Effect of Cow Urine Nitrogen Rates and Moisture Conditions on Nitrogen Mineralization in Andisol from Southern Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Samples Used for the Study
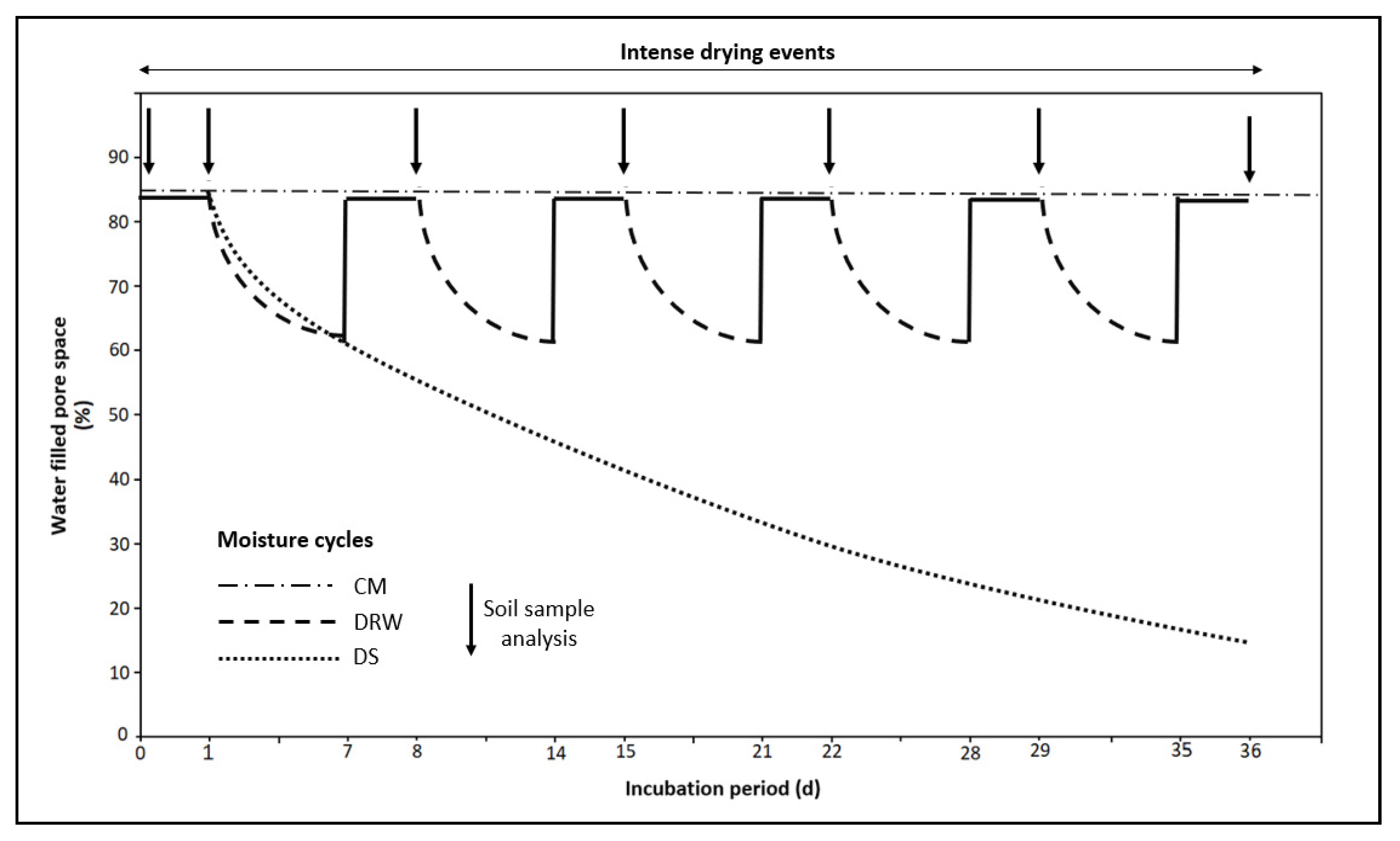
2.2. Design of the Incubation Experiment
2.3. NH4+, TON, NH3, and pH Determinations
2.4. Statistical Analysis
3. Results
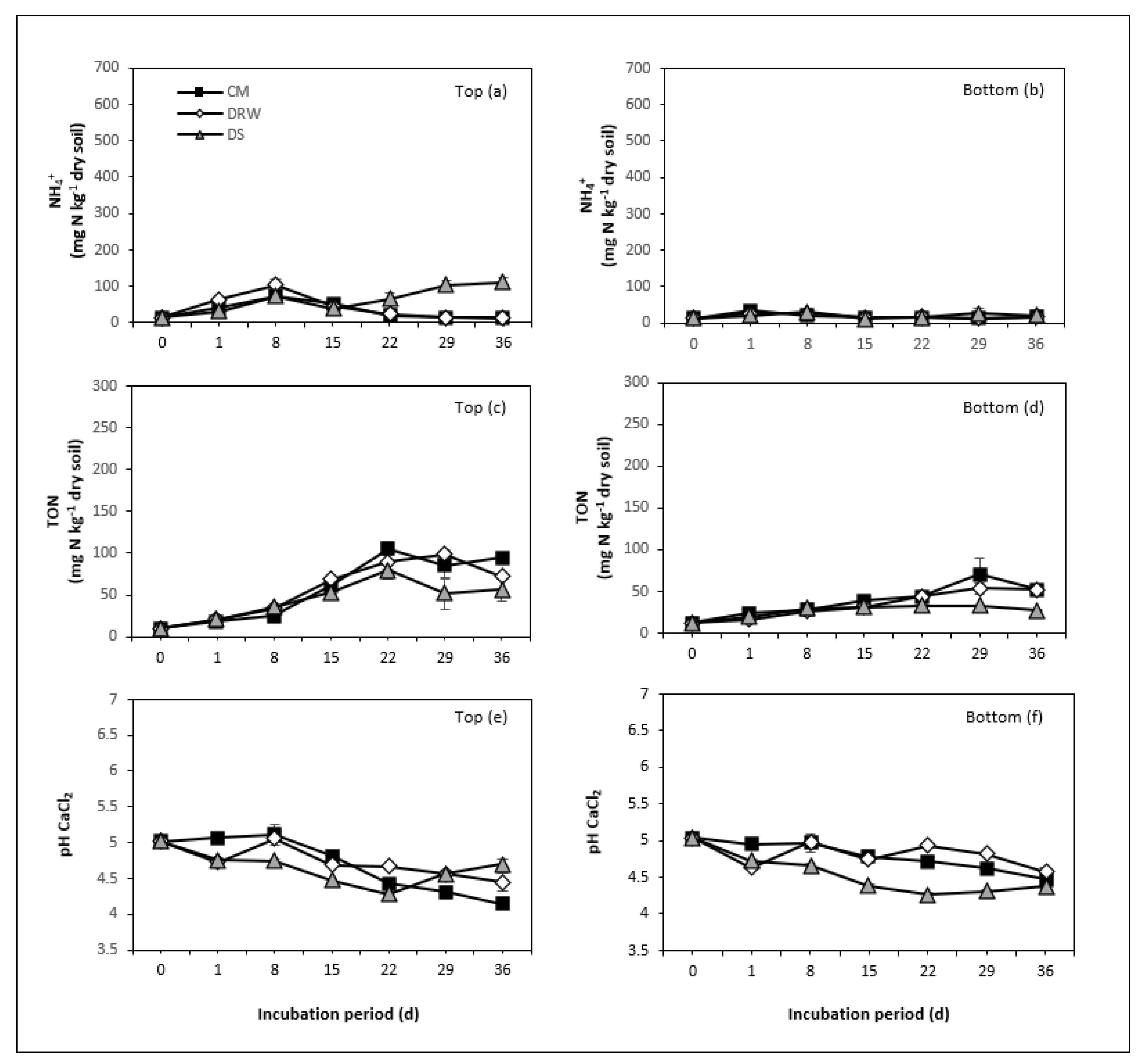
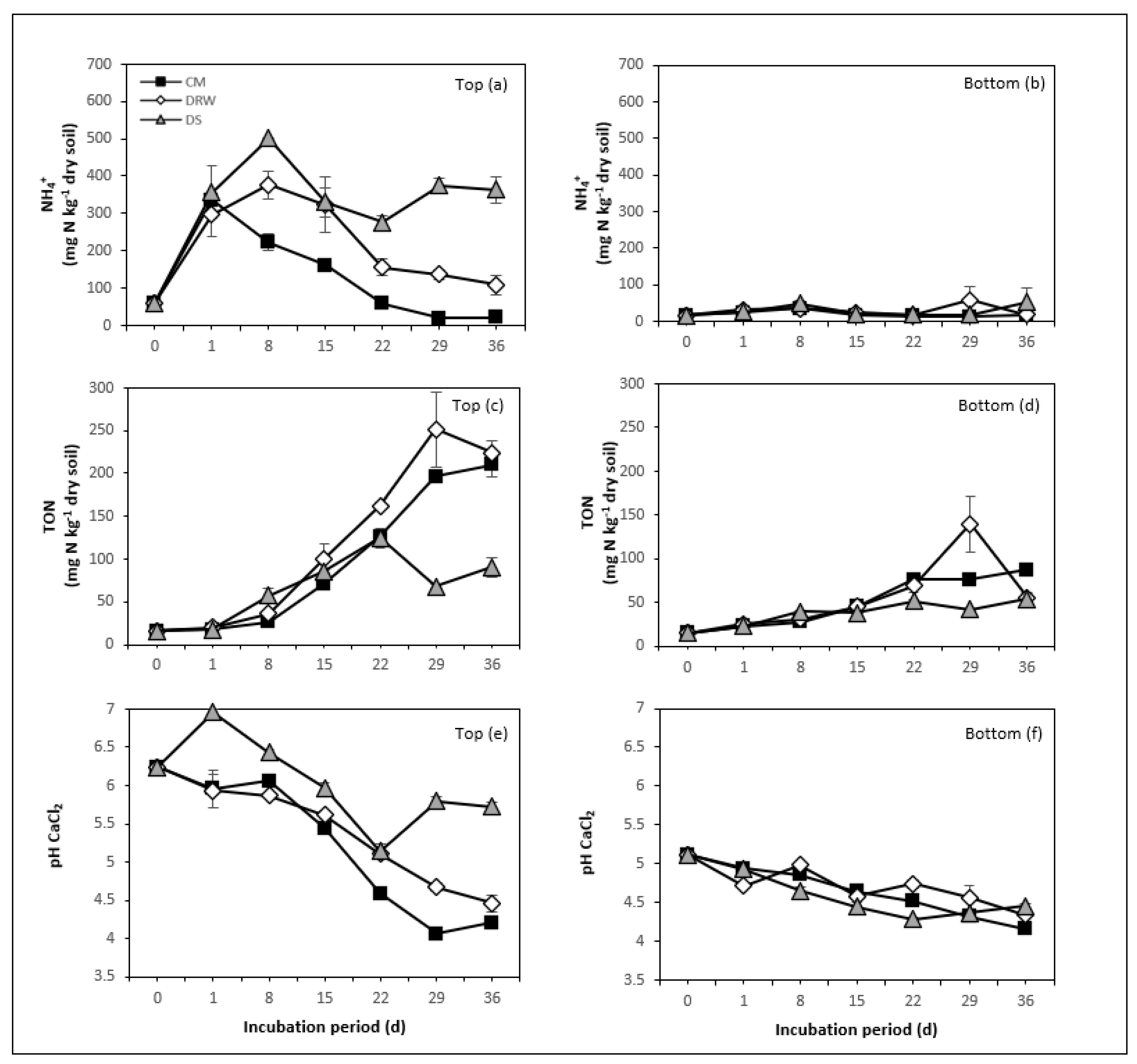
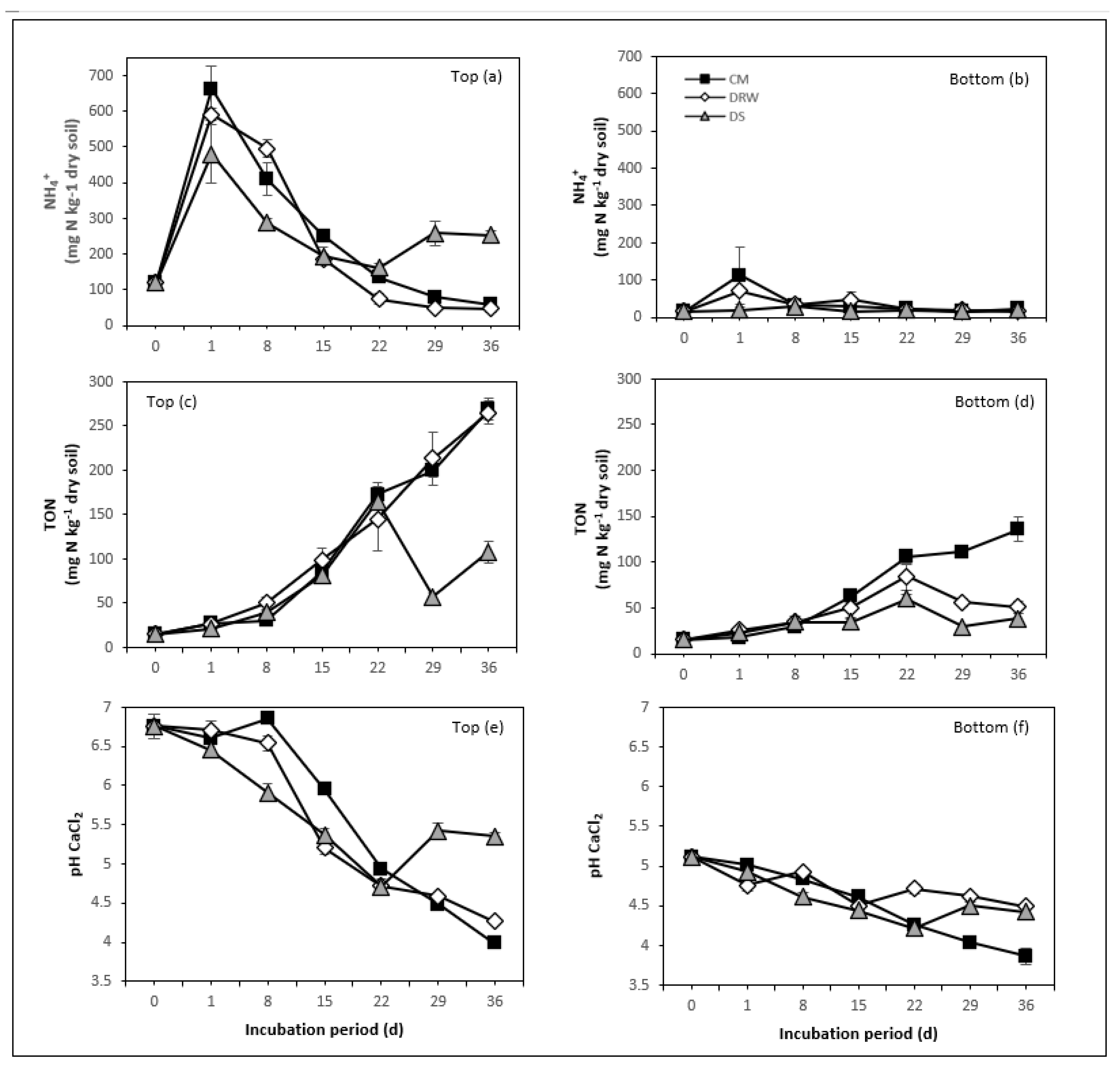
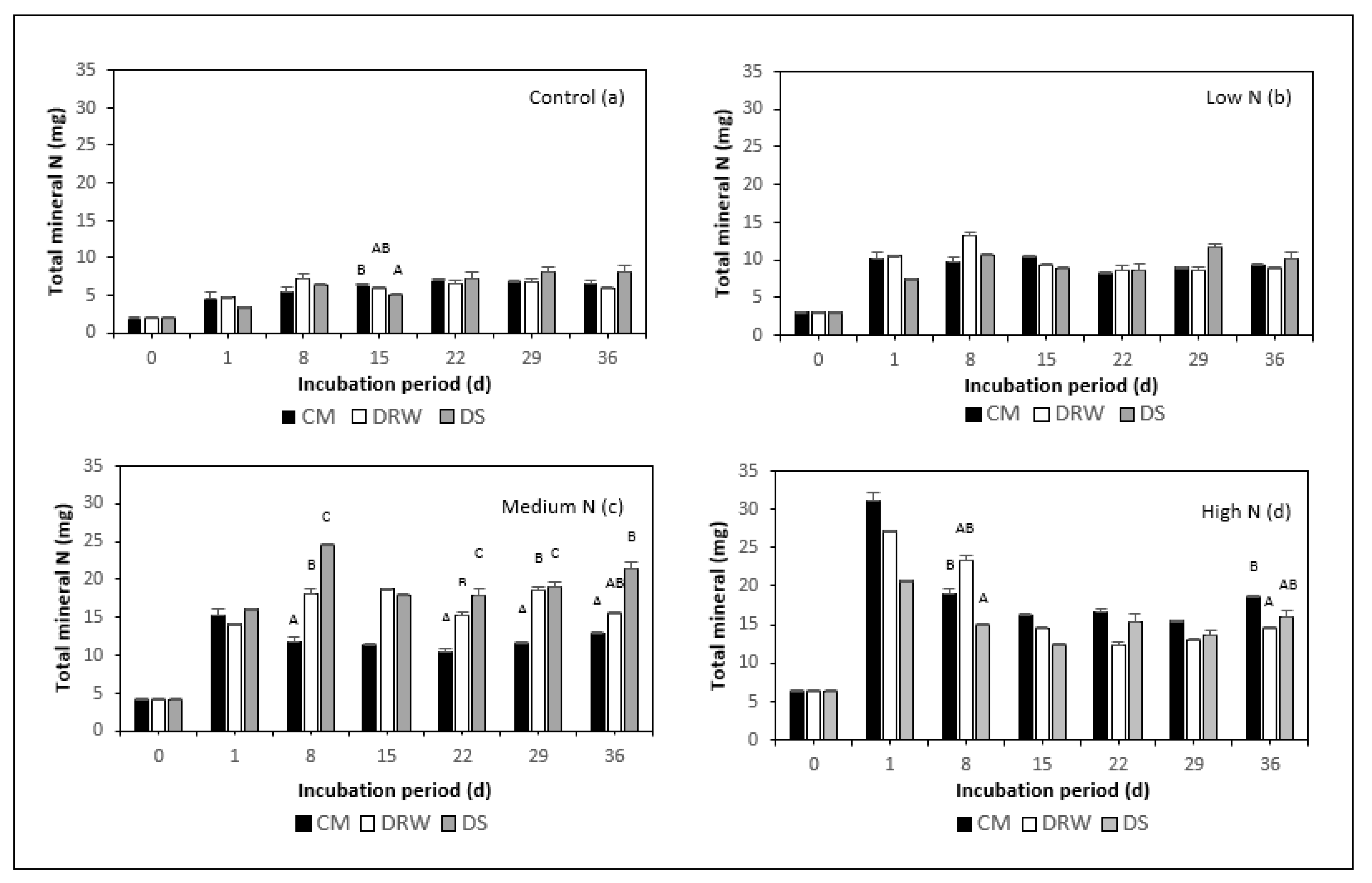
3.1. NH4+, TON, and NH3 Mineralization
3.2. Soil pH
4. Discussion
4.1. N Rate and N Mineralization
4.2. Nitrification and NH3 Oxidation
4.3. Moisture Conditions and N Mineralization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dijkstra, J.; Reynolds, C.K.; Kebreab, A.; Bannink, A.; Ellis, J.L.; France, J.; van Vuuren, A.M. Challenges in Ruminant Nutrition: Towards Minimal Nitrogen Losses in Cattle; Oltjen, W.J., Kebreab, E., Lapierre, H., Eds.; Energy and Protein Metabolism and Nutrition in Sustainable Animal Production; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 47–58. [Google Scholar]
