Effect of Ustilago maydis on the Nutritive Value and Aerobic Deterioration of Maize Silage
Abstract
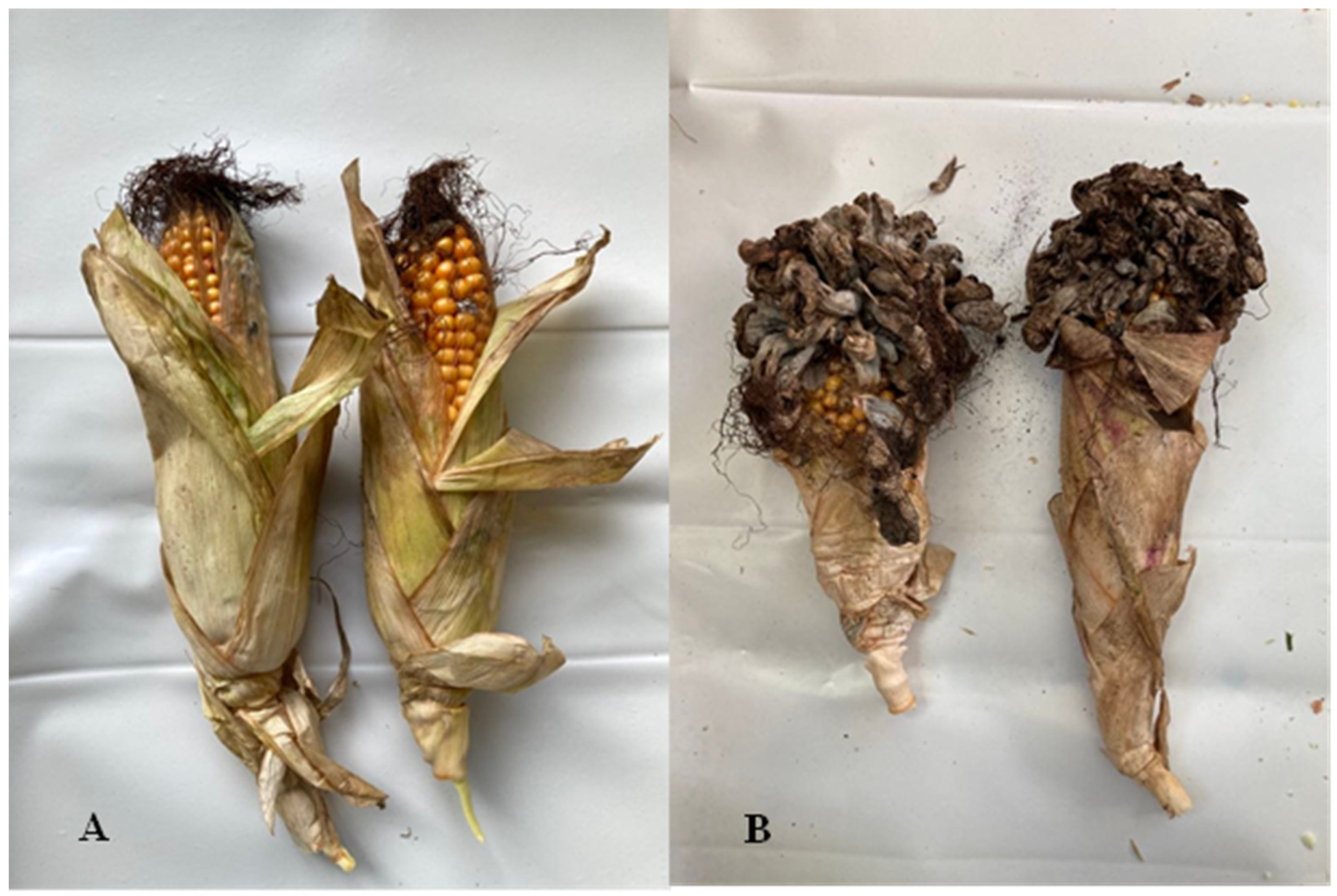
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Analysis
2.3. Statistical Analysis
3. Results
3.1. Chemical Composition of the Harvested Maize Forage
3.2. Nutritive Value of the Silage at the Opening and during Aerobic Exposure
3.3. Temperature of the Silage at the Opening and during Aerobic Exposure
3.4. Gas Concentration Measurements after the Opening of Samples
4. Discussion
4.1. Chemical Composition of the Harvested Maize Forage
4.2. Nutritive Value of the Silage at the Opening and during Aerobic Exposure
4.3. Temperature of the Silage at the Opening and during Aerobic Exposure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, H.; Yan, Y.; Li, X.; Li, X.; Shuai, Y.; Feng, G.; Ran, Q.; Cai, Y.; Li, Y.; Zhang, X. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed. Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Galicia-García, P.R.; Silva-Rojas, H.V.; Mendoza-Onofre, L.E.; Zavaleta-Mancera, H.A.; Córdova-Téllez, L.; Espinosa-Calderón, A. Selection of aggressive pathogenic and solopathogenic strains of Ustilago maydis to improve Huitlacoche production. Acta Bot. Bras. 2016, 30, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Frommer, D.; Veres, S.; Radócz, L. Susceptibility of stem infected sweet corn hybrids to common smut disease. Acta Agrar. Debr. 2018, 74, 55–57. [Google Scholar] [CrossRef]
- Morrison, E.N.; Emery, R.J.N.; Saville, B.J. Fungal derived cytokinins are necessary for normal Ustilago maydis infection of maize. Plant Pathol. 2017, 66, 726–742. [Google Scholar] [CrossRef]
