Contrasting Phenotypic Variability of Life-History Traits of Two Feral Populations of Macrolophus pygmaeus (Hemiptera: Miridae) under Two Alternative Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Life-History Traits
2.2.1. Immature Development
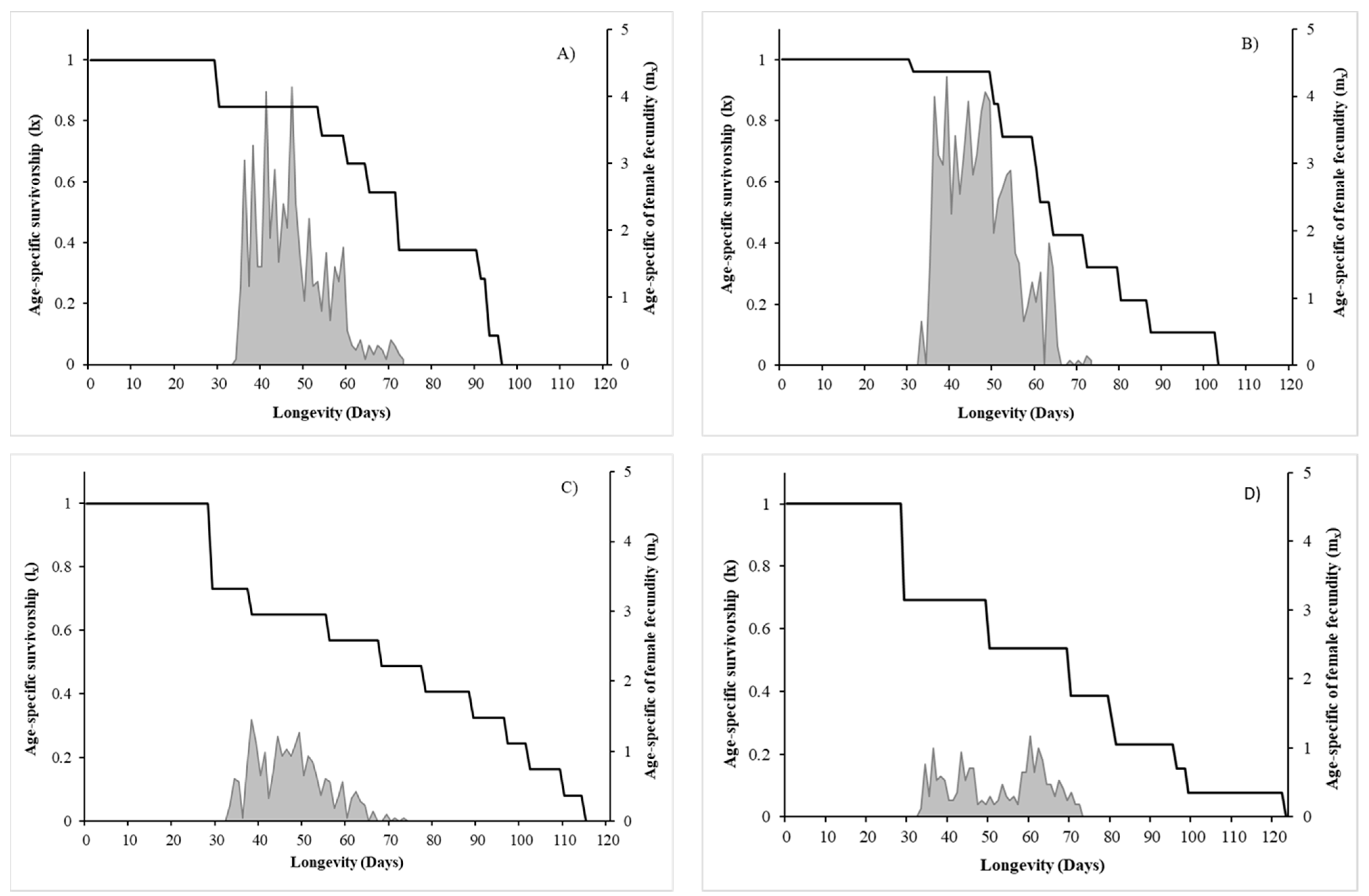
2.2.2. Longevity, Sex-Ratio and Reproductive Performance
2.3. Population Growth Parameters
2.4. Statistical Analysis
3. Results
3.1. Life-History Traits
3.1.1. Immature Development
3.1.2. Longevity, Sex-Ratio and Reproductive Performance
3.2. Population Growth Parameters
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soares, A.O.; Honěk, A.; Martinkova, Z.; Brown, P.M.J.; Borges, I. Can native geographical range, dispersal ability and development rates predict the successful establishment of alien ladybird (Coleoptera: Coccinellidae) species in Europe? Front. Ecol. Evol. 2018, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Rondoni, G.; Borges, I.; Collatz, J.; Conti, E.; Costamagna, A.; Dumont, F.; Evans, E.W.; Grez, A.A.; Howe, A.G.; Lucas, E.; et al. Exotic ladybirds for biological control of herbivorous insects—A review. Entomol. Exp. Appl. 2021, 169, 6–27. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Biondi, A.; Narciso, R.; Guedes, C.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond—The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Figueiredo, E.; Rodrigues, S.; Payer, R.; Mexia, A. Situación actual de Tuta absoluta en Portugal. Phytoma España 2010, 217, 118–120. [Google Scholar]
- Matos, T.; Figueiredo, E.; Mexia, A. Armadilhas de feromona sexual com luz para captura em massa de Tuta absoluta (Meyrick), sim ou não? Rev. De Ciências Agrárias 2012, 35, 282–286. [Google Scholar]
- Payer, R.; Figueiredo, E.; Mexia, A. Evaluation of parasitism and predation of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) by Diglyphus isaea (Walker, 1838) (Hymenoptera: Eulophidae). SHIL. Rev. lepidopterol. 2015, 43, 173–179. [Google Scholar]
- DSA (Direção de Serviços de Agricultura). Relatório de Atividades DSA 2013. 2014. Available online: http://servicos.srrn.azores.gov.pt/grastore/DRADR/RelatorioAtividades2013.pdf (accessed on 1 September 2022).
- Vieira, V. A traça-do-tomateiro Tuta absoluta (Meyrick, 1917) nas ilhas dos Açores (Lepidoptera: Gelechiidae). SHIL. Rev. lepidopterol. 2016, 44, 607–613. [Google Scholar]
- Fauvel, G.; Malausa, J.C.; Kaspar, B. Laboratory studies on the main biological characteristics of Macrolophus caliginosus (Heteroptera: Miridae). Entomophaga 1987, 32, 529–543. [Google Scholar] [CrossRef]
- Alvarado, P.; Balta, O.; Alomar, O. Efficiency of four Heteroptera as predators of Aphis gossypii and Macrosiphum euphorbiae (Hom.: Aphididae). Entomophaga 1997, 42, 215–226. [Google Scholar] [CrossRef]
