The Effect of Tillage on Faba Bean (Vicia faba L.) Nitrogen Fixation in Durum Wheat ((Triticum turgidum L. subsp. Durum (Desf))-Based Rotation under a Mediterranean Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.1.1. Crop Management
2.1.2. Soil Analysis
2.2. N Fixation Experiment and 15N-Labelled Fertilizer Treatments
2.3. Data Collection and Analysis
2.4. Estimating N2 Fixation
2.4.1. Calculation of the Proportion of N2 in Faba Bean (%Ndfa)
2.4.2. Calculation of the Amount of N Fixed by Faba Bean
2.5. Crop N Uptake and Soil N Balance
2.6. Statistical Analysis
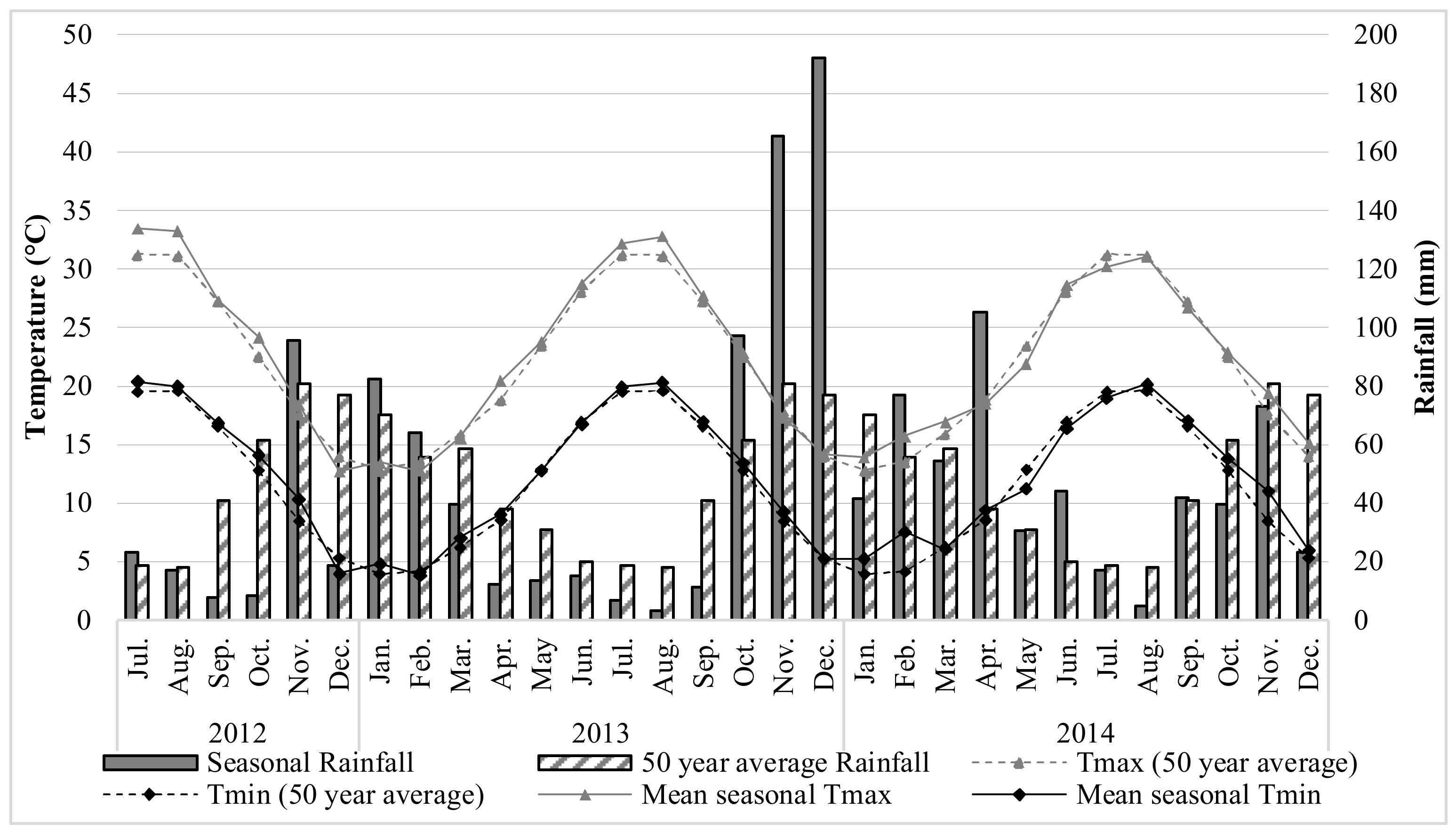
2.7. Weather Conditions
3. Results
3.1. Crop Yields
3.2. N Content and N Uptake
3.2.1. N Content in Biomass
3.2.2. Crop N Uptake
3.3. Nitrogen Fixation
Nitrogen (N) Balance
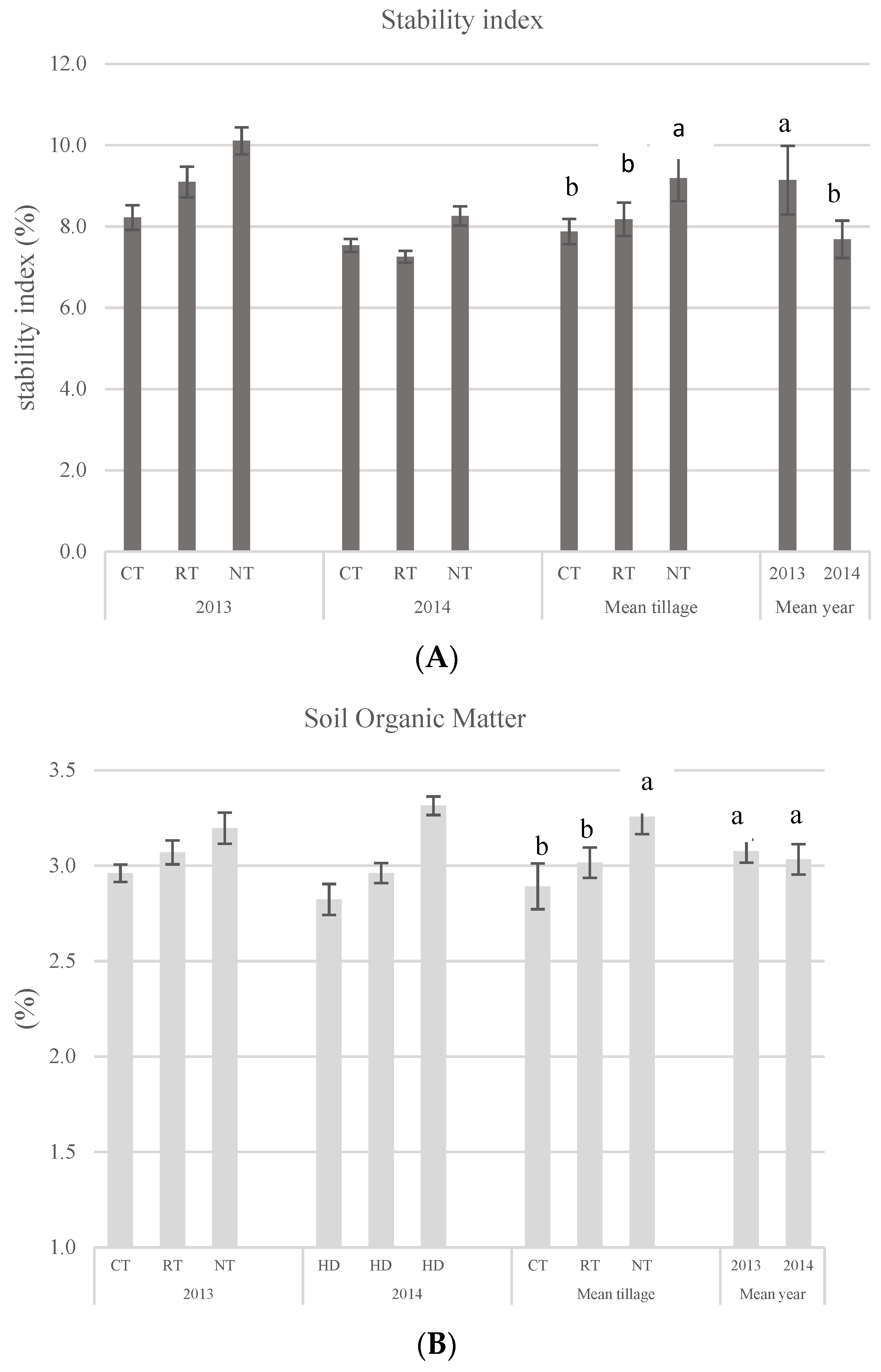
3.4. Soil Quality Parameters
4. Discussions
4.1. Yield Results
4.2. Crop N Uptake and N Balance
4.3. Nitrogen Fixation by Faba Bean
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jensen, E.S.; Hauggaard-Nielsen, H. How can increased use of biological N2 Fixation in agriculture benefit the environment? Plant Soil 2003, 252, 177–186. [Google Scholar] [CrossRef]
- Köpke, U.; Nemecek, T. Ecological services of faba bean. Field Crop. Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Peoples, M.; Bowman, A.; Gault, R.; Herridge, D.; McCallum, M.; McCormick, K.; Norton, R.; Rochester, I.; Scammell, G.; Schwenke, G. Factors regulating the contributions of fixed nitrogen by pasture and crop legumes to different farming systems of eastern Australia. Plant Soil 2001, 228, 29–41. [Google Scholar] [CrossRef]
- Ruisi, P.; Amato, G.; Badagliacca, G.; Frenda, A.S.; Giambalvo, D.; Di Miceli, G. Agro-ecological benefits of faba bean for rainfed Mediterranean cropping systems. Ital. J. Agron. 2017, 12, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Schwenke, G.D.; Peoples, M.B.; Turner, G.L.; Herridge, D.F. Does nitrogen fixation of commercial, dryland chickpea and faba bean crops in north-west New South Wales maintain or enhance soil nitrogen? Aust. J. Exp. Agric. 1998, 38, 61–70. [Google Scholar] [CrossRef]