- Abbas, H.K.; Shier, W.T.; Plasencia, J.; Weaver, M.A.; Bellaloui, N.; Kotowicz, J.K.; Butler, A.M.; Accinelli, C.; de la Torre-Hernandez, M.E.; Zablotowicz, R.M. Mycotoxin contamination in corn smut (Ustilago maydis) galls in the field and in the commercial food products. Food Control 2017, 71, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Lambie, S.C.; Kretschmer, M.; Croll, D.; Haslam, T.M.; Kunst, L.; Klose, J.; Kronstad, J.W. The putative phospholipase Lip2 counteracts oxidative damage and influences the virulence of Ustilago maydis. Mol. Plant. Pathol. 2017, 18, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Potkański, A.; Grajewski, J.; Twarużek, M.; Selwet, M.; Miklaszewska, B.; Błajet-Kosicka, A.; Szumacher-Strabel, M.; Cieślak, A.; Raczkowska-Werwińska, K. Chemical composition, fungal microflora and mycotoxin content in maize silages infected by smut (Ustilago maydis) and the effect of biological and chemical additives on silage aerobic stability. J. Anim. Feed Sci. 2010, 19, 130–142. [Google Scholar] [CrossRef]
- Aydoğdu, M.; Boyraz, N.; Kaya, Y. Effect on Yield Losses on Maize (Zea mays L.) Caused by Smut Disease (Ustilago maydis (DC) Corda). J. Turk. Phytopath. 2015, 44, 23–30. Available online: https://www.researchgate.net/publication/322831486 (accessed on 20 April 2022).
- Dolezal, P.; Nedelník, J.; Skládanka, J.; Moravcová, H.; Vyskocil, I.; Dvorácková, J.; Kalhotka, L.; Zeman, L.; Havlícek, Z.; Postulka, R.; et al. Quality of maize silage fermentation process infected with Ustilago maydis. In Proceedings of the International Symposium on Forage Quality and Conservation, São Pedro, Brazil, 15–18 November 2011; Zopollatto, M., Daniel, J.L.P., Nussio, L.G., de Sá Neto, A., Eds.; Available online: https://www.isfqcbrazil.com.br/proceedings/2011/quality-of-maize-silage-fermentation-process-infected-with-ustilago-maydis-84.pdf (accessed on 21 April 2022).