- Lantinga, E.A.; Keuning, J.A.; Groenwold, J.; Deenen, P.A.G. Distribution of excreted nitrogen by grazing cattle and its effects on sward quality, herbage production and utilization. In Animal Manure on Grassland and Fodder Crops: Fertilizer or Waste; Van der Meer, H.G., Unwin, R.J., van Dijk, T.A., Ennik, G.C., Eds.; Martinus Nijhoff: Dordrecht, The Netherlands, 1987; pp. 103–117. [Google Scholar]
- Hoogendoorn, C.; Betteridge, K.; Costall, D.; Ledgard, S. Nitrogen concentration in the urine of cattle, sheep and deer grazing acommon ryegrass/cocksfoot/white clover pasture. New Zealand J. Agric. Res. 2010, 53, 235–243. [Google Scholar] [CrossRef]
- Holmes, W. Grass: Its Production and Utilization, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1989. [Google Scholar]
- Dennis, S.; Moir, J.L.; Cameron, K.; Di, H.; Hennessy, D.; Richards, K.G. Urine patch distribution under dairy grazing at three stocking rates in Ireland. Irish J. Agric. Food Res. 2011, 50, 149–160. [Google Scholar]
- Ramírez-Sandoval, M.A.; Pinochet, D.E.; Rivero, M.J. Wetting Pattern of Cow Urine Patch in an Andisol Assessed through Bromide Concentration Distribution: A Pilot Study. Soil Syst. 2022, 6, 80. [Google Scholar] [CrossRef]
- Haynes, R.J.; Williams, P.H. Nutrient cycling y soil fertility in the grazed pasture ecosystem. Adv. Agron. 1993, 49, 119–199. [Google Scholar]
- Whitehead, D.C. Nutrient Elements in Grassland: Soil-Plant-Animal Relationships; CABI: Surrey, UK, 2000; p. 384. [Google Scholar]