- Sprent, J.I.; Bradfor, A.M.; Norton, C. Seasonal growth patterns in field beans (Vicia faba) as affected by population density, shading and its relationship with soil moisture. J. Agric. Sci. 1977, 88, 293–301. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Hauggaard-Nielsen, H. Faba bean in cropping systems. Field Crop. Res. 2010, 115, 203–216. [Google Scholar] [CrossRef] [Green Version]
- López-Bellido, L.; López-Bellido, R.; Fernández-García, P.; Muñoz-Romero, V.; Lopez-Bellido, F.J.; Lopez-Bellido, R. Carbon storage in a rainfed Mediterranean vertisol: Effects of tillage and crop rotation in a long-term experiment. Eur. J. Soil Sci. 2019, 71, 472–483. [Google Scholar] [CrossRef]
- McEwen, J.; Darby, R.J.; Hewitt, M.V.; Yeoman, D.P. Effects of field beans, fallow, lupins, oats, oilseed rape, peas, ryegrass, sunflowers and wheat on nitrogen residues in the soil and on the growth of a subsequent wheat crop. J. Agric. Sci. 1990, 115, 209–219. [Google Scholar] [CrossRef]
- Wahbi, S.; Maghraoui, T.; Hafidi, M.; Sanguin, H.; Oufdou, K.; Prin, Y.; Duponnois, R.; Galiana, A. Enhanced transfer of biologically fixed N from faba bean to intercropped wheat through mycorrhizal symbiosis. Appl. Soil Ecol. 2016, 107, 91–98. [Google Scholar] [CrossRef]
- Hardarson, G.; Atkins, C. Optimising biological N2 fixation by legumes in farming systems. Plant Soil 2003, 252, 41–54. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Biological nitrogen fixation, accumulation of soil nitrogen and nitrogen balance for white clover (Trifolium repens L.) and field pea (Pisum sativum L.) grown for seed. Field Crop. Res. 2000, 68, 49–59. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Management practices of antecedent leguminous and non-leguminous crop residues in relation to winter wheat yields, nitrogen uptake, soil nitrogen mineralization and simple nitrogen balance. Eur. J. Agron. 2002, 16, 295–308. [Google Scholar] [CrossRef]
- Mayer, J.; Buegger, F.; Jensen, E.S.; Schloter, M.; Heß, J. Residual nitrogen contribution from grain legumes to succeeding wheat and rape and related microbial process. Plant Soil 2003, 255, 541–554. [Google Scholar] [CrossRef]
- Shah, Z.; Shah, S.; Peoples, M.; Schwenke, G.; Herridge, D. Crop residue and fertiliser N effects on nitrogen fixation and yields of legume–cereal rotations and soil organic fertility. Field Crop. Res. 2003, 83, 1–11. [Google Scholar] [CrossRef]
- Unkovich, M.; Pate, J.S. An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crop. Res. 2000, 65, 211–228. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [Green Version]
