Abstract
The aim of the study was to compare the phytoremediation potential of cultivated grasses with local wild grass for soil contaminated with zinc. Two pot experiments were carried out on soil artificially contaminated with Zn. Four species of cultivated grasses were used as test plants: Poa pratensis, Lolium perenne, Festuca rubra, Festuca pratensis, and one wild, native grass: Deschampsia caespitosa. Wild grass seeds were collected from soil contaminated with heavy metals near a zinc smelter. The phytoremediation potential of grasses was determined on the basis of the tolerance index (TI), bioaccumulation (BF), and translocation (TF) factors. Differences were found between the species in the reduction in the shoot and root biomass with increasing soil contamination with Zn. The tolerance of the studied grasses to excess Zn in the soil was in the following order: D. caespitosa > L. perenne > F. rubra > F. pratensis > P. pratensis. In addition, there were differences in the accumulation and distribution of Zn between the roots and shoots, which is related to the different defense mechanisms of the studied grasses against Zn phytotoxicity. Of the five grasses tested, the highest phytoremediation potential was shown by D. caespitosa. This grass had a significantly higher tolerance to Zn and a lower transfer of Zn from the roots to shoots than the other cultivated grasses tested. All four cultivated grasses can be useful for phytostabilization because they accumulated Zn mainly in the roots and limited its translocation to the shoots. Unlike wild grass seeds, cultivated grass seeds are readily available commercially and can be used for the phytoremediation of HM-contaminated sites.
1. Introduction
The accumulation of heavy metals in soil is a serious threat due to their high toxicity and long biological half-life. These contaminants are absorbed by plant roots and have a high capacity to pass through the food chain into human and animal organisms [1]. Therefore, the remediation of heavy metals in soil systems has been an urgent issue to be solved. Remediation by plants, called phytoremediation, is generally considered an environmentally friendly, cheap, minimally invasive, and socially acceptable method. There are two most popular phytoremediation techniques—phytoextraction and phytostabilization [2]. Phytoextraction involves the uptake from the soil and translocation of heavy metals into easily harvestable above-ground plant parts, which are removed from the contaminated area. Plants used for this purpose should be characterized by rapid growth, high biomass, and high tolerance to heavy metals [3]. Phytostabilization involves immobilization and reduced mobility of heavy metals. Metals can be accumulated by the roots, absorbed on their surface, or precipitated in the rhizosphere. Plants ideal for phytostabilization should have a well-developed root system and be tolerant of high concentrations of heavy metals. It is also important that they exhibit a high accumulation of metals in the roots and a low translocation of metals from the roots to shoots [4,5,6,7]. Plants suitable for phytoremediation tolerate high levels of HM in the soil because they have developed numerous mechanisms that adapt them to defend themselves against metals [8,9,10,11]. Metal detoxification within the plant involves the induction of an antioxidant mechanism involving various compounds that neutralize reactive oxygen species and the exclusion of metals from metabolic processes by complexing and depositing them in the cytoplasm, vacuoles, or apoplasm [12,13,14,15].
Zinc phytotoxicity is considered one of the most important environmental problems. This is due to the extensive dispersion of Zn in the environment and reaching phytotoxic levels in many soils, which is caused by contaminants of anthropogenic origin, such as fertilizers, pesticides, sewage sludge, steel mills, incinerators, mines, and galvanized products [16]. Zinc is readily taken up by plants and the intensity of its accumulation in tissues depends on the species and cultivar [17]. Excessive Zn concentration in the plant disrupts many metabolic and physiological processes, resulting in reduced growth of the shoots and plant roots [18].
Grasses are plants suitable for the phytoremediation of Zn-contaminated soils. They are characterized by rapid growth, a well-developed root system, high biomass, and a perennial growth cycle [19,20]. In addition, they can accumulate large amounts of heavy metals in both the roots and shoots [21,22,23]. Metal-contaminated areas are generally colonized spontaneously by wildflower species, including grasses that have adapted well to adverse growth conditions. The few papers comparing the metal tolerance of wild species growing in HM-contaminated areas with analogous species from uncontaminated areas show that wild species are generally more tolerant. However, some cultivated grasses are also suitable for phytoremediation [24,25]. Knowledge of the suitability for phytoremediation of commonly cultivated grasses is of great practical importance because, unlike wild grasses, their seeds are readily available commercially and can be used for the phytoremediation of HM-contaminated sites. The aim of our study was to determine the Zn tolerance of several common cultivated grass species and to compare their phytoremediation potential with wild local grass from the area contaminated with Zn.