- Ramírez-Sandoval, M.; Pinochet, D.; Rivero, M.J. Soil Dynamics and Nitrogen Absorption by a Natural Grassland under Cow Urine and Dung Patches in an Andisol in Southern Chile. Agronomy 2022, 12, 719. [Google Scholar] [CrossRef]
- Di, H.; Cameron, K. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycling Agroecosyst. 2002, 46, 237–256. [Google Scholar] [CrossRef]
- Saarijärvi, K.; Virkajärvi, P. Nitrogen dynamics of cattle dung y urine patches on intensively managed boreal pasture. J. Agr. Sci. 2009, 147, 479–491. [Google Scholar] [CrossRef]
- Cárdenas, L.M.; Misselbrook, T.M.; Hodgson, C.; Donovan, N.; Gilhespy, S.; Smith, K.A.; Dhanoa, M.S.; Chadwick, D. Effect of the application of cattle urine with or without the nitrification inhibitor DCD, and dung on greenhouse gas emissions from a UK grassland soil. Agric. Ecosyst. Environ. 2016, 235, 229–241. [Google Scholar] [CrossRef]
- Jarvis, S.; Pain, B. Ammonia volatilization from agricultural land. Proc. Fertil. Soc. 1990, 298, 1–35. [Google Scholar]
- van Groenigen, J.W.; Kuikman, P.J.; de Groot, W.J.; Velthof, G.L. Nitrous oxide emission from urinetreated soil as influenced by urine composition and soil physical conditions. Soil Biol. Biochem. 2005, 37, 463–473. [Google Scholar] [CrossRef]