- Whiteley, J.A.; Gonzalez, A. Biotic nitrogen fixation in the bryosphere is inhibited more by drought than warming. Oecologia 2016, 181, 1243–1258. [Google Scholar] [CrossRef]
- De Vita, P.; Di Paolo, E.; Fecondo, G.; Di Fonzo, N.; Pisante, M. No-tillage and conventional tillage effects on durum wheat yield, grain quality and soil moisture content in southern Italy. Soil Tillage Res. 2007, 92, 69–78. [Google Scholar] [CrossRef]
- Souza, A.; Colozzi-Filho, A.; Giller, K.E. The Soil Microbial Community and Soil Tillage, Soil Tillage in Agroecosystems; El Titi, A., Ed.; CRC Press: London, UK, 2003; pp. 51–81. [Google Scholar]
- Torabian, S.; Farhangi-Abriz, S.; Denton, M.D. Do tillage systems influence nitrogen fixation in legumes? A review. Soil Tillage Res. 2018, 185, 113–121. [Google Scholar] [CrossRef]
- López-Bellido, L.; López-Bellido, R.J.; Redondo, R.; Benítez, J. Faba bean nitrogen fixation in a wheat-based rotation under rainfed Mediterranean conditions: Effect of tillage system. Field Crop. Res. 2006, 98, 253–260. [Google Scholar] [CrossRef]
- Fernández, R.O.; Fernández, P.G.; Cervera, J.G.; Torres, F.P. Soil properties and crop yields after 21 years of direct drilling trials in southern Spain. Soil Tillage Res. 2007, 94, 47–54. [Google Scholar] [CrossRef]
- Ali, S.A.; Tedone, L.; Verdini, L.; Cazzato, E.; De Mastro, G. Wheat Response to No-Tillage and Nitrogen Fertilization in a Long-Term Faba Bean-Based Rotation. Agronomy 2019, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Iqbal, A.; He, L.; Zhao, Q.; Wei, S.; Ali, I.; Ullah, S.; Yan, B.; Jiang, L. Long-Term No-Tillage and Straw Retention Management Enhances Soil Bacterial Community Diversity and Soil Properties in Southern China. Agronomy 2020, 10, 1233. [Google Scholar] [CrossRef]
- Cai, L.; Guo, Z.; Zhang, J.; Gai, Z.; Liu, J.; Meng, Q.; Liu, X. No tillage and residue mulching method on bacterial community diversity regulation in a black soil region of Northeastern China. PLoS ONE 2021, 16, e0256970. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Tedone, L.; De Mastro, G. A comparison of the energy consumption of rainfed durum wheat under different management scenarios in southern Italy. Energy 2013, 61, 308–318. [Google Scholar] [CrossRef]
