Can Sugarcane Yield and Health Be Altered with Fully Mechanized Management?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Implementation
2.3. Plant Sampling
2.4. Analysis of Microbial Diversity
2.5. Statistical Analyses
3. Results
3.1. Sugarcane Yields
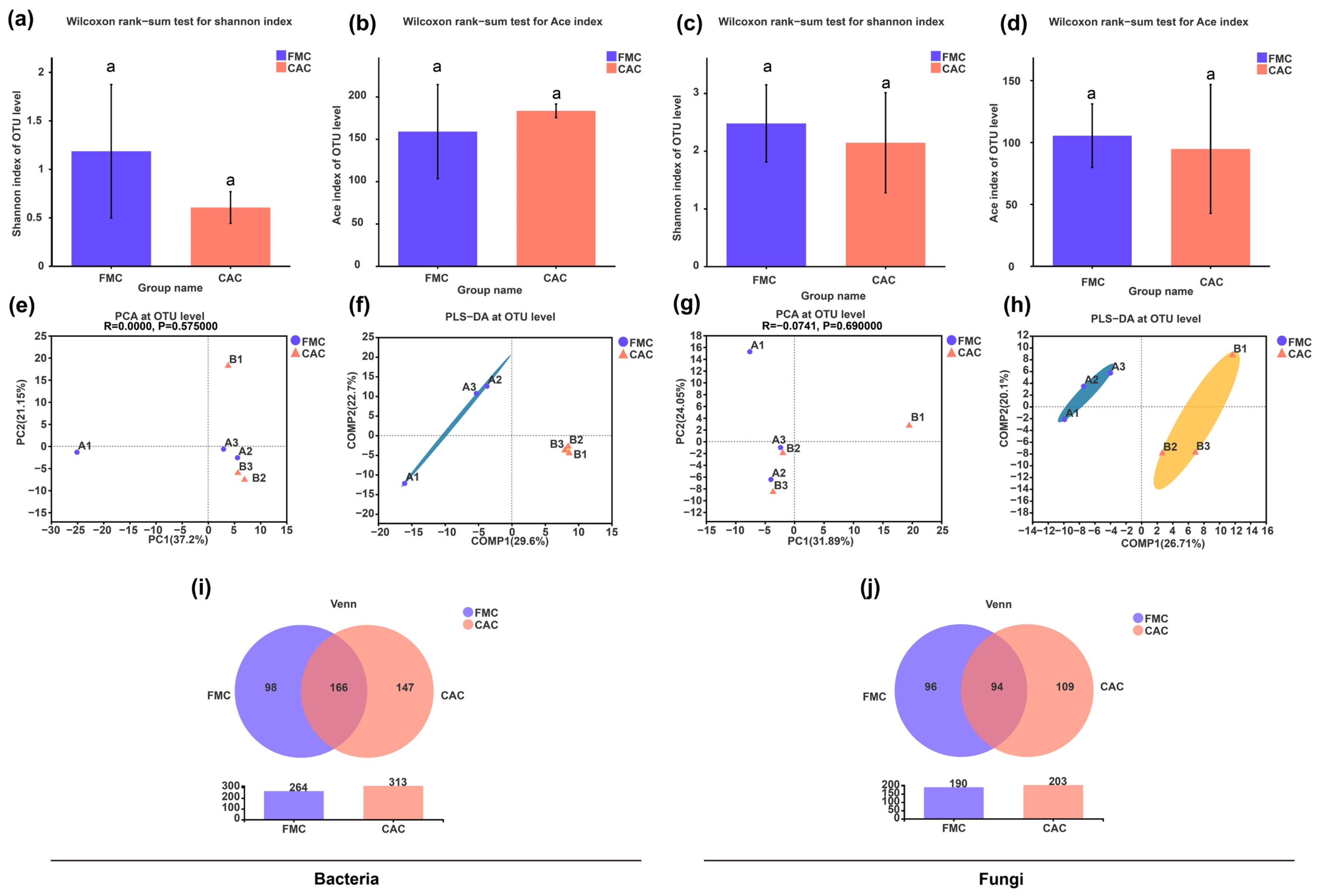
3.2. Diversity of Endophytic Bacteria and Fungi in Sugarcane Stems
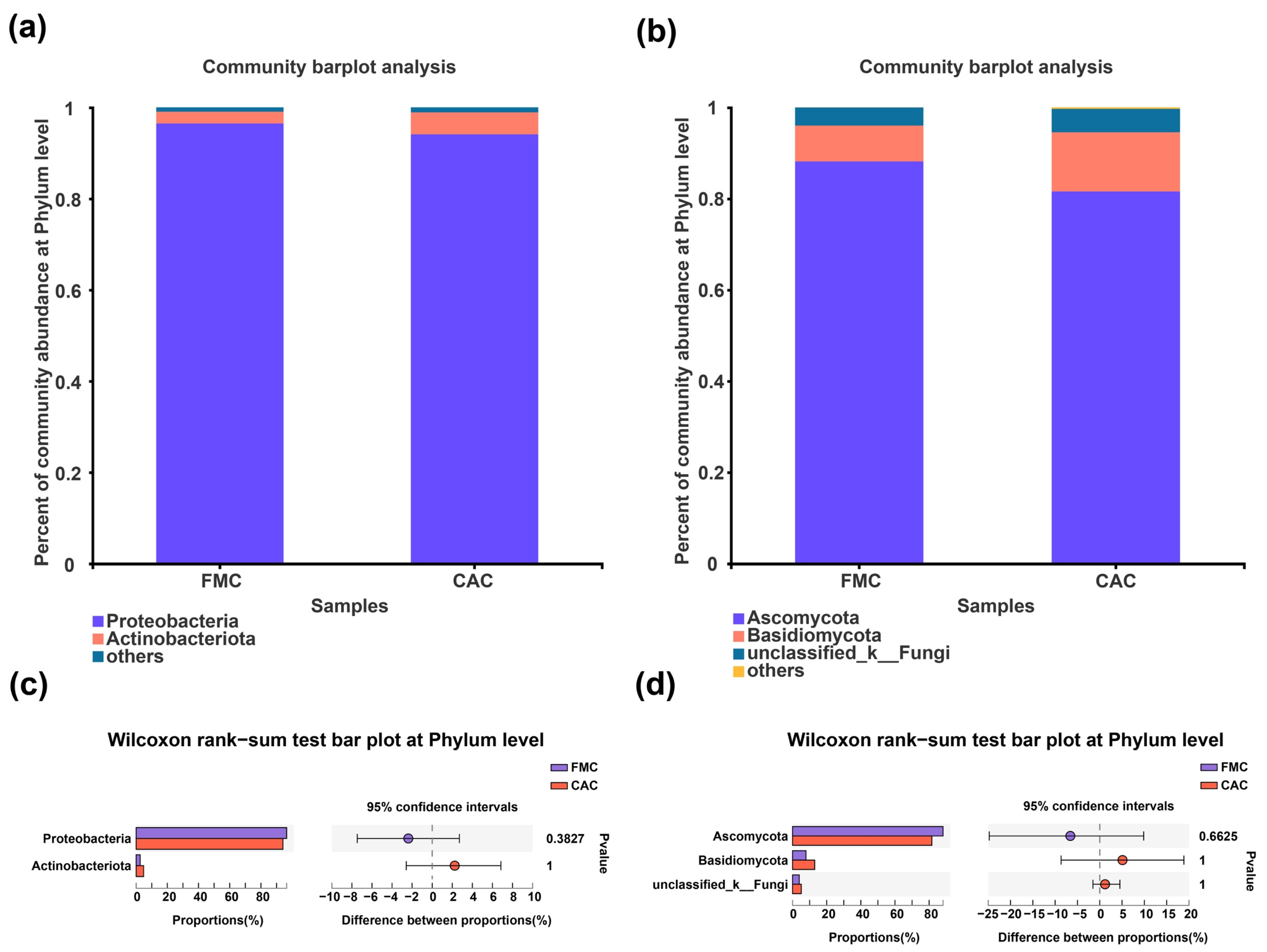
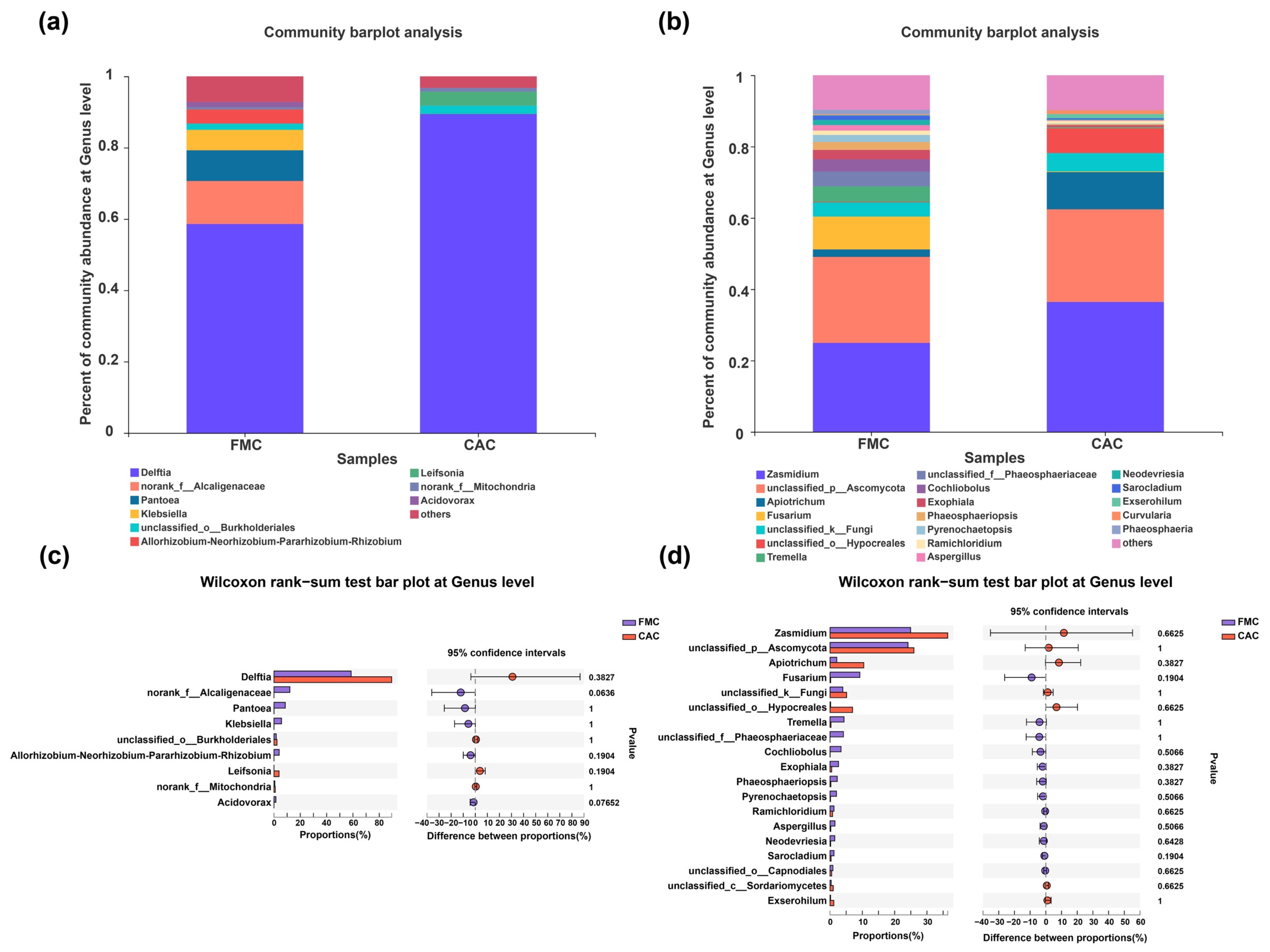
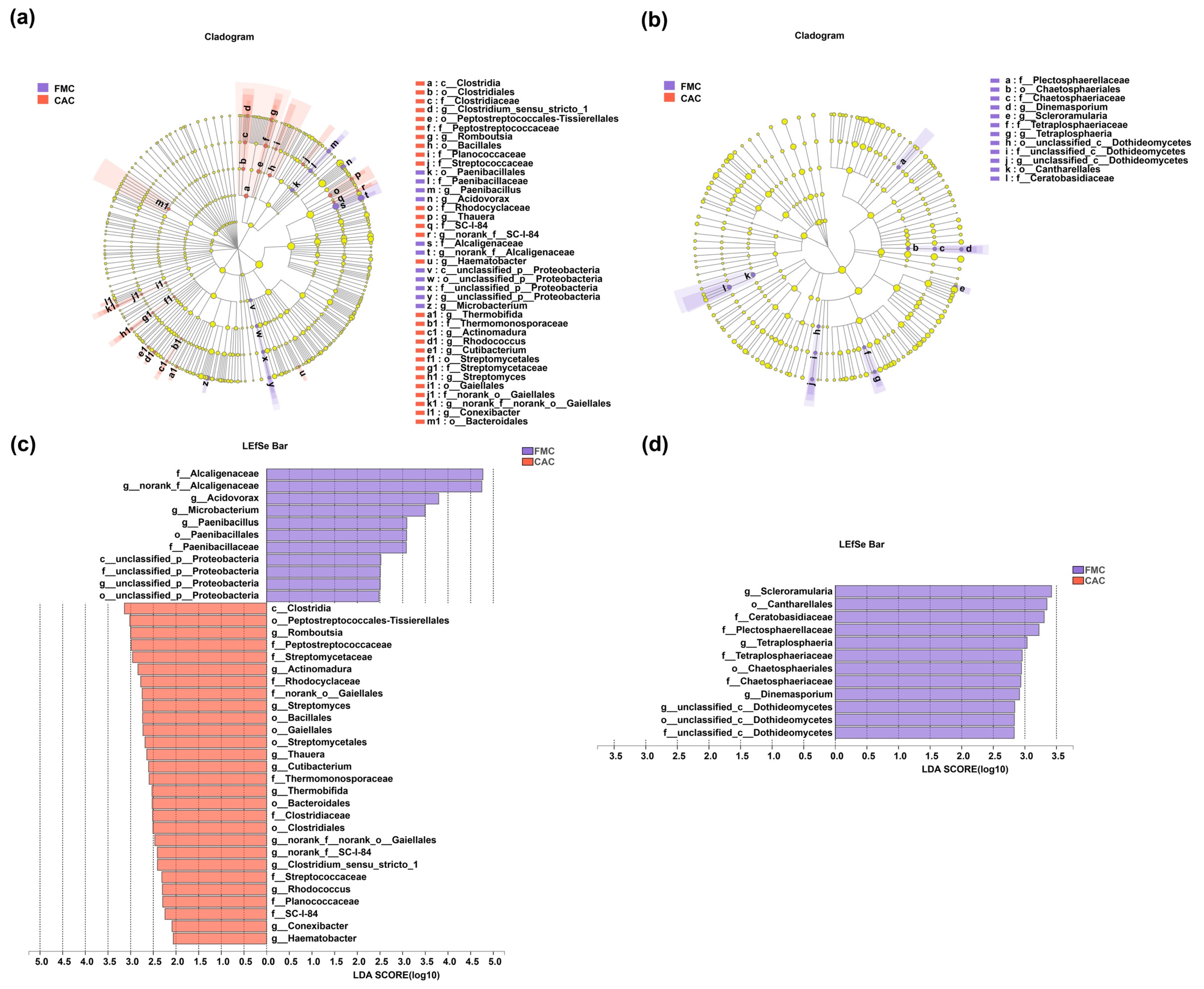
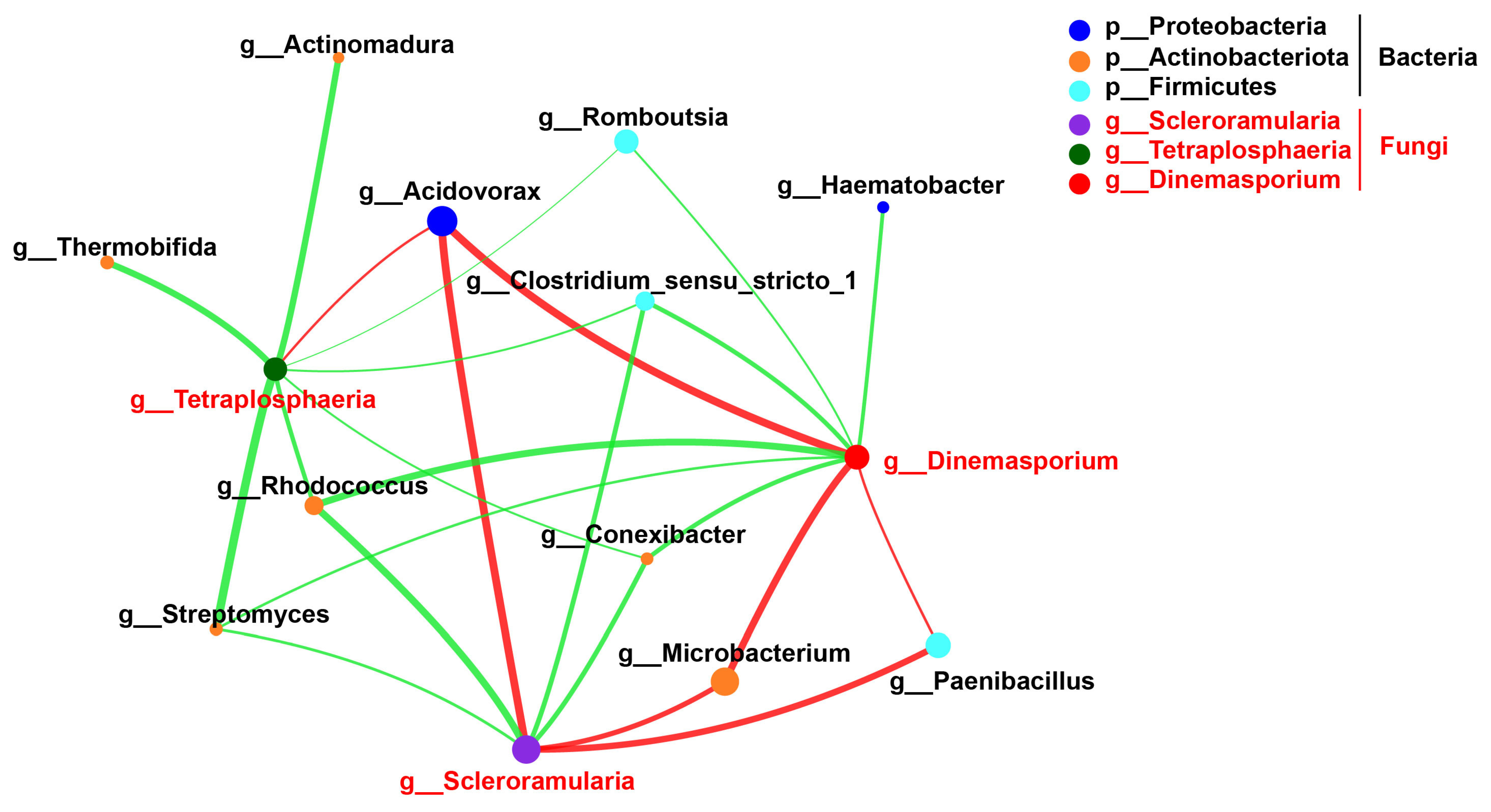
3.3. Composition of Endophytic Bacteria and Fungi in Sugarcane Stems at Different Levels
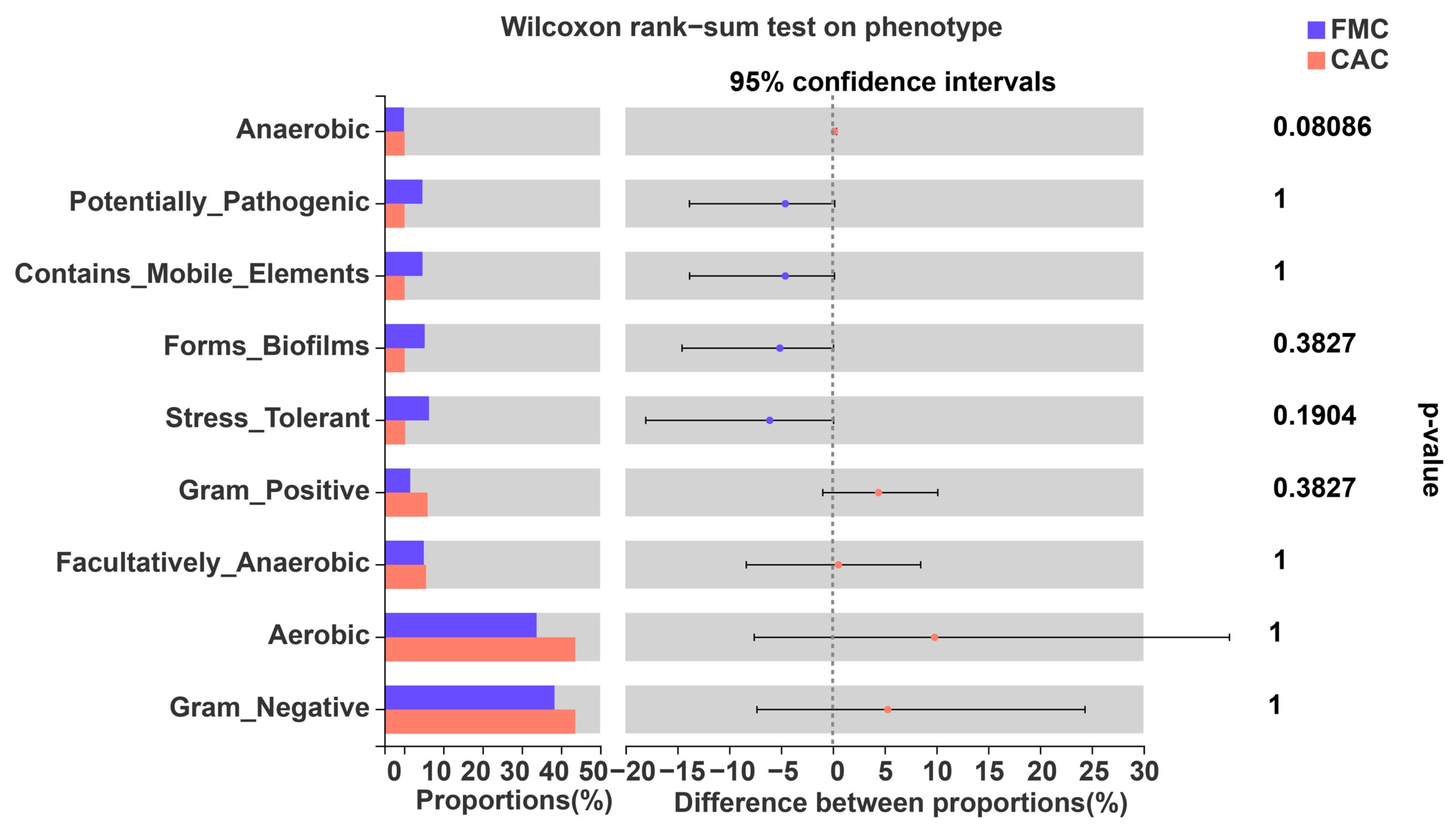
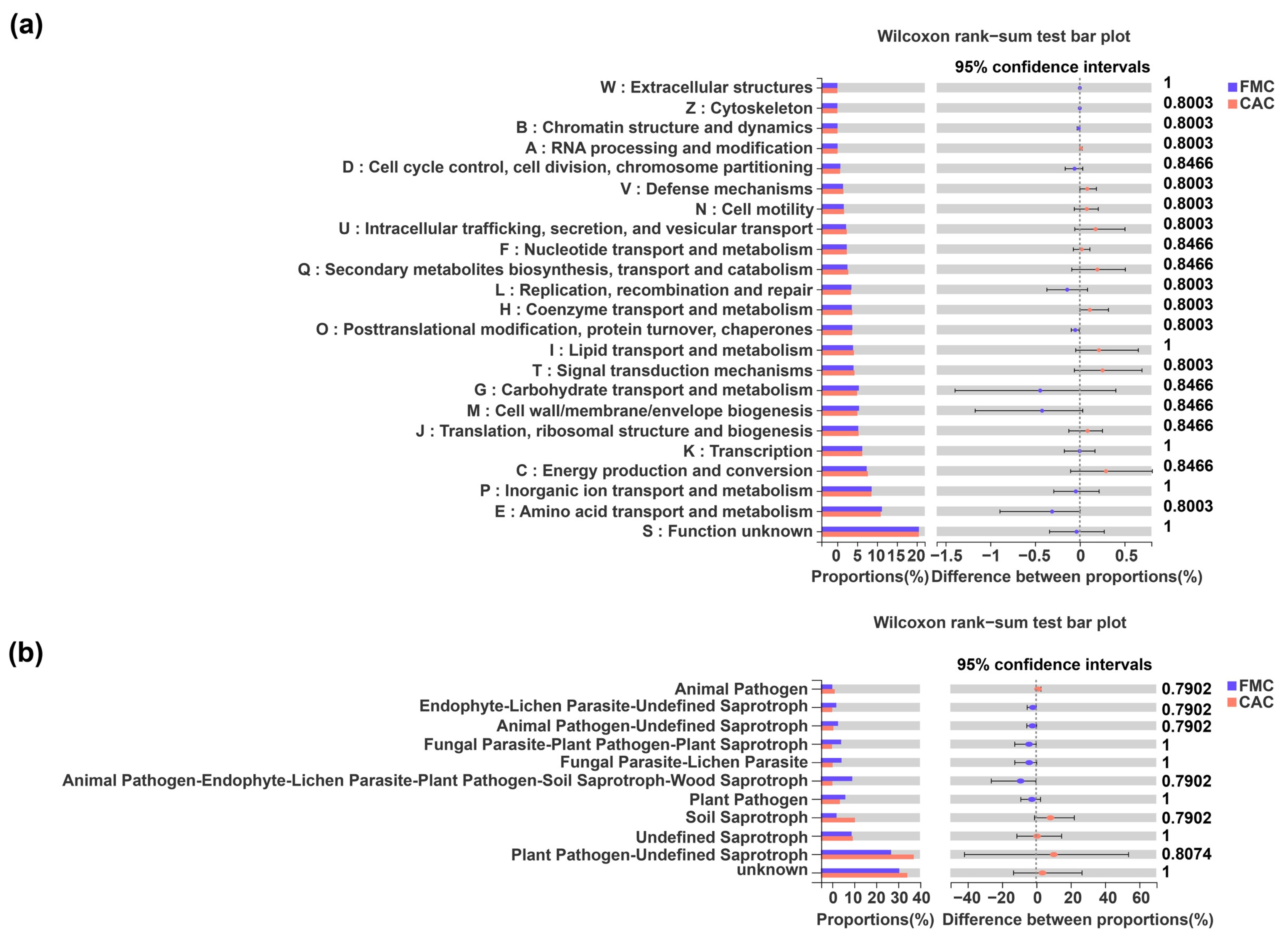
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.K.; Singh, P.; Li, H.B.; Song, Q.Q.; Guo, D.J.; Solanki, M.K.; Verma, K.K.; Malviya, M.K.; Song, X.P.; Lakshmanan, P.; et al. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane: A comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Dong, F.; Liu, Q.; Lin, W.; Hu, C.; Yuan, Z. Soil metagenomics reveals effects of continuous sugarcane cropping on the structure and functional pathway of rhizospheric microbial community. Front. Microbiol. 