- Barnadas, I.; Gabarra, R.; Albajes, R. Predatory capacity of two mirid bugs preying on Bemisia tabaci. Entomol. Entomol. Exp. Appl. 1998, 86, 215–219. [Google Scholar] [CrossRef]
- Riudavets, J.; Castañé, C. Identification and evaluation of native predators of Frankliniella occidentalis (Thysanoptera: Thripidae) in the Mediterranean. Environ. Entomol. 1998, 27, 86–93. [Google Scholar] [CrossRef]
- Margaritopoulos, J.T.; Tsitsipis, J.A.; Perdikis, D.C. Biological characteristics of the mirids Macrolophus costalis and Macrolophus pygmaeus preying on the tobacco form of Myzus persicae (Hemiptera: Aphididade). Bull. Entomol. Res. 2003, 93, 39–45. [Google Scholar] [CrossRef]
- Perdikis, D.; Kapaxidi, E.; Papadoulis, G. Biological control of insect and mite pests in greenhouse solanaceous crops. Eur. J. Plant Sci. Biotechnol. 2008, 2, 125–144. [Google Scholar]
- Arnó, J.; Sorribas, R.; Prat, M.; Matas, M.; Pozo, C.; Rodríguez, D.; Garreta, A.; Gómez, A.; Gabarra, R. Tuta absoluta, a new pest in IPM tomatoes in the northeast of Spain. IOBC/WPRS Bull. 2009, 49, 203–208. [Google Scholar]
- Calvo, J.; Blockmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- Urbaneja, A.; Montón, H.; Mollá, O. Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J. Appl. Entomol. 2009, 133, 292–296. [Google Scholar] [CrossRef]
- Urbaneja, A.; Gonzalez-Cabrera, J.; Arnó, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Kerzhner, I.M.; Josifov, M. Cimicomorpha II: Miridae. In Catalogue of the Heteroptera of the Palaearctic Region; Aukema, B., Rieger, C., Eds.; Netherlands Entomological Society: Wageningen, The Netherlands, 1999; p. 577. [Google Scholar]
- Sanchez, J.A.; Spina, M.L.; Perera, O.P. Analysis of the population structure of Macrolophus pygmaeus (Rambur) (Hemiptera: Miridae) in the Palaearctic region using microsatellite markers. Ecol. Evol. 2012, 2, 3145–3159. [Google Scholar] [CrossRef] [PubMed]
- Streito, J.-C.; Clouet, C.; Hamdi, F.; Gauthier, N. Population genetic structure of the biological control agent Macrolophus pygmaeus in Mediterranean agroecosystems. Insect Sci. 2017, 24, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Mollá, O.; Biondi, A.; Alonso-Valiente, M.; Urbaneja, A. A comparative life history study of two mirid bugs preying on Tuta absoluta and Ephestia kuehniella eggs on tomato crops: Implications for biological control. BioControl 2014, 57, 175–183. [Google Scholar] [CrossRef]
- Sylla, S.; Brévault, T.; Diarra, K.; Bearez, P.; Desneux, N. Life-history traits of Macrolophus pygmaeus with different prey foods. PLoS ONE 2016, 11, e0166610. [Google Scholar] [CrossRef]