- Ali, S.A.; Tedone, L.; Verdini, L.; De Mastro, G. Effect of different crop management systems on rainfed durum wheat greenhouse gas emissions and carbon footprint under Mediterranean conditions. J. Clean. Prod. 2017, 140, 608–621. [Google Scholar] [CrossRef]
- López-Bellido, R.J.; López-Bellido, L.; Benítez-Vega, J.; Muñoz-Romero, V.; López-Bellido, F.J.; Redondo, R. Chickpea and faba bean nitrogen fixation in a Mediterranean rainfed Vertisol: Effect of the tillage system. Eur. J. Agron. 2011, 34, 222–230. [Google Scholar] [CrossRef]
- Indorante, S.J.; Hammer, R.D.; Koenig, P.G.; Follmer, L.R. Particle-Size Analysis by a Modified Pipette Procedure. Soil Sci. Soc. Am. J. 1990, 54, 560–563. [Google Scholar] [CrossRef]
- Klute, A. (Ed.) Water retention: Laboratory methods. In Methods of Soil Analysis: Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy, Soil Society of America: Madison, WI, USA, 1986; pp. 635–662. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar]
- A Weaver, M.; Krutz, L.J.; Zablotowicz, R.M.; Reddy, K.N. Effects of glyphosate on soil microbial communities and its mineralization in a Mississippi soil. Pest Manag. Sci. 2007, 63, 388–393. [Google Scholar] [CrossRef]
- Wilding, L.P.; Drees, L.R. Spatial Variability and Pedology. In Pedogenesis and Soil Taxonomy I: Concepts and Interactions; Wilding, L.P., Smeck, N.E., Hall, G.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 83–116. [Google Scholar]
- Pagliai, M.; Vignozzi, N.; Pellegrini, S. Soil structure and the effect of management practices. Soil Tillage Res. 2004, 79, 131–143. [Google Scholar] [CrossRef]
- Hayat, R.S.M.T.; Ali, S.; Siddique, M.T.; Chatha, T.H. Biological nitrogen fixation of summer legumes and their residual effects on subsequent rainfed wheat yield. Pak. J. Bot. 2008, 40, 711–722. [Google Scholar]
- Evans, J.; McNeill, A.M.; Unkovich, M.J.; Fettell, N.A.; Heenan, D.P. Net nitrogen balances for cool-season grain legume crops and contributions to wheat nitrogen uptake: A review. Aust. J. Exp. Agric. 2001, 41, 347. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Volpi, I.; Antichi, D.; Ambus, P.L.; Bonari, E.; O Di Nasso, N.N.; Bosco, S. Minimum tillage mitigated soil N2O emissions and maximized crop yield in faba bean in a Mediterranean environment. Soil Tillage Res. 2018, 178, 11–21. [Google Scholar] [CrossRef]