- Doak, B.W. Some chemical changes in the nitrogenous constituents of urine when voided on pasture. J. Agric. Sci. 1952, 42, 162–171. [Google Scholar] [CrossRef]
- Sherlock, R.; Goh, K. Dynamics of ammonia volatilisation from simulated urine patches and aqueous urea applied to pasture. II. Theoretical derivation of a simplified model. Fertil. Res. 1985, 6, 3–22. [Google Scholar] [CrossRef]
- Venterea, R.T.; Rolston, D.E. Nitric and nitrous oxide emissions following fertilizer application to agricultural soil: Biotic and abiotic mechanisms and kinetics. J. Geophys. Res. 2000, 105, 15117–15129. [Google Scholar] [CrossRef]
- De Boer, W.; Gunnewiek, P.K.; Veenhuis, M.; Bock, E.; Laanbroek, H.J. Nitrification at low pH by aggregated chemolithotrophic bacteria. Appl. Environ. Microbiol. 1991, 57, 3600–3604. [Google Scholar] [CrossRef]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, 94–98. [Google Scholar] [CrossRef]
- Malhi, S.; McGill, W. Nitrification in three Alberta soils: Effect of temperature, moisture and substrate concentration. Soil Biol. Biochem. 1982, 14, 393–399. [Google Scholar] [CrossRef]
- Clough, T.; Sherlock, R.; Mautner, M.; Milligan, D.; Wilson, P.; Freeman, C.; McEwan, M. Emission of nitrogen oxides and ammonia from varying rates of applied synthetic urine and correlations with soil chemistry. Aust. J. Soil Res. 2003, 41, 421–438. [Google Scholar] [CrossRef]