2. Materials and Methods
2.1. Pot Experiments
In order to study the tolerance of grasses to soil zinc contamination, two identical pot experiments were carried out in the greenhouse, one in spring (III–V) and one in autumn (IX–X). In both experiments, pots were filled with 2 kg of the same soil brought from a field located in Jelcz-Laskowice near Wroclaw, Poland. The physical and chemical properties of the soil are presented in Table 1. Soil with relatively low pH and a low sorption complex was used in the experiments to increase Zn mobility.

Table 1.
Properties of soils used in the experiment.
Before sowing grasses, the experimental soil was artificially contaminated with zinc in the form of ZnSO4∙7H2O, resulting in 4 levels of Zn content in the soil: Zn0- without contamination, Zn1-240 mg kg−1, Zn2-500 mg kg−1, and Zn3-900 mg kg−1. Five grass species were used as test plants: Deschampsia caespitosa, Poa pratensis (c. Niweta), Lolium perenne (c. Kinga), Festuca rubra (c. Adio), and Festuca pratensis (c. Fantasia). As a result, 20 treatments (4 Zn levels × 5 grasses) were tested in a single experiment, each in 4 replications.
Seeds of all grass species, with the exception of D. caespitosa, came from the Nieznanice Breeding and Production Plant, which is part of the Malopolska Plant Breeding company. Seeds of D. caespitosa and samples of the soil on which it grew were collected from a wasteland near a zinc smelter in Bukowno near Olkusz, Poland (Figure 1). Chemical analyses of soil samples where D. caespitosa grew showed contamination with Zn, Cd, and Pb (Table 2).

Figure 1.
A wasteland near a zinc smelter in Bukowno near Olkusz, Poland, colonized by Deschampsia caespitosa.

Table 2.
The concentration of metals in the soil from the D. caespitosa site in Bukowno near Olkusz.
Grasses were sown 2 weeks after Zn was applied to the soil. Initially, more seeds were sown, and after about 2 weeks, 30 individuals per pot for F. rubra, F. pratensis, and L. perenne, and 60 individuals per pot for D. caespitosa and P. pratensis, were left. One week after thinning, NPK fertilization was applied at a rate of 50 mg N, 8.5 mg P, and 27.5 mg K per pot, watering the plants with the fertilizer solution. Plants were harvested 2 months after sowing. Grass shoots were cut 5 mm above the ground. The roots were removed from the pots, cleaned of soil, and washed thoroughly with tap water, followed by a 2 h rinse in distilled water using a rotary stirrer. The shoots and roots were then dried (24 h at 50 °C and 3 h at 100 °C, respectively), carefully weighed, finely ground, and subjected to chemical analyses.
2.2. Chemical Analyses
All chemical analyses were performed at the Main Laboratory of the Institute of Fertilisation and Soil Science in Pulawy, accredited by the Polish Centre for Accreditation (certificate number AB 339 based on the PN-EN ISO/IEC 17025 [26] standard.
Soil Zn content was determined by the FAAS method after digestion in aqua regia [27]. Total organic carbon (TOC) content was determined by the Thiurin method using potassium dichromate [28], pH by the potentiometric method in 1 mol KCl dm−3 [29], and texture by the aerometric method [30]. In addition, the contents of available phosphorus and potassium were determined in the soil by the Enger–Riehm method [31,32] and magnesium by the Schachtschabel method [33].
Zn in the shoots and roots was determined by the FAAS method, after prior dry-ashing of the material in a muffle furnace and digesting it with 20% nitric acid [34].
2.3. Calculation of the Tolerance Index and Bioaccumulation and Translocation Factors
In order to compare the tolerance of the tested grasses to excess zinc, the tolerance index (TI) was calculated according to the Wilkins formula [35], in the authors’ modification. This index expresses the ratio of the biomass on the contaminated treatment to the biomass of the plants on the control:
To compare metal accumulation in plants, a bioaccumulation factor (BF) was calculated for the shoots and roots using the following formulas, according to Melo et al. [36]:
The translocation of metals from roots to above-ground parts was determined based on the translocation factor (TF), expressed by the following formula [36]:
2.4. Statistical Calculations
As the results of the spring and autumn experiments were very similar to each other, it was decided to average them. All results of plant biomass and Zn concentration in the shoots and roots were given as the means from these two experiments. ANOVA calculations were performed using Statgraphics v 5.0 software (StatPoint Technologies, Inc., Warrenton, VA, USA). Multiple comparisons among groups were made with Tukey’s honest significant difference test (p < 0.05).