2021, 12, 369. [Google Scholar] [CrossRef]
- Yang, S.D.; Xiao, J.; Liang, T.; Tan, H.W. Response of bacterial compositions to the use of slow-release fertilizers with long-acting agents and synergists. Appl. Soil Ecol. 2023, 182, 104699. [Google Scholar] [CrossRef]
- Tayyab, M.; Yang, Z.; Zhang, C.; Islam, W.; Lin, W.; Zhang, H. Sugarcane monoculture drives microbial community composition, activity and abundance of agricultural-related microorganisms. Environ. Sci. Pollut. Res. 2021, 28, 48080–48096. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.D.; Xiao, J.; Liang, T.; He, W.Z.; Tan, H.W. Response of soil biological properties and bacterial diversity to different levels of nitrogen application in sugarcane fields. AMB Express 2021, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Fatah, G.S.A.; Verona, S.L.; Nugraheni, S.D. The analysis of labor efficiency on sugarcane cultivation through mechanization application. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012127. [Google Scholar] [CrossRef]
- Tayyab, M.; Fallah, N.; Zhang, C.; Pang, Z.; Islam, W.; Lin, S.; Lin, W.; Zhang, H. Sugarcane cultivar-dependent changes in assemblage of soil rhizosphere fungal communities in subtropical ecosystem. Environ. Sci. Pollut. Res. 2022, 29, 20795–20807. [Google Scholar] [CrossRef]
- Tayyab, M.; Islam, W.; Noman, A.; Pang, Z.; Li, S.; Lin, S.; Lin, W.; Zhang, H. Sugarcane cultivars manipulate rhizosphere bacterial communities’ structure and composition of agriculturally important keystone taxa. 3 Biotech 2022, 12, 32. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A.; Sahu, K.P.; Patel, A.; Reddy, B.; Sheoran, N.; Charishma, K.; Rajashekara, H.; Bhagat, S.; Rathour, R. Deciphering core-microbiome of rice leaf endosphere: Revelation by metagenomic and microbiological analysis of aromatic and non-aromatic genotypes grown in three geographical zones. Microbiol. Res. 2021, 246, 126704. [Google Scholar] [CrossRef]