- Ruisi, P.; Giambalvo, D.; Di Miceli, G.; Frenda, A.S.; Saia, S.; Amato, G. Tillage Effects on Yield and Nitrogen Fixation of Legumes in Mediterranean Conditions. Agron. J. 2012, 104, 1459–1466. [Google Scholar] [CrossRef] [Green Version]
- Siddique, K.; Loss, S.P.; Regan, K.L.; Jettner, R.L. Adaptation and seed yield of cool season grain legumes in Mediterranean environments of south-western Australia. Aust. J. Agric. Res. 1999, 50, 375. [Google Scholar] [CrossRef]
- Giambalvo, D.; Ruisi, P.; Saia, S.; DI Miceli, G.; Frenda, A.S.; Amato, G. Faba bean grain yield, N2 fixation, and weed infestation in a long-term tillage experiment under rainfed Mediterranean conditions. Plant Soil 2012, 360, 215–227. [Google Scholar] [CrossRef]
- Mazzoncini, M.; Di Bene, C.; Coli, A.; Antichi, D.; Petri, M.; Bonari, E. Rainfed Wheat and Soybean Productivity in a Long-Term Tillage Experiment in Central Italy. Agron. J. 2008, 100, 1418–1429. [Google Scholar] [CrossRef]
- López-Bellido, R.J.; López-Bellido, L.; López-Bellido, F.J.; Castillo, J.E. Faba bean (Vicia faba L.) response to tillage and soil residual nitrogen in a continuous rotation with wheat (Triticum aestivum L.) under rainfed Mediterranean conditions. Agron. J. 2003, 95, 1253–1261. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Baddeley, J.A.; Watson, C.A. Models of biological nitrogen fixation of legumes. In Sustainable Agriculture Volume 2; Springer: Dordrecht, The Netherlands, 2011; pp. 883–905. [Google Scholar]
- Aslam, M.; Mahmood, I.A.; Peoples, M.B.; Schwenke, G.D.; Herridge, D.F. Contribution of chickpea nitrogen fixation to increased wheat production and soil organic fertility in rainfed cropping. Biol. Fertil. Soils 2003, 38, 59–64. [Google Scholar] [CrossRef]
- Turpin, E.J.; Herridge, D.F.; Robertson, M.J. Nitrogen fixation and soil nitrate interactions in field-grown chickpea (Cicer arietinum) and faba bean (Vicia faba). Aust. J. Agric. Res. 2002, 53, 599–608. [Google Scholar] [CrossRef]
- Van Kessel, C.; Hartley, C. Agricultural management of grain legumes: Has it led to an increase in nitrogen fixation? Field Crop. Res. 2000, 65, 165–181. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Peoples, M.B.; Hauggaard-Nielsen, H.; Jensen, E.S. The potential environmental benefits and risks derived from legumes in rotations. Nitrogen Fixat. Crop. Prod. 2015, 52, 349–385. [Google Scholar] [CrossRef]