3. Results
3.1. Impact of Zinc on Grass Biomass
The plant response to excess Zn in the soil was manifested by a significant reduction in shoot and root biomass at increasing levels of soil contamination from Zn1 to Zn3. Depending on the grass species and soil Zn content, this response varied.
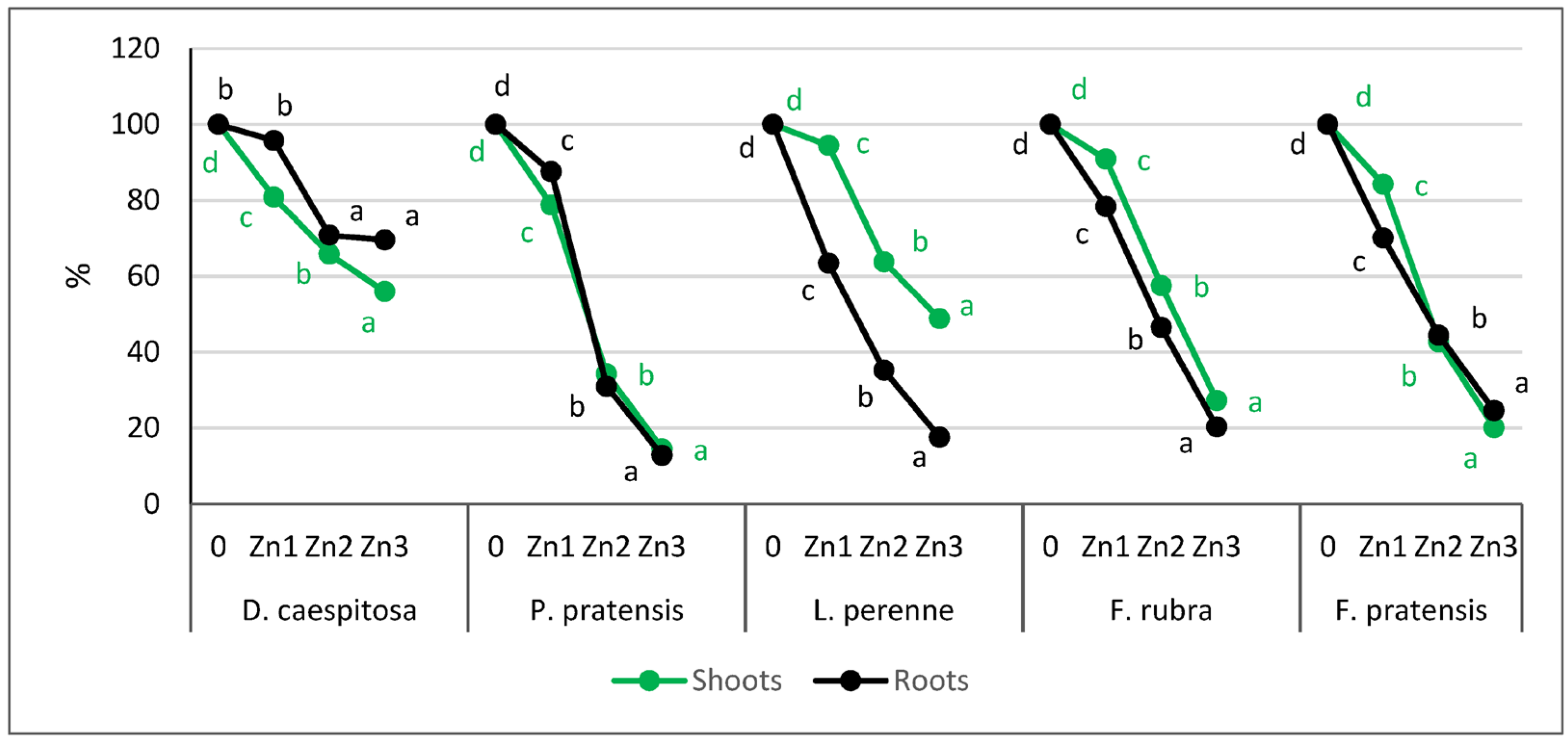
D. caespitosa showed a greater reduction in shoot biomass than in root biomass. There was a 19%, 34%, and 44% reduction in shoot biomass on Zn1, Zn2, and Zn3, respectively, compared to the control (Figure 2). The roots, on the other hand, had lower biomass only on Zn2 and Zn3, where the reduction was 30% at both contamination levels.