- Hill, M.G.; Mauchline, N.A.; Hall, A.J.; Stannard, K.A. Life table parameters of two armoured scale insect (Hemiptera: Diaspididae) species on resistant and susceptible kiwifruit (Actinidia spp.) germplasm. N. Z. J. Crop Hortic. Sci. 2009, 37, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Borges, I.; Soares, A.O.; Hemptinne, J.-L. Contrasting population growth parameters of the aphidophagous Scymnus nubilus and the coccidophagous Nephus reunioni. BioControl 2013, 58, 351–357. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, W.; Sun, Y.; Feng, J. Development of insect life tables: Comparison of two demographic methods of Delia antiqua (Diptera: Anthomyiidae) on different hosts. Sci. Rep. 2017, 7, 4821. [Google Scholar] [CrossRef] [Green Version]
- Carey, J.R. Applied Demography for Biologists: With Special Emphasis on Insects; Oxford University Press: New York, NY, USA, 1993; p. 206. [Google Scholar]
- Pianka, E.R. Evolutionary Ecology; Addison Wesley Educational Publishers, Inc.: San Francisco, CA, USA, 2000; p. 411. [Google Scholar]
- Begon, M.; Townsend, C.R.; Harper, J.L. Ecology: From Individuals to Ecosystems; Blackwell Publishing: Oxford, MI, USA, 2006; p. 750. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods; Blackwell Science Ltd.: Oxford, UK, 2000; p. 593. [Google Scholar]
- Cabral, S.; Soares, A.O.; Moura, R.; Garcia, P. Suitability of Aphis fabae, Myzus persicae (Homoptera: Aphididae) and Aleyrodes proletella (Homoptera: Aleyrodidae) as prey for Coccinella undecimpunctata (Coleoptera: Coccinellidae). Biol. Control 2006, 39, 434–440. [Google Scholar] [CrossRef]
- Kontodimas, D.; Milonas, P.G.; Stathas, G.J.; Economou, L.P.; Kavallieratos, N.G. Life table parameters of the pseudococcid predators Nephus includens and Nephus bisignatus (Coleoptera: Coccinellidae). Eur. J. Entomol. 2007, 104, 415. [Google Scholar] [CrossRef] [Green Version]
- Mota, J.A.; Soares, A.O.; Garcia, P. Temperature dependence for development of the whitefly predator Clitostethus arcuatus (Rossi). BioControl 2008, 53, 603–613. [Google Scholar] [CrossRef]
- de Maia, A.H.N.; Luiz, A.J.B.; Campanhola, C. Statistical inference on associated fertility life table parameters using jackknife technique: Computational aspects. J. Econ. Entomol. 2000, 93, 511–518. [Google Scholar] [CrossRef]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.; Lee, S.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.R.; Klein, J.P.; Zhang, M.-J. Comparing treatments in the presence of crossing survival curves: An application to bone marrow transplantation. Biometrics 2008, 64, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Bouliotis, G.; Billingham, L. Crossing survival curves: Alternatives to the log-rank test. Trials 2011, 12, A137. [Google Scholar] [CrossRef]