- Somers, C.; Girkin, N.; Rippey, B.; Lanigan, G.; Richards, K. The effects of urine nitrogen application rate on nitrogen transformations in grassland soils. J. Agric. Sci. 2019, 157, 1–8. [Google Scholar] [CrossRef]
- Baral, K.R.; Thomsen, A.G.; Olesen, J.E.; Petersen, S.O. Controls of nitrous oxide emission after simulated cattle urine deposition. Agric. Ecosyst. Environ. 2014, 188, 103–110. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources, WRB, 2nd ed.; World Soil Resources Reports 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Shoji, S.; Nanzyo, M.; Dahlgren, R. Volcanic Ash Soils: Genesis, Properties and Utilization; Developments in Soil Science; Elsevier Science: Amsterdam, The Netherlands, 1994; p. 288. [Google Scholar]
- Matus, F.; Garrido, E.; Sepúlveda, N.; Cárcamo, I.; Panichini, M.; Zagal, E. Relationship between extractable Al and organic C in volcanic soils of Chile. Geoderma 2008, 148, 180–188. [Google Scholar] [CrossRef]
- Dörner, J.; Dec, D.; Peng, X.; Horn, R. Effect of land use change on the dynamic behaviour of structural properties of an Andisol in southern Chile under saturated and unsaturated hydraulic conditions. Geoderma 2010, 159, 189–197. [Google Scholar] [CrossRef]
- Soil Survey Staff. Natural Resources Conservation Services; USDA: Washington, DC, USA, 2010.