- Peoples, M.B.; Unkovich, M.J.; Herridge, D.F. Measuring symbiotic nitrogen fixation by legumes. In Agronomy Monograph 52; Emerich, D.W., Krighman, H.B., Eds.; ASA: Madison, WI, USA, 2015; pp. 125–170. [Google Scholar] [CrossRef]
- De Mastro, F.; Traversa, A.; Brunetti, G.; Debiase, G.; Cocozza, C.; Nigro, F. Soil culturable microorganisms as affected by different soil managements in a two year wheat-faba bean rotation. Appl. Soil Ecol. 2020, 149, 103533. [Google Scholar] [CrossRef]
- Hughes, J.F.; Herridge, D.F. Effect of tillage on yield, nodulation and nitrogen fixation of soybeans in far north-coastal New South Wales. Aust. J. Exp. Agric. 1989, 29, 671–677. [Google Scholar] [CrossRef]


| Characteristics | M. Unit | Value |
|---|---|---|
| Total nitrogen | g kg−1 | 1.7 ± 0.22 |
| Available P | mg kg−1 | 27.6 ± 0.51 |
| Nitrate | ppm | 14.3 ± 0.39 |
| Organic matter | % | 2.8 ± 0.22 |
| Total lime | % | 8.8 ± 0.39 |
| Exchangeable Na (ESP) | % | 1.9 ± 0.15 |
| pH | - | 7.72 ± 0.17 |
| Exchangeable K2O | mg kg−1 | 227 ± 5.0 |
| Total carbonate | g kg−1 | 15.0 ± 1.2 |
| Active carbonate | g kg−1 | 5.0 ± 0.18 |
| Soil salinity (ECe; saturated paste extract method) | dS m−1 | 0.95 ± 0.03 |
| Year | Treatment | Flowering | Maturity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Faba Bean | Wheat | Faba Bean | Wheat | ||||||
| Total Aboveground Biomass (t ha−1) | Grain (t ha−1) | Straw (t ha−1) | Harvest index | Grain (t ha−1) | Straw (t ha−1) | Total Biomass (t ha−1) | |||
| 2013 | CT | 4.64 | 5.20 | 3.29 | 6.42 | 0.34 | 5.88 | 5.60 | 11.48 |
| RT | 4.65 | 5.30 | 2.36 | 6.64 | 0.26 | 5.86 | 5.15 | 11.01 | |
| NT | 4.58 | 5.49 | 2.84 | 6.92 | 0.29 | 6.40 | 4.43 | 10.83 | |
| 2014 | CT | 4.83 | 4.71 | 3.27 | 6.13 | 0.35 | 4.74 | 8.11 | 12.84 |
| RT | 5.02 | 5.16 | 2.75 | 5.78 | 0.32 | 4.89 | 7.79 | 12.67 | |
| NT | 5.26 | 5.49 | 2.94 | 6.33 | 0.32 | 6.56 | 7.95 | 14.51 | |
| Mean tillage (T) | |||||||||
| CT | 4.74 a | 4.95 b | 3.28 a | 6.27 a | 0.34 a | 5.31 b | 6.85 a | 12.16 ab | |
| RT | 4.84 a | 5.23 ab | 2.55 b | 6.21 a | 0.29 b | 5.37 b | 6.47 ab | 11.84 b | |
| NT | 4.92 a | 5.49 a | 2.89 ab | 6.63 a | 0.30 b | 6.48 a | 6.19 b | 12.67 a | |
| ns | * | * | ns | * | ** | * | * | ||
| Mean year (Y) | |||||||||
| 2013 | 4.62 a | 5.33 a | 2.83 a | 6.66 a | 0.30 b | 6.05 a | 5.06 b | 11.11 a | |