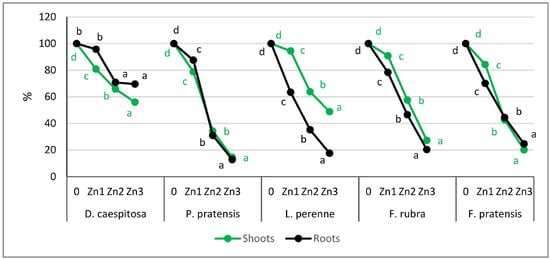
Figure 2.
Relative yield (control treatment = 100%). Values marked with the same letters within one species do not differ significantly, according to Tukey’s test (p < 0.05).
L. perenne reacted differently to excess Zn than D. caespitosa. This grass showed a much greater reduction in root biomass than in shoot biomass. The Zn1 level caused only a 6% reduction in shoot biomass, while on Zn2 and Zn3, the reduction was 36 and 51%, respectively. In contrast, the roots already on Zn1 reduced their mass by 37%, and at subsequent levels of contamination, root biomass was reduced by 65 and 82% compared to the control.
The response of the other three grasses to Zn was similar to each other, especially at Zn2 and Zn3 contamination levels. P. pratensis showed a 21%, 66%, and 86% decrease in shoot biomass and a 13%, 69%, and 87% decrease in root biomass on Zn1, Zn2, and Zn3, respectively. F. rubra responded with a 9%, 43%, and 73% decrease in shoot biomass and a 22%, 54%, and 80% decrease in root weight. For F. pratensis, increasing Zn levels reduced the shoot biomass by 16%, 57%, and 80%, and the roots by 30%, 56%, and 75%, compared to the control.
The average tolerance index, calculated for the three Zn levels combined, showed that the least toxic effect of Zn on shoot biomass occurred for D. caespitosa and L. perenne. This index for the mentioned grasses was 68% and 69%, which means that they produced only about 30% less biomass of the shoots compared to plants growing on non-contaminated soil with Zn (Table 3). The other grasses (F. rubra, F. pratensis, and P. pratensis) responded to Zn by reducing shoot biomass to 58%, 49%, and 42% compared to the control, respectively. Considering the roots, differences in mean tolerance indices among grasses were bigger than for the shoots. Index values for the roots ranged from 79% (D. caespitosa) to 39% (L. perenne).

Table 3.
Tolerance index.
The tolerance index calculated on the basis of the total biomass of the shoots and roots relative to the corresponding sum on the control allows ranking the grass species tested from most to least tolerant to excess Zn in the soil: D. caespitosa > L. perenne > F. rubra > F. pratensis > P. pratensis. The difference between D. caespitosa and P. pratensis in their response to excess Zn in the soil was already visible during the growing of the plants (Figure 3) and also when the roots were uprooted from the soil after plant harvest (Figure 4).

Figure 3.
Grasses growing in zinc-contaminated soil. Pot number/Zn level: 82/0, 86/Zn1, 89/Zn2, 94/Zn3; 97/0, 103/Zn1, 105/Zn2, and 111/Zn3.

Figure 4.
Grass roots. Pot number/Zn level: 82/0, 86/Zn1, 89/Zn2, 94/Zn3; 97/0, 103/Zn1, 105/Zn2, and 111/Zn3.