- Ashajyothi, M.; Kumar, A.; Sheoran, N.; Ganesan, P.; Gogoi, R.; Subbaiyan, G.K.; Bhattacharya, R. Black pepper (Piper nigrum L.) associated endophytic Pseudomonas putida BP25 alters root phenotype and induces defense in rice (Oryza sativa L.) against blast disease incited by Magnaporthe oryzae. Biol. Control 2020, 143, 104181. [Google Scholar] [CrossRef]
- Teheran-Sierra, L.G.; Funnicelli, M.I.G.; de Carvalho, L.A.L.; Ferro, M.I.T.; Soares, M.A.; Pinheiro, D.G. Bacterial communities associated with sugarcane under different agricultural management exhibit a diversity of plant growth-promoting traits and evidence of synergistic effect. Microbiol. Res. 2021, 247, 126729. [Google Scholar] [CrossRef] [PubMed]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.V.N.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B.; et al. The Endophytic Microbiome as a Hotspot of Synergistic Interactions, with Prospects of Plant Growth Promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, S.Y.; Sun, Y.; Yang, S.D.; He, Y. Differences of rhizospheric and endophytic bacteria are recruited by different watermelon phenotypes relating to rind colors formation. Sci. Rep. 2022, 12, 6360. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Dong, W.; Yan, D.H. Organs, cultivars, soil, and fruit properties affect structure of endophytic mycobiota of Pinggu peach trees. Microorganisms 2019, 7, 322. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.N.; Kui, L.; Singh, P.; Liu, L.F.; Xie, L.Y.; He, L.L.; Li, F.S. Identification and characterization of bacillus subtilis B9: A diazotrophic plant growth-promoting endophytic bacterium isolated from sugarcane root. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Sun, Y.; Wu, S.; Liang, W.; Yang, S. Effects of mechanical weeding on soil fertility and microbial community structure in star anise (Illicium verum Hook.f.) plantations. PLoS ONE 2022, 17, e0266949. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef]
- Bruto, M.; Prigent-Combaret, C.; Muller, D.; Moënne-Loccoz, Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci. Rep. 2014, 4, 6261. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I. Isolation, functional characterization and efficacy of biofilm-forming rhizobacteria under abiotic stress conditions. Antonie Van Leeuw. 