- Cole, N.A.; Rush, C.M.; Greene, L.W. Influence of Corn Smut on the Palatability and Digestibility of Corn Silage. Prof. Anim. Sci. 2001, 17, 287–294. [Google Scholar] [CrossRef]
- Smith, D.R.; White, D.G. Diseases of Corn. In Corn and Corn Improvement, 3rd ed.; Sprague, G.F., Dudley, J.W., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1988; p. 715. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Pahlow, G.; Hünting, K. Gärungsbiologische Grundlagen und biochemische Prozesse der Silagebereitung. In Praxishandbuch Futter- und Substratkonservierung, 8th ed.; Deutsche Landwirtschafts-Gesellschaft e.V: Frankfurt am Main, Germany, 2011; pp. 73–82. [Google Scholar]
- European and Mediterranean Plant Protection Organization. PP 1/019(4) Seed-Borne Cereal Fungi; European and Mediterranean Plant Protection Organization: Paris, France, 2020. [Google Scholar]
- Ferrero, F.; Tabacco, E.; Piano, S.; Casale, M.; Borreani, G. Temperature during conservation in laboratory silos affects fermentation profile and aerobic stability of corn silage treated with Lactobacillus buchneri, Lactobacillus hilgardii, and their combination. J. Dairy Sci. 2021, 104, 1696–1713. [Google Scholar] [CrossRef] [PubMed]
- Serva, L.; Marchesini, G.; Chinello, M.; Contiero, B.; Tenti, S.; Mirisola, M.; Grandis, D.; Andrighetto, I. Use of near-infrared spectroscopy and multivariate approach for estimating silage fermentation quality from freshly harvested maize. Ital. J. Anim. Sci. 2021, 20, 859–871. [Google Scholar] [CrossRef]
- Gerlach, K.; Roß, F.; Weiß, K.; Büscher, W.; Südekum, K.-H. Changes in maize silage fermentation products during aerobic deterioration and effects on dry matter intake by goats. Agric. Food Sci. 2013, 22, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Weiermüller, J.; Akermann, A.; Laudensack, W.; Chodorski, J.; Blank, L.M.; Ulber, R. Brewers’ spent grain as carbon source for itaconate production with engineered Ustilago maydis. Bioresour. Technol. 2021, 336, 125262. [Google Scholar] [CrossRef]
- Ruan, X.; Ma, L.; Zhang, Y.; Wang, Q.; Gao, X. Dissection of the Complex Transcription and Metabolism Regulation Networks Associated with Maize Resistance to Ustilago maydis. Genes 2021, 12, 1789. [Google Scholar] [CrossRef]
- Cuervo-Parra, J.A.; Pérez España, V.H.; Zavala-González, E.A.; Peralta-Gil, M.; Aparicio Burgos, J.E.; Romero-Cortes, T. Trichoderma Asperellum strains as potential biological control agents against Fusarium verticillioides and Ustilago maydis in maize. Biocontrol Sci. Technol. 2022, 32, 624–647. [Google Scholar] [CrossRef]
- Richter, G.H.; Flachowsky, G.; Schneider, A.; Wirth, R.; Schwartze, J.; Jahreis, G. Investigations about the influence of blister smut (Ustilago zeae) on feed value of maize for silage making. Wirtsch. Futter 1994, 40, 161–169. Available online: https://agris.fao.org/agris-search/search.do?recordID=DE95A0964 (accessed on 21 April 2022).