- Li, H.; Han, D.; Hou, Y.; Chen, H.; Chen, Z. Statistical Inference Methods for Two Crossing Survival Curves: A Comparison of Methods. PLoS ONE 2015, 10, e0116774. [Google Scholar] [CrossRef]
- Dormuth, I.; Liu, T.; Xu, J.; Yu, M.; Pauly, M.; Marc Ditzhaus, M. Which test for crossing survival curves? A user’s guideline. BMC Med. Res. Methodol. 2022, 22, 34. [Google Scholar] [CrossRef]
- Bompard, A.; Jaworski, C.C.; Bearez, P.; Desneux, N. Sharing a predator: Can an invasive alien pest affect the predation on a local pest? Pop. Ecol. 2013, 55, 433–440. [Google Scholar] [CrossRef]
- Calvo, F.J.; Lorente, M.J.; Stansly, P.A.; Belda, J.E. Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol. Exp. Appl. 2012, 143, 111–119. [Google Scholar] [CrossRef]
- Perdikis, D.; Lykouressis, D. Effects of various items, host plants, and temperatures on the development and survival of Macrolophus pygmaeus Rambur (Hemiptera: Miridae). Biol. Control 2000, 17, 55–60. [Google Scholar] [CrossRef]
- Lykouressis, D.; Giatropoulos, A.; Perdikis, D.; Favas, C. Assessing the suitability of noncultivated plants and associated insect prey as food sources for the omnivorous predator Macrolophus pygmaeus (Hemiptera: Miridae). Biol. Control 2008, 44, 142–148. [Google Scholar] [CrossRef]
- Perdikis, D.; Lykouressis, D. Macrolophus pygmaeus (Hemiptera: Miridae) population parameters and biological characteristics when feeding on eggplant and tomato without prey. J. Econ. Entomol. 2004, 97, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- INE–Instituto Nacional de Estatística. Inquérito à Estrutura das Explorações Agrícolas-2016–Destaque. 2017. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_destaques&DESTAQUESdest_boui=281413215&DESTAQUESmodo=2 (accessed on 1 September 2022).
- Laikre, L.; Schwartz, M.K.; Waples, R.S.; Ryman, N. Compromising genetic diversity in the wild: Unmonitored large-scale release of plants and animals. TREE 2010, 25, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Sethuraman, A.; Janzen, F.J.; Obrycki, J. Population genetics of the predatory lady beetle Hippodamia convergens. Biol. Control 2015, 84, 1–10. [Google Scholar] [CrossRef]
- Fleming, D.E.; Roehrdanz, R.L.; Allen, K.C.; Musser, F.R. Comparisons of Lygus lineolaris (Hemiptera: Miridae) populations from two distinct geographical regions of Mississippi. Environ. Entomol. 2015, 44, 898–906. [Google Scholar] [CrossRef]
| Azores | Portugal Mainland | |||
|---|---|---|---|---|
| E. kuehniella (N) | T. absoluta (N) | E. kuehniella (N) | T. absoluta(N) | |
| Egg | 11.37 ± 0.11 (30) a | 12.00 ± 0.00 (30) b | ||
| Immature | 18.18 ± 0.10 (17) a | 18.53 ± 0.15 (17) a | 17.29 ± 0.17 (17) a | 17.65 ± 0.12 (17) a |
| Azores | Portugal Mainland | ||
|---|---|---|---|