3.2. Zinc Content in Soil and Plants
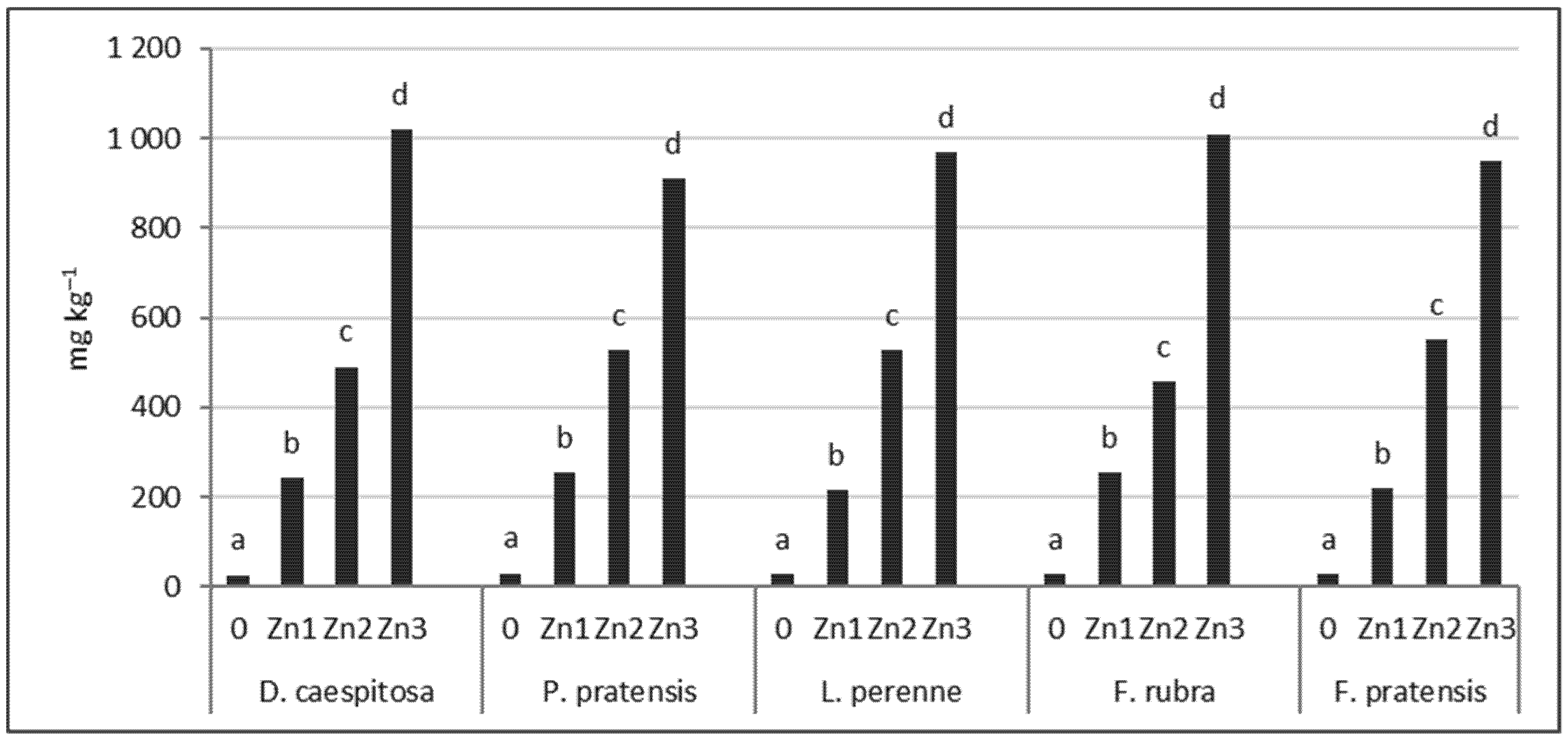
The soil Zn content after plant harvest was similar for the five grasses tested (Figure 5). At the Zn1 level, the Zn concentration was 217–254 mg kg−1; at the Zn2 level, it ranged from 456–553 mg kg−1; and at the Zn3 level, it ranged from 910–1020 mg kg−1. It should be noted that the Zn content of the soil from the D. caespitosa seed collection site (Bukowno near Olkusz) oscillated between the Zn2 and Zn3 levels used in our experiments (Table 2, Figure 5).
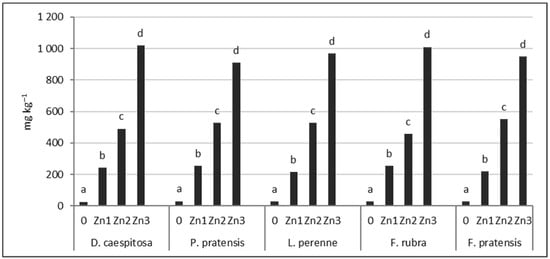
Figure 5.
Zn concentration in soil after harvest. Values marked with the same letters within one species do not differ significantly, according to Tukey’s test (p < 0.05).