- Pezzolla, D.; Cardenas, L.M.; Mian, I.A.; Carswell, A.; Donovan, N.; Dhanoa, M.S.; Blackwell, M.S. Responses of carbon, nitrogen and phosphorus to two consecutive drying–rewetting cycles in soils. J. Plant Nutr. Soil Sci. 2019, 182, 217–228. [Google Scholar] [CrossRef]
- Sadzawka, A.; Carrasco, M.; Grez, R.; Mora, M.; Flores, H.; Neaman, A. Métodos de Análisis Recomendados Para los Suelos de Chile. Serie Actas INIA; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2006; Volume 34, p. 164. [Google Scholar]
- Watson, C.; Kilpatrick, D.; Cooper, J. The effect of increasing application rate of granular calcium ammonium nitrate on net nitrification in a laboratory study of grassland soils. Fertiliser Res. 1994, 40, 155–161. [Google Scholar] [CrossRef]
- Smith, A.P.; Bond-Lamberty, B.; Benscoter, B.W.; Tfaily, M.M.; Hinkle, C.R.; Liu, C.; Bailey, V.L. Free ammonia inhibition of nitrification in river sediments leading to nitrite accumulation. J. Environ. Qual. 1997, 26, 1049–1055. [Google Scholar] [CrossRef]
- Clough, T.J.; Kelliher, F.M.; Sherlock, R.R.; Ford, C.D. Lime and soil moisture effects on nitrous oxide and dinitrogen emissions from a pasture soil. Soil Sci. Soc. Am. J. 2004, 68, 1600–1609. [Google Scholar] [CrossRef]
- Khan, S.; Clough, T.; Goh, K.; Sherlock, R. Influence of soil pH on NOx and N2O emissions from bovine urine applied to soil columns. N. Z. J. Agric. Res. 2011, 54, 285–301. [Google Scholar] [CrossRef]
- Whitehead, D.C.; Bristow, A.W. Transformations of nitrogen following the application of 15N-labelled cattle urine to an established grass sward. J. Appl. Ecol. 1990, 27, 667–678. [Google Scholar] [CrossRef]
- Ball, R.; Keeney, D.; Thoebald, P.; Nes, P. Nitrogen balance in urine-affected areas of a New Zealand pasture. Agron. J. 1979, 71, 309–314. [Google Scholar] [CrossRef]
- Sherlock, R.; Goh, K. Dynamics of ammonia volatilization from simulated urine patches and aqueous urea applied to pasture. Fertil. Res. 1984, 5, 181–195. [Google Scholar] [CrossRef]
- Silva, R.G.; Cameron, K.C.; Di, H.; Hendry, T. A lysimeter study of the impact of cow urine, dairy shed effluent, and nitrogen fertiliser on nitrate leaching. Aust. J. Soil Res. 1999, 37, 357–369. [Google Scholar] [CrossRef]
- Bussink, D.; Oenema, O. Ammonia volatilization from dairy farming systems in temperate areas: A review. Nutr. Cycling Agroecosyst. 1998, 51, 19–33. [Google Scholar] [CrossRef]
- Selbie, D.R.; Buckthought, L.E.; Shepherd, M. The Challenge of the Urine Patch for Managing Nitrogen in Grazed Pasture Systems. Adv. Agron. 2015, 129, 229–292. [Google Scholar]
- Cárdenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Gregory, A.S.; Matthews, G.P.; Whalley, W.R.; Misselbrook, T.H.; Scholefield, D.; Well, R. Effect of soil saturation on denitrification in a grassland soil. Biogeosciences 2017, 14, 4691–4710. [Google Scholar] [CrossRef]
- Keeney, D.; MacGregor, A. Short-term cycling of 15N-urea in a ryegrass-white clover pasture. N. Z. J. Agric. Res. 1978, 21, 443–448. [Google Scholar] [CrossRef]
- Williams, P.H.; Haynes, R.J. Comparison of initial wetting pattern, nutrient concentrations in soil solution and fate of 15N-labelled urine in sheep and cattle urine patch areas of pasture soil. Plant Soil 1994, 162, 49–59. [Google Scholar] [CrossRef]
- Thompson, R.; Fillery, I. Fate of urea nitrogen in sheep urine applied to soil at different times of the year in the pasture-wheat rotation in south Western Australia. Aust. J. Agric. Res. 1998, 49, 495–510. [Google Scholar] [CrossRef]
- Alfaro, M.; Salazar, F.; Hube, S.; Ramírez, L.; Mora, L. Ammonia and nitrous oxide emissions as affected by nitrification and urease inhibitors. J. Soil Sci. Plant Nutr. 2018, 18, 479–486. [Google Scholar]
- Bolan, N.S.; Saggar, S.; Luo, J.; Bhandral, R.; Singh, J. Gaseous emissions of nitrogen from grazed pastures: Processes, measurements and modelling, environmental implications, and mitigation. Adv. Agron. 2004, 84, 37–120. [Google Scholar]
- Taylor, A.E.; Myrold, D.D.; Bottomley, P.J. Temperature affects the kinetics of nitrite oxidation and nitrification coupling in four agricultural soils. Soil Biol. Biochem. 2019, 136, 107523. [Google Scholar] [CrossRef]
- He, J.Z.; Shen, J.P.; Zhang, L.M.; Zhu, Y.G.; Zheng, Y.M.; Xu, M.G.; Di, H. Quantitative analyses of the abundance andcomposition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environm. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Lehtovirta, L.E.; Prosser, J.I.; Nicol, G.W. Soil pH regulates the abundance and diversity of Group1.1c Crenarchaeota. FEMS Microbiol. Ecol. 2009, 70, 367–376. [Google Scholar] [CrossRef]
- Könneke, M.; Bernhard, A.E.; José, R.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef]
- Offre, P.; Prosser, J.I.; Nicol, G.W. Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol. Ecol. 2009, 7, 99–108. [Google Scholar] [CrossRef]
- De Boer, W.; Kowalchuk, G.A. Nitrification in acid soils: Micro-organisms and mechanisms. Soil Biol. Biochem. 2001, 33, 853–866. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Shen, J.-P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.-Z. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 2010, 72, 386–394. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Wessén, E.; Söderström, M.; Stenberg, M.; Bru, D.; Hellman, M.; Welsh, A.; Thomsen, F.; Klemedtson, L.; Philippot, L.; Hallin, S. Spatial distribution of ammonia-oxidizing bacteria and archaea across a 44-hectare farm related to ecosystem functioning. ISME J. 2011, 5, 1213–1225. [Google Scholar] [CrossRef]