- Brüning, D.; Gerlach, K.; Weiß, K.; Südekum, K.-H. Effect of compaction, delayed sealing and aerobic exposure on maize silage quality and on formation of volatile organic compounds. Grass Forage Sci. 2018, 73, 53–66. [Google Scholar] [CrossRef]
- Yuan, X.; Guo, G.; Wen, A.; Desta, S.T.; Wang, J.; Wang, Y.; Shao, T. The effect of different additives on the fermentation quality, in vitro digestibility and aerobic stability of a total mixed ration silage. Anim. Feed Sci. Technol. 2015, 207, 41–50. [Google Scholar] [CrossRef]
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry matter and nutritional losses during aerobic deterioration of corn and sorghum silages as influenced by different lactic acid bacteria inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Filya, I.; Sucu, E. The effects of lactic acid bacteria on the fermentation, aerobic stability and nutritive value of maize silage. Grass Forage Sci. 2010, 65, 446–455. [Google Scholar] [CrossRef]
- Filya, I. Nutritive value and aerobic stability of whole crop maize silage harvested at four stages of maturity. Anim. Feed Sci. Technol. 2004, 116, 141–150. [Google Scholar] [CrossRef]
- Venslovas, E.; Merkevičiūtė-Venslovė, L.; Mankevičienė, A.; Kochiieru, Y.; Šlepetienė, A.; Cesevičienė, J. The prevalence of mycotoxins and their relation to nutrient composition of maize and grass silage. Zemdirbyste-Agriculture 2021, 108, 147–152. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunade, I.M.; Arriola, K.G.; Jiang, Y.; Driver, J.P.; Staples, C.R.; Adesogan, A.T. Effects of 3 sequestering agents on milk aflatoxin M1 concentration and the performance and immune status of dairy cows fed diets artificially contaminated with aflatoxin B1. J. Dairy Sci. 2016, 99, 6263–6273. [Google Scholar] [CrossRef] [PubMed]
- Ashbell, G.; Weinberg, Z.G.; Hen, Y.; Filya, I. The effects of temperature on the aerobic stability of wheat and corn silages. J. Ind. Microbiol. Biotechnol. 2002, 28, 261–263. [Google Scholar] [CrossRef]
- Andrieu, B.; Demey, V. On-farm corn silage investigation: Multi-analyses on silage practices, silage quality and its effect on aerobic stability. In Proceedings of the XVII International Silage Conference, Piracicaba, Brazil, 1–3 July 2015; Daniel, J.L.P., Morais, G., Junges, D., Nussio, L.G., Eds.; Available online: https://www.isfqcbrazil.com.br/proceedings/2015/Proceedings-of-the-XVII-International-Silage-Conference-Brazil-2015.pdf (accessed on 10 May 2022).

| Treatment | DM | CP | CA | Starch | CF | NDF | ADF |
|---|---|---|---|---|---|---|---|
| g kg−1 | |||||||
| Smut-free maize | 333.1 ± 3.7 b | 71.3 ± 1.8 a | 40.8 ± 0.8 a | 257.4 ± 7.2 c | 216.6 ± 4.4 a | 399.4 ± 8.2 b | 221.4 ± 4.4 b |
| Smut-infected maize (50%) | 329.1 ± 5.2 b | 71.1 ± 1.9 a | 41.1 ± 1.1 a | 225.4 ± 10.6 b | 217.5 ± 13.7 a | 375.7 ± 9.4 a | 202.3 ± 7.5 a |