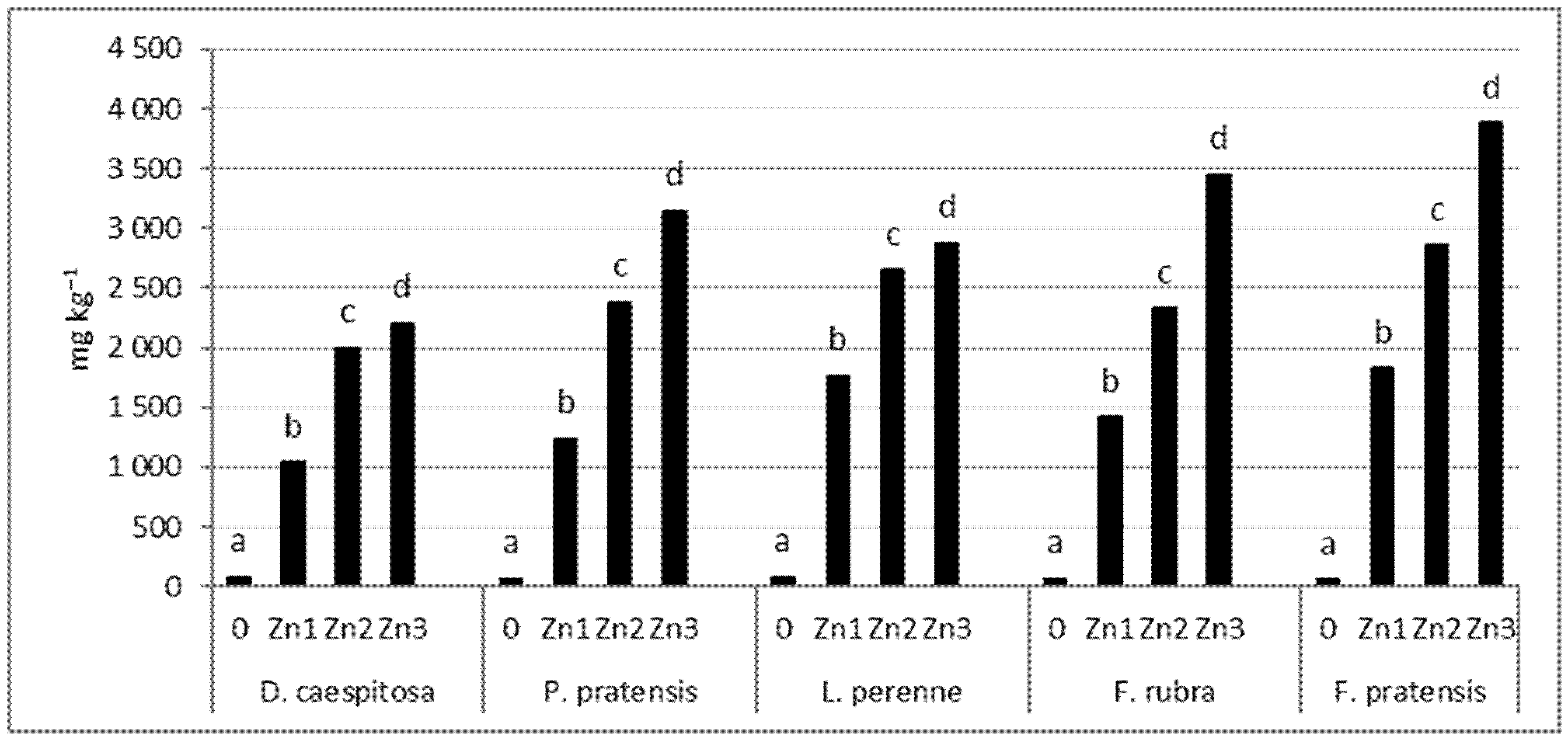
The concentration of Zn in grass shoots increases significantly with the increase in the level of soil contamination (Figure 6). The smallest Zn increase in the shoots was found for D. caespitosa. This grass accumulated only 13–28 times more zinc at Zn1−Zn3 levels than the control. In contrast, F. pratensis showed the greatest increase in shoot Zn concentration. This grass showed a 33–69-fold increase in Zn concentration at Zn1−Zn3 levels compared to the control. P. pratensis, L. perenne, and F. rubra showed a 18–46, 23–37, and 23–56-fold analogous increases in Zn concentration in the shoots, respectively.
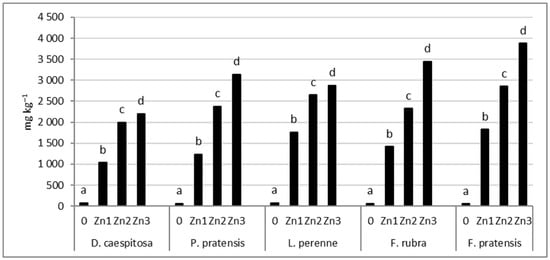
Figure 6.
Zn concentration in shoots. Values marked with the same letters within one species do not differ significantly, according to Tukey’s test (p < 0.05).