- Yao, H.; Gao, Y.; Nicol, G.W.; Campbell, C.D.; Prosser, J.I.; Zhang, L.; Han, W.; Singh, B.K. Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl. Environ. Microbiol. 2011, 77, 4618–4625. [Google Scholar] [CrossRef]
- Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol. Ecol. 2010, 74, 566–574. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Hu, H.-W.; Shen, J.-P.; He, J.-Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Wang, S.; Ma, B.; Ge, S.; Wang, Z.; Huang, H.; Zhang, J.; Zhang, L. Pathways and organisms involved in ammonia oxidation and nitrous oxide emission. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2213–2296. [Google Scholar] [CrossRef]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Change Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]
- Olfs, H.W.; Neu, A.; Werner, W. Soil N transformations after applicationof 15N-labeled biomass in incubation experiments with repeated soil drying and rewetting. J. Plant Nutr. Soil Sci. 2004, 167, 147–152. [Google Scholar] [CrossRef]
- Canarini, A.; Dijkstra, F.A. Dry-rewetting cycles regulate wheat carbon rhizodeposition, stabilization and nitrogen cycling. Soil Biol. Biochem. 2015, 81, 195–203. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Hentschel, K.; Borken, W.; Matzner, E. Leaching losses of inorganic N and DOC following repeated drying and wetting of a spruce forest soil. Plant Soil. 2007, 300, 21–34. [Google Scholar] [CrossRef]
- Harrison-Kirk, T.; Beare, M.; Meenken, E.; Condron, L. Soil organic matter and texture affect responses to dry/wet cycles: Changes in soil organic matter fractions and relationships with C and N mineralisation. Soil Biol. Biochem. 2014, 74, 50–60. [Google Scholar] [CrossRef]
- Miller, A.E.; Schimel, J.P.; Meixner, T.; Sickman, J.O.; Melack, J.M. Episodic rewetting enhances carbon and nitrogen release from chaparral soils. Soil Biol. Biochem. 2005, 37, 2195–2204. [Google Scholar] [CrossRef]
- Fuchslueger, L.; Kastl, E.-M.; Bauer, F.; Kienzl, S.; Hasibeder, R.; Ladreiter-Knauss, T.; Schmitt, M.; Bahn, M.; Schloter, M.; Richter, A. Effects of drought on nitrogen turnover and abundances of ammonia-oxidizers in mountain grassland. Biogeosciences 2014, 11, 6003–6015. [Google Scholar] [CrossRef]
- Rudaz, A.O.; Davidson, E.A.; Firestone, M.K. Sources of nitrous-oxide production following wetting of dry soil. FEMS Microbiol. Ecol. 1991, 8, 117–124. [Google Scholar] [CrossRef]
- Rey, A.; Petsikos, C.; Jarvis, P.; Grace, J. Effect of temperature and moisture on rates of carbon mineralization ina Mediterranean oak forest soil under controlled and field conditions. Eur. J. Soil Sci. 2005, 56, 589–599. [Google Scholar] [CrossRef]
- Franzluebbers, A.; Haney, R.; Honeycutt, C.; Schomberg, H.-H.; Hons, F. Flush of carbon dioxide following rewetting of dried soil relates to active organic pools. Soil Sci. Soc. Am. J. 2000, 64, 613–623. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci. Soc. Am. J. 2003, 67, 798–805. [Google Scholar] [CrossRef]
- Pesaro, M.; Nicollier, G.; Zeyer, J.; Widmer, F. Impact of drying-rewetting stress on microbial communities and activities and on degradation of two crop protection products. Appl. Environ. Microbiol. 2004, 70, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Beare, M.H.; Hendrix, P.; Cabrera, M.; Coleman, D. Aggregate protected and unprotected organic-matter pools in conventional-tillage and no-tillage soils. Soil Sci. Soc. Am. J. 1994, 58, 787–795. [Google Scholar] [CrossRef]
- Hassink, J.; Whitmore, A.P. A model of the physical protection of organic matter in soils. Soil Sci. Soc. Am. J. 1997, 61, 131–139. [Google Scholar] [CrossRef]
- Malamoud, K.; McBratney, A.B.; Minasny, B.; Field, D.J. Modeling how carbon affects soil structure. Geoderma 2009, 149, 19–26. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil. 2002, 241, 155–176. [Google Scholar]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.; de Moraes Sa, J.C.; Albrecht, A. Soil organic matter, biota and aggregation in temperate and tropical soils—Effects of no-tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef]
- Helfrich, M.; Ludwig, B.; Potthoff, M.; Flessa, H. Effect of litter quality and soil fungi on macroaggregate dynamics and associated partitioning of litter carbon and nitrogen. Soil Biol. Biochem. 2008, 40, 1823–1835. [Google Scholar] [CrossRef]
- Nikolaidis, N.P.; Bidoglio, G. Soil organic matter dynamics and structure. Sustain. Agric. Rev. 2013, 12, 175–199. [Google Scholar]
- Lundquist, E.; Jackson, L.; Scow, K. Wet dry cycles affect DOC in two California agricultural soils. Soil Biol. Biochem. 1999, 31, 1031–1038. [Google Scholar] [CrossRef]
- Smith, A.P.; Bond-Lamberty, B.; Benscoter, B.W.; Tfaily, M.M.; Hinkle, C.R.; Liu, C.; Bailey, V.L. Shifts in pore connectivity from precipitation versus groundwater rewetting increases soil carbon loss after drought. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Cosentino, D.; Chenu, C.; Le Bissonnais, Y. Aggregate stability and microbial community dynamics under drying–wetting cycles in a silt loam soil. Soil Biol. Biochem. 2006, 38, 2053–2062. [Google Scholar] [CrossRef]
