve the nutritional value of an important commodity crop. Front. Plant Sci. 2014, 5, 440. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Li, D.; Li, X.; Gao, Y.; Li, W.; Li, H.; Liu, J.; Liu, H.; Chen, W.; Luo, J.; et al. Metabolome-based genome-wide association study of maize kernel leads to novel biochemical insights. Nat. Commun. 2014, 5, 3438. [Google Scholar] [CrossRef] [PubMed]
- Rayyan, S.; Fossen, T.; Andersen, Ø.M.J. Flavone C-glycosides from seeds of fenugreek, Trigonella foenum-graecum L. J. Agric. Food Chem. 2010, 58, 7211–7217. [Google Scholar] [CrossRef]
- Rayyan, S.; Fossen, T.; Andersen, Ø.M.J. Flavone C-glycosides from leaves of oxalis triangularis. J. Agric. Food Chem. 2005, 53, 10057–10060. [Google Scholar] [CrossRef] [PubMed]
- Casati, P.; Walbot, V. Differential accumulation of Maysin and rhamnosylisoorientin in leaves of high-altitudes landraces of maize after UV-B exposure. Plant Cell Environ. 2005, 28, 788–799. [Google Scholar] [CrossRef]
- Rector, B.G.; Snook, M.E.; Widstrom, N.W. Effect of husk characters on resistance to corn earworm (Lepidoptera: Noctuidae) in high-Maysin maize populations. J. Econ. Entomol. 2002, 95, 1303–1307. [Google Scholar] [CrossRef]
- Paraginski, R.T.; Vanier, N.L.; Berrios, J.D.J.; de Oliveira, M.; Elias, M.C. Physicochemical and pasting properties of maize as affected by storage temperature. J. Stored Prod. Res. 2017, 59, 209–214. [Google Scholar] [CrossRef]
- Akaffou, F.A.; Koffi, D.M.; Cisse, M.; Niamké, S.L. Physicochemical and functional properties of flours from three purple maize varieties named “Violet de Katiola” in Côte d’Ivoire. Asian Food Sci. J. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Gong, K.; Chen, L.; Li, X.; Sun, L.; Liu, K. Effects of germination combined with extrusion on the nutritional composition, functional properties and polyphenol profile and related in vitro hypoglycemic effect of whole grain corn. J. Cereal Sci. 2018, 83, 1–8. [Google Scholar] [CrossRef]
- Ren, X.; Liang, Q.; Zhang, X.; Hou, T.; Li, S.; Ma, H. Stability and antioxidant activities of corn protein hydrolysates under simulated gastrointestinal digestion. Cereal Chem. 2018, 95, 760–769. [Google Scholar] [CrossRef]
| Sample | % Carbon | % Hydrogen | % Sulfur | % Nitrogen | % Protein | % Hydrolysis Degree |
|---|---|---|---|---|---|---|
| PCF | 41.00 ± 0.28 c | 8.70 ± 0.06 c | 0.10 ± 0.01 a | 1.61 ± 0.03 a | 9.94 ± 0.28 a | N. D |
| PCPC25 | 59.05 ± 0.28 d | 9.97 ± 0.10 c | 0.40 ± 0.00 c | 7.81 ± 0.01 b | 48.79 ± 0.15 b | N. D |
| GH25 | 16.52 ± 1.00 b | 1.35 ± 0.23 a | 0.16 ± 0.02 a | 1.78 ± 0.08 a | 11.10 ± 0.29 a | 77.24 ± 0.25 a |
| DH25 | 12.07 ± 0.13 a | 2.59 ± 0.09 b | 0.58 ± 0.03 d | 1.67 ± 0.03 a | 10.44 ± 0.08 a | 78.61 ± 0.29 a |
| PCPC35 | 59.74 ± 0.18 d | 10.14 ± 0.09 | 0.33 ± 0.01 b | 7.31 ± 0.02 b | 45.67 ± 0.02 b | N. D |
| GH35 | 13.04 ± 0.58 a | 1.19 ± 0.09 a | 0.11 ± 0.00 a | 1.74 ± 0.05 a | 10.85 ± 0.04 a | 76.23 ± 0.32 a |
| DH35 | 13.30 ± 0.27 a | 2.46 ± 0.05 b | 0.08 ± 0.00 a | 1.71 ± 0.02 a | 10.67 ± 0.04 a | 76.64 ± 0.19 a |
| Sample | Total Flavanol Content (TFLC) mg RE/100 g DW | Total Flavonoids Content (TFC) mg RE/100 g DW | Total Anthocyanins Content (TAC) mg Cy3-glu/g DW | Total Pro-Anthocyanins Content (TPA) mg CE/g DW |
|---|---|---|---|---|