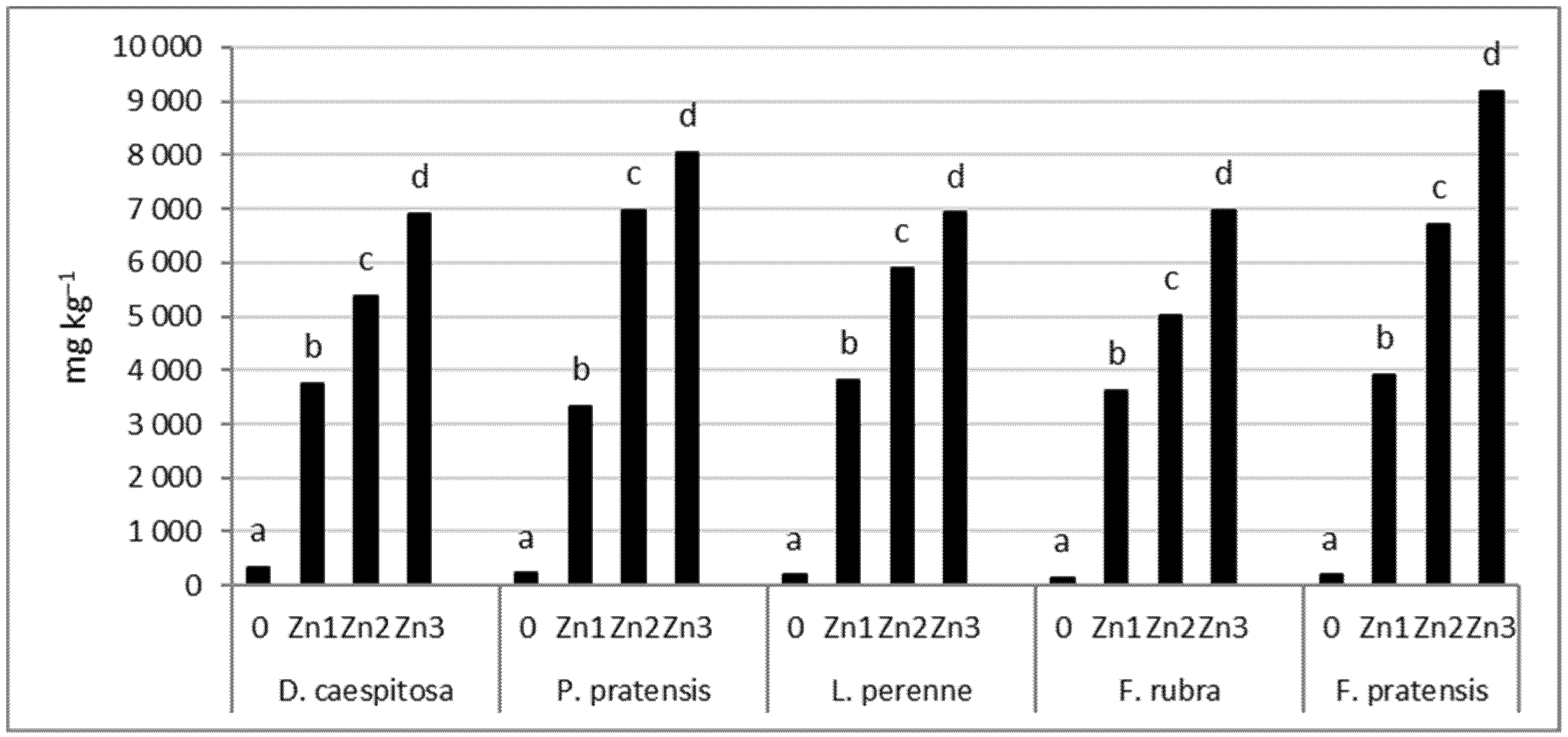
The concentration of Zn in the roots increased significantly with increasing soil contamination with Zn (Figure 7). As with the shoots, the smallest increase was observed in D. caespitosa. This grass accumulated 11–21 times more Zn in the roots at Zn1–Zn3 levels compared to the control. This can be followed by P. pratensis and L. perenne, which had 15–36 and 20–37 times higher Zn concentrations in the roots, respectively. The greatest increase in Zn content in the roots relative to plants growing on uncontaminated soil was recorded for both species of the genus Festuca. For F. pratensis, 21–48 and for F. rubra, 25–47 times more of this element was found at Zn1–Zn3 levels compared to the control.