| Smut-infected maize (100%) | 308.6 ± 5.0 a | 70.5 ± 1.7 a | 48.5 ± 2.1 b | 172.4 ± 10.9 a | 243.0 ± 6.7 b | 380.7 ± 10.0 ab | 201.4 ± 2.9 a |
| Item | Treatment | Days of Aerobic Exposure | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 28 | ||
| DM g kg−1 | Smut-free maize silage | 301.2 ± 3.8 aA | 335.1 ± 5.1 b | 351.0 ± 5.7 bC | 404.0 ± 3.7 cC | 496.0 ± 13.1 dC |
| Smut-infected maize silage (50%) | 314.0 ± 7.9 aAB | 327.7 ± 8.6 ab | 328.0 ± 6.7 abB | 317.0 ± 6.1 aB | 344.0 ± 5.6 bB | |
| Smut-infected maize silage (100%) | 324.8 ± 5.9 bB | 328.0 ± 7.0 b | 306.0 ± 5.4 aA | 301.0 ± 8.5 aA | 289.0 ± 6.2 aA | |
| pH | Smut-free maize silage | 4.10 ± 0.04 A | 4.00 ± 0.05 A | 4.10 ± 0.08 A | 4.02 ± 0.05 A | 4.10 ± 0.05 A |
| Smut-infected maize silage (50%) | 4.10 ± 0.04 A | 4.10 ± 0.04 A | 4.12 ± 0.05 A | 4.12 ± 0.05 A | 4.20 ± 0.05 A | |
| Smut-infected maize silage (100%) | 4.30 ± 0.04 aB | 4.30 ± 0.03 aB | 4.30 ± 0.04 aB | 4.25 ± 0.04 aB | 5.30 ± 0.04 bB | |
| CP g kg−1 | Smut-free maize silage | 93.8 ± 3.2 aB | 93.7 ± 1.0 a | 88.8 ± 1.2 a | 92.4 ± 0.4 a | 99.8 ± 3.1 bA |
| Smut-infected maize silage (50%) | 93.5 ± 0.3 B | 89.3 ± 4.5 | 92.8 ± 0.8 | 91.6 ± 1.1 | 90.0 ± 2.7 A | |
| Smut-infected maize silage (100%) | 87.2 ± 2.6 aA | 94.8 ± 0.9 a | 91.4 ± 3.1 a | 90.5 ± 1.8 a | 121.7 ± 6.9 bB | |
| CL g kg−1 | Smut-free maize silage | 24.9 ± 0.4 A | 24.5 ± 0.3 A | 23.3 ± 2.5 A | 22.7 ± 0.3 A | 23.1 ± 0.9 |
| Smut-infected maize silage (50%) | 26.8 ± 0.8 bB | 24.8 ± 0.3 abA | 25.4 ± 0.6 abA | 25.6 ± 1.2 abB | 24.7 ± 0.7 a | |
| Smut-infected maize silage (100%) | 30.7 ± 0.5 bC | 27.7 ± 0.7 bB | 35.2 ± 1.6 cB | 30.2 ± 0.8 bC | 23.5 ± 2.1 a | |
| CA g kg−1 | Smut-free maize silage | 54.1 ± 5.7 | 52.9 ± 1.5 | 55.7 ± 2.8 | 55.3 ± 1.9 | 55.0 ± 1.3 B |
| Smut-infected maize silage (50%) | 54.1 ± 1.2 | 50.3 ± 2.1 | 52.9 ± 0.7 | 53.1 ± 4.8 | 48.4 ± 3.4 A | |
| Smut-infected maize silage (100%) | 46.8 ± 2.8 ab | 53.0 ± 3.7 b | 53.5 ± 2.6 b | 49.8 ± 4.6 ab | 44.2 ± 1.3 aA | |
| Starch g kg−1 | Smut-free maize silage | 245.9 ± 7.9 bB | 202.3 ± 15.8 aB | 222.7 ± 11.7 abC | 216.9 ± 1.5 aB | 217.9 ± 10.7 abB |
| Smut-infected maize silage (50%) | 233.4 ± 9.9 bB | 181.4 ± 9.1 aB | 188.7 ± 1.2 aB | 196.2 ± 14.0 aB | 200.1 ± 20.2 abAB | |
| Smut-infected maize silage (100%) | 162.5 ± 18.2 bA | 117.3 ± 8.4 aA | 139.8 ± 2.7 abA | 159.1 ± 8.0 bA | 166.7 ± 15.3 bA | |
| CF g kg−1 | Smut-free maize silage | 188.0 ± 5.6 | 200.3 ± 8.4 B | 194.1 ± 5.0 | 197.0 ± 10.3 | 193.4 ± 4.6 B |
| Smut-infected maize silage (50%) | 192.9 ± 6.7 ab | 181.2 ± 7.1 aA | 198.1 ± 3.4 b | 199.3 ± 1.9 b | 186.0 ± 6.8 abB | |
| Smut-infected maize silage (100%) | 199.8 ± 6.8 bc | 211.8 ± 5.1 cB | 205.4 ± 5.2 c | 189.9 ± 4.8 b | 138.0 ± 1.2 aA | |