| E. kuehniella (N) | T. absoluta (N) | E. kuehniella (N) | T. absoluta(N) |
| 0.85 (17) | 0.96 (17) | 0.73 (17) | 0.69 (17) |
| Azores | Portugal Mainland | Significance | ||||
|---|---|---|---|---|---|---|
| Biological Traits | E. kuehniella (N) | T. absoluta (N) | E. kuehniella (N) | T. absoluta (N) | Region | Prey |
| Sex-ratio (Females:Males) | 0.65:0.35 | 0.65:0.35 | 0.42:0.58 | 0.42:0.58 | n.s. | n.s. |
| Female body weight (mg) | 1.09 ± 0.03 (17) | 1.10 ± 0.03 (17) | 1.04 ± 0.05 (8) | 0.97 ± 0.04 (8) | Az. > P.m. | n.s. |
| Male body weight (mg) | 0.65 ± 0.04 (9) | 0.68 ± 0.03 (8) | 0.67 ± 0.02 (11) | 0.61 ± 0.03 (8) | n.s. | n.s. |
| Pre-oviposition time (days) | 5.88 ± 1.62 (8) a | 4.11 ± 0.42 (9) a | 7.17 ± 2.26 (6) a | 14.33 ± 4.94 (6) b | ||
| Lifetime fertility (n. of nymphs) | 77.89 ± 19.14 (9) | 113.67 ± 20.36 (9) | 44.89 ± 18.51 (9) | 35.00 ± 14.46 (9) | Az. > P.m. | n.s. |
| Oviposition period (days) | 26.51 ± 3.16 (8) | 22.00 ± 2.89 (9) | 20.17 ± 4.66 (9) | 16.00 ± 3.07 (9) | n.s. | n.s. |
| Female longevity (days) | 48.44 ± 5.34 (9) | 38.33 ± 5.80 (9) | 54.78 ± 8.69 (9) | 50.56 ± 7.97 (9) | n.s. | n.s. |
| Azores | Portugal Mainland | Significance | ||||
|---|---|---|---|---|---|---|
| E. kuehniella | T. absoluta | K. kuehniella | T. absoluta | Region | Prey | |
| rm | 0.082± 0.005 a | 0.114 ± 0.004 b | 0.062 ± 0.009 ac | 0.054 ± 0.010 c | ||
| λ | 1.085 ± 0.005 a | 1.121 ± 0.004 b | 1.064 ± 0.009 ac | 1.056 ± 0.010 c | ||
| R0 | 41.16 ± 10.12 a | 74.86 ± 12.78 b | 15.25 ± 5.13 a | 12.09 ± 4.19 a | ||
| 45.72 ± 1.09 ab | 36.69 ± 0.64 a | 45.47 ± 2.30 ab | 47.37 ± 4.45 b | |||
| DT | 8.42 ± 0.50 | 5.76 ± 0.31 | 10.88 ± 1.94 | 12.07 ± 3.15 | Az. < P.m. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, I.; Oliveira, L.; Durão, A.C.; Arruda, P.; Figueiredo, E.; Franco, J.C.; Lucas, E.; Soares, A.O. Contrasting Phenotypic Variability of Life-History Traits of Two Feral Populations of Macrolophus pygmaeus (Hemiptera: Miridae) under Two Alternative Diets. Agronomy 2023, 13, 118. https://doi.org/10.3390/agronomy13010118
Borges I, Oliveira L, Durão AC, Arruda P, Figueiredo E, Franco JC, Lucas E, Soares AO. Contrasting Phenotypic Variability of Life-History Traits of Two Feral Populations of Macrolophus pygmaeus (Hemiptera: Miridae) under Two Alternative Diets. Agronomy. 2023; 13(1):118. https://doi.org/10.3390/agronomy13010118
Chicago/Turabian StyleBorges, Isabel, Luísa Oliveira, Ana C. Durão, Patrícia Arruda, Elisabete Figueiredo, José Carlos Franco, Eric Lucas, and António O. Soares. 2023. "Contrasting Phenotypic Variability of Life-History Traits of Two Feral Populations of Macrolophus pygmaeus (Hemiptera: Miridae) under Two Alternative Diets" Agronomy 13, no. 1: 118. https://doi.org/10.3390/agronomy13010118