| 2014 | 5.04 a | 5.12 a | 2.99 a | 6.08 b | 0.33 a | 5.39 b | 7.95 a | 13.34 b | |
| ns | ns | ns | * | * | ** | ** | * | ||
| Interaction | |||||||||
| T × Y | ns | ns | ns | ns | ns | * | * | ns | |
| Total mean | 4.83 | 5.22 | 2.91 | 6.37 | 0.31 | 5.72 | 6.50 | 12.22 | |
| Treatment | Flowering | Maturity | |||||
|---|---|---|---|---|---|---|---|
| Year | Faba Bean | Wheat | Faba Bean | Wheat | |||
| Total Aboveground Biomass | Grain | Straw | Grain | Straw | |||
| 2013 | CT | 5.23 | 3.42 | 4.84 | 1.23 | 2.60 | 0.60 |
| RT | 4.31 | 3.32 | 4.73 | 1.39 | 2.45 | 0.79 | |
| NT | 5.06 | 2.06 | 4.94 | 1.27 | 1.73 | 0.34 | |
| 2014 | CT | 3.80 | 2.20 | 4.69 | 1.81 | 2.37 | 0.90 |
| RT | 3.23 | 2.12 | 4.80 | 1.44 | 2.38 | 0.94 | |
| NT | 3.22 | 1.89 | 4.81 | 1.48 | 2.12 | 0.50 | |
| Mean tillage (T) | |||||||
| CT | 4.51 a | 2.81 a | 4.77 a | 1.52 a | 2.49 a | 0.75 b | |
| RT | 3.77 b | 2.72 a | 4.77 a | 1.41 a | 2.41 a | 0.86 a | |
| NT | 4.14 ab | 1.97 b | 4.87 a | 1.38 a | 1.93 b | 0.42 c | |
| ** | ** | ns | ns | * | ** | ||
| Mean year (Y) | |||||||
| 2013 | 4.86 a | 2.94 a | 4.84 a | 1.29 a | 2.26 a | 0.58 b | |
| 2014 | 3.42 b | 2.07 b | 4.77 a | 1.58 a | 2.29 a | 0.78 a | |
| ** | * | ns | ns | ns | ns | ||
| Interaction | |||||||
| T × Y | * | ns | ns | ns | ns | ns | |
| Total mean | 4.14 | 2.50 | 4.80 | 1.09 | 2.28 | 0.68 | |
| Year | Treatment | Flowering | Maturity | ||||
|---|---|---|---|---|---|---|---|
| Faba Bean | Wheat | Faba Bean | Wheat | ||||
| Total Aboveground Biomass (kg ha−1) | Grain (kg ha−1) | Straw (kg ha−1) | Grain (kg ha−1) | Straw (kg ha−1) | |||
| 2013 | CT | 242.5 | 177.9 | 159.3 | 78.6 | 252.7 | 35.2 |
| RT | 200.1 | 176.0 | 111.5 | 92.1 | 220.2 | 46.3 | |
| NT | 231.5 | 113.2 | 140.2 | 88.1 | 169.4 | 22.0 | |
| 2014 | CT | 183.7 | 103.4 | 153.4 | 111.2 | 223.2 | 42. |
| RT | 162.3 | 109.7 | 132.1 | 83.4 | 203.2 | 45.7 | |
| NT | 169.1 | 103.6 | 141.5 | 93.6 | 196.9 | 32.7 | |
| Mean tillage (T) | |||||||
| CT | 213.1 a | 140.7 a | 156.4 a | 94.9 a | 227.9 a | 38.8 b | |
| RT | 181.2 b | 142.8 a | 121.8 b | 87.8 a | 211.7 b | 46.0 a | |
| NT | 200.3 a | 108.4 b | 140.9 ab | 90.9 a | 203.2 b | 27.3 c | |
| ** | ** | * | ns | ** | *** | ||
| Mean year (Y) | |||||||
| 2013 | 224.7 a | 155.7 a | 137.0 b | 86.3 a | 214.1 a | 34.5 b | |