- Park, E.-J.; Sul, W.J.; Smucker, A.J. Glucose additions to aggregates subjected to drying/wetting cycles promote carbon sequestration and aggregate stability. Soil Biol. Biochem. 2007, 39, 2758–2768. [Google Scholar] [CrossRef]
- Sun, D.; Li, K.; Bi, Q.; Zhu, J.; Zhang, Q.; Jin, C.; Lu, L.; Lin, X. Effects of organic amendment on soil aggregation and microbial community composition during drying-rewetting alternation. Sci. Total Environ. 2017, 574, 735–743. [Google Scholar] [CrossRef]
- Kalbitz, K.; Meyer, A.; Yang, R.; Gerstberger, P. Response of dissolved organic matter in the forest floor to long-term manipulation of litter and throughfall inputs. Biogeochemistry 2007, 86, 301–318. [Google Scholar] [CrossRef]
- Müller, R. Osmoadaptation in bacteria and archaea: Common principles and differences. Environ. Microbiol. 2001, 12, 743–754. [Google Scholar]
- Placella, S.A.; Firestone, M.K. Transcriptional response of nitrifying communities to wetting of dry soil. Appl. Environ. Microbiol. 2013, 79, 3294–3302. [Google Scholar] [CrossRef]
- Thion, C.; Prosser, J.I. Differential response of nonadapted ammonia-oxidising archaea and bacteria to drying-rewetting stress. FEMS Microbiol. Ecol. 2014, 90, 380–389. [Google Scholar] [CrossRef] [PubMed]


| Property | Unit | Value |
|---|---|---|
| Soil type | - | Silandic Andosol; Eutric, Siltic [24] |
| Texture | - | Silty clay loam—silt loam [24] |
| NH4+ | mg kg−1 dry soil | 14.14 ± 1.43 |
| TON | mg kg−1 dry soil | 11.21 ± 0.23 |
| pH | 5.55 ± 0.50 | |
| P | mg kg−1 dry soil | 26.11 ± 7.14 |
| K | cmol kg−1 dry soil | 0.33 ± 0.16 |
| Mg | cmol kg−1 dry soil | 0.56 ± 0.24 |
| Ca | cmol kg−1 dry soil | 4.27 ± 2.24 |
| Na | cmol kg−1 dry soil | 0.16 ± 0.04 |
| Al+3 | cmol kg−1 dry soil | 0.54 ± 0.35 |
| Al+3 sat. | % | 12.86 ± 13.57 |
| Organic matter | g g−1 dry soil | 0.168 ± 0.015 |
| Particle density | g cm−3 | 2.24 |
| Bulk density | g cm−3 | 0.65 |
| Water content for packing | g g−1 dry soil | 0.42 |
| Incubation Day | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 15 | 22 | 29 | 36 | ||||||||
| Variable | Effect | Top | Bottom | Top | Bottom | Top | Bottom | Top | Bottom | Top | Bottom | Top | Bottom |
| NH4+ | |||||||||||||
| N rate | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | |
| Moisture cycle | n.s. | n.s. | 0.0147 | n.s. | n.s. | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | <0.0001 | n.s. | |
| N rate × moisture cycle | n.s. | n.s. | 0.0229 | n.s. | 0.0031 | n.s. | 0.0001 | n.s. | 0.0024 | 0.0302 | <0.0001 | n.s. | |
| Total oxidized nitrogen (TON) | |||||||||||||
| N rate | n.s. | n.s. | n.s. | n.s. | 0.0003 | 0.0001 | <0.0001 | <0.0001 | 0.0048 | <0.0001 | <0.0001 | <0.0001 | |
| Moisture cycle | n.s. | n.s. | <0.0001 | n.s. | n.s. | n.s. | n.s. | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| N rate × moisture cycle | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.0021 | n.s. | 0.0008 | |
| Total mineral N | N rate | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| moisture cycle | n.s. | 0.041 | n.s. | 0.0228 | 0.0107 | 0.0209 | |||||||
| N rate × moisture cycle | n.s. | 0.0184 | 0.0086 | 0.001 | 0.0002 | 0.0106 | |||||||
| % of N applied | N rate | n.s. | 0.0007 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| Moisture cycle | n.s. | n.s. | n.s. | 0.0002 | 0.0002 | n.s. | |||||||
| N rate × moisture cycle | n.s. | n.s. | 0.0286 | <0.0001 | <0.0001 | 0.0071 | |||||||
| NH3 | N rate | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| Moisture cycle | n.s. | n.s. | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| N rate × moisture cycle | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| pH CaCl2 | N rate | <0.0001 | 0.006 | <0.0001 | n.s. | <0.0001 | 0.0007 | <0.0001 | <0.0001 | <0.0001 | 0.0004 | 0.0004 | <0.0001 |
| Moisture cycle | 0.0127 | <0.0001 | 0.0101 | 0.0216 | 0.0007 | < 0.0001 | 0.0057 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| N rate × moisture cycle | <0.0001 | n.s. | 0.001 | n.s. | <0.0001 | 0.0019 | <0.0001 | 0.0011 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Sandoval, M.; Pinochet, D.; Rivero, M.J.; Cardenas, L.M. Effect of Cow Urine Nitrogen Rates and Moisture Conditions on Nitrogen Mineralization in Andisol from Southern Chile. Agronomy 2023, 13, 10. https://doi.org/10.3390/agronomy13010010
Ramírez-Sandoval M, Pinochet D, Rivero MJ, Cardenas LM. Effect of Cow Urine Nitrogen Rates and Moisture Conditions on Nitrogen Mineralization in Andisol from Southern Chile. Agronomy. 2023; 13(1):10. https://doi.org/10.3390/agronomy13010010
Chicago/Turabian StyleRamírez-Sandoval, Magdalena, Dante Pinochet, M. Jordana Rivero, and Laura M. Cardenas. 2023. "Effect of Cow Urine Nitrogen Rates and Moisture Conditions on Nitrogen Mineralization in Andisol from Southern Chile" Agronomy 13, no. 1: 10. https://doi.org/10.3390/agronomy13010010
APA StyleRamírez-Sandoval, M., Pinochet, D., Rivero, M. J., & Cardenas, L. M. (2023). Effect of Cow Urine Nitrogen Rates and Moisture Conditions on Nitrogen Mineralization in Andisol from Southern Chile. Agronomy, 13(1), 10. https://doi.org/10.3390/agronomy13010010