| PCF | 460.90 ± 0.49 a | 503.07 ± 0.27 a | 1609.59 ± 0.02 d | 56.98 ± 0.00 b |
| PCPC25 | 4228.54 ± 1.69 b | 2039.38 ± 0.27 d | 1157.35 ± 0.02 b | 67.00 ± 3.54 a |
| GH25 | 630.57 ± 0.47 a | 1603.66 ± 0.26 c | 66.52 ± 0.01 a | 35.08 ± 3.42 c |
| DH25 | 690.03 ± 0.85 a | 1326.37 ± 0.27 b | 181.45 ± 0.01 a | 36.80 ± 3.59 c |
| PCPC35 | 11091.37 ± 6.11 c | 7975.59 ± 0.59 e | 1620.24 ± 0.01 c | 57.73 ± 3.36 b |
| GH35 | 1097.28 ± 1.09 a | 1539.54 ± 0.23 c | 116.35 ± 0.01 a | 28.95 ± 0.00 d |
| DH35 | 783.07 ± 0.78 a | 1796.30 ± 0.14 d | 88.88 ± 0.01 a | 37.25 ± 0.00 c |
| N° | Name | Phenol Type | Retention Time (min) | [M − H]− m/z | WM (Da) |
|---|---|---|---|---|---|
| 1 | Apigenin | Flavonoid/Flavonol | 3.004 | 270.2 | 270.237 |
| 2 | 7,4’-Dihydroxy-3’-methoxyflavone | Flavonoid/Flavonol | 3.570 | 284.4 | 284.263 |
| 3 | (+) Catechin | Flavonoid/Flavonol | 5.362 | 290.4 | 290.268 |
| 4 | Quercetin | Flavonoid/Flavonol | 2.984 | 302.3 | 302.236 |
| 5 | Dihydroquercetin/Taxifolin | Flavonoid/Flavonol | 5.743 | 304.3 | 304.252 |
| 6 | Myricetin | Flavonoid/Flavonol | 2.742 | 318.3 | 318.235 |
| 7 | p-Coumaric acid 4-O-glucoside | Phenolic acid | 3.809 | 326.4 | 326.299 |
| 8 | Gallic acid 4-O-glucoside | Phenolic acid | 5.903 | 332.4 | 332.260 |
| Sample | % Water Absorption Capacity (WAC) | % Oil Absorption Capacity (OAC) |
|---|---|---|
| PCF | 26.90 ± 0.52 a | 22.08 ± 0.21 b |
| PCPC25 | 27.77 ± 2.97 a | 24.94 ± 1.22 a |
| GH25 | 19.04 ± 0.93 a,b | 19.05 ± 1.40 b,c |
| DH25 | 13.48 ± 0.00 a | 11.32 ± 0.07 a |
| PCPC35 | 29.89 ± 5.47 a | 23.80 ± 1.19 ab |
| GH35 | 19.26 ± 2.20 a,b | 16.45 ± 2.04 b |
| DH35 | 13.17 ± 0.50 a | 11.31 ± 0.60 a |
| Sample | ABTS (μmol TE/g DW) | FRAP (μmol TE/g DW) | DPPH (μmol TE/g DW) |
|---|---|---|---|
| Purple corn flour | 63772 ± 1.00 e | 47292 ± 2.64 c | 93203 ± 1.18 c |
| PCPC25 | 570.97 ± 0.07 d | 772.85 ± 0.29 b | 90.60± 20.90 b |
| GH25 | 142.00 ± 0.00 b | 64.73 ± 0.01 a | 74.72 ± 8.00 a |
| DH25 | 74.12 ± 0.01 a | 59.42 ± 0.02 a | 85.30 ± 7.90 b |
| PCPC35 | 402.75 ± 0.09 c | 747.02 ± 0.09 b | 83.98 ± 13.69 b |
| GH35 | 107.52 ± 0.04 b | 53.89 ± 0.00 a | 57.34 ± 7.95 a |
| DH35 | 77.10 ± 0.01 a | 59.16 ± 0.01 a | 67.12 ± 7.92 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilcacundo, E.; Montalvo, V.; Sanaguano, H.; Moran, R.; Carrillo, W.; García, A. Identification of Phytochemical Compounds, Functional Properties and Antioxidant Activity of Germinated Purple Corn Protein Concentrate and Its Gastrointestinal Hydrolysates. Agronomy 2022, 12, 2217. https://doi.org/10.3390/agronomy12092217
Vilcacundo E, Montalvo V, Sanaguano H, Moran R, Carrillo W, García A. Identification of Phytochemical Compounds, Functional Properties and Antioxidant Activity of Germinated Purple Corn Protein Concentrate and Its Gastrointestinal Hydrolysates. Agronomy. 2022; 12(9):2217. https://doi.org/10.3390/agronomy12092217
Chicago/Turabian StyleVilcacundo, Edgar, Vinicio Montalvo, Herminia Sanaguano, Roberto Moran, Wilman Carrillo, and Antón García. 2022. "Identification of Phytochemical Compounds, Functional Properties and Antioxidant Activity of Germinated Purple Corn Protein Concentrate and Its Gastrointestinal Hydrolysates" Agronomy 12, no. 9: 2217. https://doi.org/10.3390/agronomy12092217