2019, 112, 1827–1839. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.Y.; Sun, Y.; Yang, S.D.; Tan, H.W. Effect of intercropping with different legume crops on endophytic bacterial diversity of sugarcanes. Chin. J. Trop. Crops 2021, 42, 3188–3198. (In Chinese) [Google Scholar] [CrossRef]
- Mehnaz, S. Bacteria in Agrobiology: Crop Ecosystems. Plant Growth-Promoting Bacteria Associated with Sugarcane. In Bacteria in Agrobiology: Crop Ecosystems; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 165–187. [Google Scholar]
- Bigott, Y.; Gallego, S.; Montemurro, N.; Breuil, M.C.; Pérez, S.; Michas, A.; Martin-Laurent, F.; Schröder, P. Fate and impact of wastewater-borne micropollutants in lettuce and the root- associated bacteria. Sci. Total Environ. 2022, 831, 154674. [Google Scholar] [CrossRef] [PubMed]
- Siani, R.; Stabl, G.; Gutjahr, C.; Schloter, M.; Radl, V. Acidovorax pan-genome reveals specific functional traits for plant beneficial and pathogenic plant-associations. Microb. Genom. 2021, 7, 000666. [Google Scholar] [CrossRef] [PubMed]
- Giordano, P.R.; Chaves, A.M.; Mitkowski, N.A.; Vargas, J.M. Identification, characterization, and distribution of Acidovorax avenae subsp. avenae associated with creeping bentgrass etiolation and decline. Plant Dis. 2012, 96, 1736–1742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, F.; Niu, S.; Lin, X.; Pei, S.; Jiang, L.; Tian, Y.; Zhang, G. Description of Microbacterium luteum sp. nov.; Microbacterium cremeum sp. nov.; and Microbacterium atlanticum sp. nov.; three novel C50 carotenoid producing bacteria. J. Microbiol. 2021, 59, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef]
- Li, H.; Sun, G.; Batzer, J.C.; Crous, P.W.; Groenewald, J.Z.; Karakaya, A.; Gleason, M.L. Scleroramularia gen. nov. associated with sooty blotch and flyspeck of apple and pawpaw from the Northern Hemisphere. Fungal Divers. 2011, 46, 53–66. [Google Scholar] [CrossRef]
- Krohn, K.; Sohrab, M.H.; van Ree, T.; Draeger, S.; Schulz, B.; Antus, S.; Kurtán, T. Dinemasones A, B and C—New bioactive metabolites from the endophytic gungus Dinemasporium strigosum. Eur. J. Org. Chem. 2008, 2008, 5638–5646. [Google Scholar] [CrossRef]