| NDF g kg−1 | Smut-free maize silage | 390.0 ± 14.1 A | 409.2 ± 5.2 A | 397.4 ± 7.8 A | 400.3 ± 8.4 A | 396.0 ± 3.7 A |
| Smut-infected maize silage (50%) | 403.2 ± 9.6 A | 395.0 ± 14.4 A | 416.5 ± 5.8 B | 406.3 ± 5.1 A | 402.5 ± 4.2 A | |
| Smut-infected maize silage (100%) | 435.3 ± 7.4 B | 466.2 ± 4.5 B | 448.4 ± 4.4 C | 430.5 ± 8.9 B | 466.4 ± 12.1 B | |
| ADF g kg−1 | Smut-free maize silage | 210.2 ± 7.0 | 222.4 ± 9.4 B | 217.2 ± 5.7 | 216.2 ± 12.4 B | 212.9 ± 4.4 |
| Smut-infected maize silage (50%) | 202.2 ± 9.4 | 190.4 ± 9.1 A | 209.7 ± 5.4 | 210.6 ± 2.1 AB | 193.3 ± 8.9 | |
| Smut-infected maize silage (100%) | 204.2 ± 15.1 bc | 227.6 ± 6.9 cB | 217.9 ± 4.2 c | 192.9 ± 9.4 bA | 160.1 ± 6.0 aA | |
| ME MJ/kg | Smut-free maize silage | 10.87 ± 0.14 | 10.71 ± 0.11 A | 10.77 ± 0.06 B | 10.74 ± 0.16 | 10.79 ± 0.07 A |
| Smut-infected maize silage (50%) | 10.88 ± 0.07 abc | 11.01 ± 0.12 cB | 10.74 ± 0.05 abB | 10.73 ± 0.08 a | 10.96 ± 0.08 bcB | |
| Smut-infected maize silage (100%) | 10.71 ± 0.09 ab | 10.50 ± 0.10 aA | 10.61 ± 0.03 aA | 10.89 ± 0.11 b | 11.67 ± 0.02 cB | |
| NEL MJ/kg | Smut-free maize silage | 6.57 ± 0.08 | 6.45 ± 0.08 AB | 6.50 ± 0.05 B | 6.47 ± 0.12 | 6.51 ± 0.05 A |
| Smut-infected maize silage (50%) | 6.55 ± 0.06 | 6.63 ± 0.13 B | 6.47 ± 0.04 B | 6.46 ±0.04 | 6.61 ± 0.07 A | |
| Smut-infected maize silage (100%) | 6.45 ± 0.07 bc | 6.27 ± 0.06 aA | 6.37 ± 0.02 abA | 6.57 ± 0.07 c | 7.15 ± 0.02 dB | |
| Treatment | CH4 | O2 | CO2 | H2S |
|---|---|---|---|---|
| ppm | ||||
| Smut-free silage | 3.6 ± 2.7 | 0 | 303.2 ± 121.5 | 14 ± 5.5 |
| Smut-infected silage (50%) | 1.8 ± 2.6 | 0 | 430.8 ± 181.5 | 8 ± 4.5 |
| Smut-infected silage (100%) | 2.6 ± 2.5 | 0 | 531.0 ± 173.6 | 8 ± 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkevičiūte-Venslovė, L.; Venslovas, E.; Mankevičienė, A.; Šlepetienė, A.; Cesevičienė, J. Effect of Ustilago maydis on the Nutritive Value and Aerobic Deterioration of Maize Silage. Agronomy 2023, 13, 111. https://doi.org/10.3390/agronomy13010111
Merkevičiūte-Venslovė L, Venslovas E, Mankevičienė A, Šlepetienė A, Cesevičienė J. Effect of Ustilago maydis on the Nutritive Value and Aerobic Deterioration of Maize Silage. Agronomy. 2023; 13(1):111. https://doi.org/10.3390/agronomy13010111
Chicago/Turabian StyleMerkevičiūte-Venslovė, Lauksmė, Eimantas Venslovas, Audronė Mankevičienė, Alvyra Šlepetienė, and Jurgita Cesevičienė. 2023. "Effect of Ustilago maydis on the Nutritive Value and Aerobic Deterioration of Maize Silage" Agronomy 13, no. 1: 111. https://doi.org/10.3390/agronomy13010111
APA StyleMerkevičiūte-Venslovė, L., Venslovas, E., Mankevičienė, A., Šlepetienė, A., & Cesevičienė, J. (2023). Effect of Ustilago maydis on the Nutritive Value and Aerobic Deterioration of Maize Silage. Agronomy, 13(1), 111. https://doi.org/10.3390/agronomy13010111