| 2014 | 171.7 b | 105.6 b | 142.4 a | 96.1 a | 207.8 a | 40.3 a | |
| ** | *** | * | ns | ns | * | ||
| Interaction | |||||||
| T × Y | * | ** | ns | ns | ns | * | |
| Total mean | 198.2 | 130.6 | 139.7 | 91.2 | 210.9 | 37.4 | |
| Year | Treatments | Flowering | Maturity |
|---|---|---|---|
| Ndfa (%) | |||
| 2013 | CT | 64.6 | 79.3 |
| RT | 63.5 | 73.7 | |
| NT | 72.9 | 79.3 | |
| 2014 | CT | 56.8 | 80.8 |
| RT | 61.8 | 79.6 | |
| NT | 66.4 | 79.3 | |
| Mean tillage (T) | |||
| CT | 60.7 b | 80.0 a | |
| RT | 62.7 b | 76.7 b | |
| NT | 69.6 a | 79.3 a | |
| ** | * | ||
| Mean year (Y) | |||
| 2013 | 67.0 a | 77.4 a | |
| 2014 | 61.7 b | 79.9 a | |
| ** | ns | ||
| Interaction | |||
| T × Y | * | ns | |
| Total mean | 64.3 | 78.7 | |
| Year | Treatments | Flowering | Maturity | Mean |
|---|---|---|---|---|
| 2013 | CT | 147.5 | 186.7 | 167.1 |
| RT | 119.7 | 146.9 | 133.3 | |
| NT | 159.5 | 178.0 | 168.8 | |
| 2014 | CT | 115.9 | 213.7 | 164.8 |
| RT | 111.4 | 175.1 | 143.3 | |
| NT | 119.2 | 186.4 | 152.8 | |
| Mean tillage (T) | ||||
| CT | 131.7 a | 200.2 a | 166.0 a | |
| RT | 115.6 b | 161.0 b | 138.3 b | |
| NT | 139.4 a | 182.2 ab | 160.8 a | |
| ** | ** | * | ||
| Mean year (Y) | ||||
| 2013 | 142.2 a | 170.6 a | 156.4 a | |
| 2014 | 115.5 b | 191.8 a | 153.60 a | |
| *** | ns | ** | ||
| Interaction | ||||
| T × Y | * | ns | ns | |
| Total mean | 128.2 | 183.6 | 155.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedone, L.; Alhajj Ali, S.; De Mastro, G. The Effect of Tillage on Faba Bean (Vicia faba L.) Nitrogen Fixation in Durum Wheat ((Triticum turgidum L. subsp. Durum (Desf))-Based Rotation under a Mediterranean Climate. Agronomy 2023, 13, 105. https://doi.org/10.3390/agronomy13010105
Tedone L, Alhajj Ali S, De Mastro G. The Effect of Tillage on Faba Bean (Vicia faba L.) Nitrogen Fixation in Durum Wheat ((Triticum turgidum L. subsp. Durum (Desf))-Based Rotation under a Mediterranean Climate. Agronomy. 2023; 13(1):105. https://doi.org/10.3390/agronomy13010105
Chicago/Turabian StyleTedone, Luigi, Salem Alhajj Ali, and Giuseppe De Mastro. 2023. "The Effect of Tillage on Faba Bean (Vicia faba L.) Nitrogen Fixation in Durum Wheat ((Triticum turgidum L. subsp. Durum (Desf))-Based Rotation under a Mediterranean Climate" Agronomy 13, no. 1: 105. https://doi.org/10.3390/agronomy13010105