| Treatments | 2019 | 2020 | 2021 |
|---|---|---|---|
| FMC | 100.25 ± 2.01 a | 98.58 ± 0.89 a | 101.94 ± 0.80 a |
| CAC | 88.06 ± 0.64 a | 84.34 ± 0.67 a | 88.21 ± 0.71 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Liang, T.; Yang, S.; Tan, H. Can Sugarcane Yield and Health Be Altered with Fully Mechanized Management? Agronomy 2023, 13, 153. https://doi.org/10.3390/agronomy13010153
Xiao J, Liang T, Yang S, Tan H. Can Sugarcane Yield and Health Be Altered with Fully Mechanized Management? Agronomy. 2023; 13(1):153. https://doi.org/10.3390/agronomy13010153
Chicago/Turabian StyleXiao, Jian, Tian Liang, Shangdong Yang, and Hongwei Tan. 2023. "Can Sugarcane Yield and Health Be Altered with Fully Mechanized Management?" Agronomy 13, no. 1: 153. https://doi.org/10.3390/agronomy13010153
APA StyleXiao, J., Liang, T., Yang, S., & Tan, H. (2023). Can Sugarcane Yield and Health Be Altered with Fully Mechanized Management? Agronomy, 13(1), 153. https://doi.org/10.3390/agronomy13010153