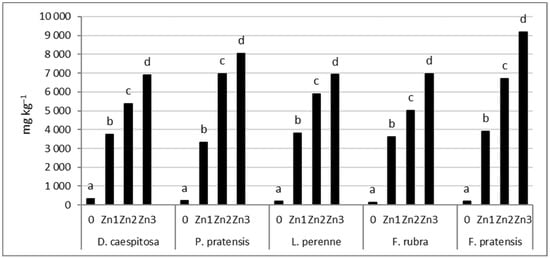
Figure 7.
Zn concentration in roots. Values marked with the same letters within one species do not differ significantly, according to Tukey’s test (p < 0.05).
3.3. Bioaccumulation (BF) and Translocation Factors (TF)
BFshoot and BFroot decreased for all tested grasses along with the increase in Zn in the soil from Zn1 to Zn3, although in a variable manner depending on the grass species (Table 4). In general, the percentage reduction in both factors within a single species was similar. For D. caespitosa, the BFshoot value for Zn3 decreased by 54% relative to Zn1, and BFroot by 56%. For the three cultivated grasses (L. perenne, F. pratensis, and F. rubra), BFshoot decreased by 63%, 51%, and 40%, and BFroot by 60%, 46%, and 52%, respectively. P. pratensis showed the weakest response to increasing Zn in soil. The BFshoot and BFroot of this grass only decreased by 30% on Zn3 compared to Zn1.

Table 4.
Bioaccumulation (BF) and translocation factors (TF).
BFshoot, calculated as averages of the three levels of Zn in the soil, allowed all grasses to be ranked according to the amount of metal accumulation in the shoots, as follows: D. caespitosa < P. pratensis < F. rubra < L. perenne < F. pratensis (Table 4). According to similarly calculated BFroot values, the grasses accumulated Zn in the roots in the following order: F. rubra < D. caespitosa < P. pratensis < L. perenne < F. pratensis.
The grasses tested differed in Zn distribution between the roots and shoots, as evidenced by different translocation factors (TFs) (Table 4). Some grasses reduced Zn transport from the roots to shoots as Zn1 to Zn3 contamination increased. Thus, for L. perenne and F. pratensis, a reduction in TF was observed from 0.46 at the Zn1 level to 0.42 at the Zn3 level. For other grasses, an increase in the TF value for Zn3 compared to Zn1 was observed. For F. rubra, the TF value increased from 0.39 to 0.49. At the same time, D. caespitosa and P. pratensis showed relatively stable TF. For both grasses, the increase in the TF value for Zn3 compared to Zn1 was small, ranging from 0.28 to 0.29 and 0.37 to 0.39, respectively.
The mean TF calculated for the three levels of Zn in the soil showed that D. caespitosa translocated the least Zn from the roots to shoots among the five grasses tested (Table 4). This grass contained 31% Zn in the shoots compared to the amount accumulated in the roots (TF = 0.31). In contrast, TF values for the cultivated grasses ranged from 0.36 to 0.45. The ranking of the grasses in order from least to most Zn translocation from the roots to shoots was as follows: D. caespitosa > P. pratensis > F. rubra = F. pratensis > L. perenne.
4. Discussion
In general, there is a belief among researchers that native plant species are better used for phytoremediation than foreign or altered species through breeding [37,38,39,40]. This is because native species are adapted to the climatic and soil conditions of the area to be cleaned. In recent years, many studies have been performed on the suitability of different native plant species for the phytoremediation of heavy metal-contaminated areas [25,41,42]. Most studies have focused on grasses, which are pioneers in colonizing devastated areas [43,44,45,46]. In our study, we compared the suitability of wild grass (D. caespitosa) and four cultivated grasses for the phytoremediation of Zn-contaminated soil.
Plants used for phytoremediation should generally have a high tolerance to contamination, as measured by the tolerance index (TI). In addition, plants suitable for the phytoextraction of metals from the soil should be characterized by high biomass and high bioaccumulation of metals in the shoots (BFshoot > 1). In contrast, plants intended for phytostabilization should be characterized by high bioaccumulation of metal in the roots (BFroot > 1). At the same time, they should show a weak transfer of metal from the roots to the above-ground part, which is expressed by the low translocation factor (TF < 1) [47,48,49]. In our study, all the grasses tested showed high Zn accumulation in the shoots. The BFshoot ranged from 3.5 to 5.9, classifying these grasses as useful for the phytoextraction of Zn from the soil. It is not possible to fully assess their suitability for this purpose, as no data on the final biomass of the grasses were obtained (plants were cut 2 months after sowing). However, the suitability of the tested grasses for phytostabilization can be assessed on the basis of the tolerance index (TI), BFroots, and TF. Depending on the species, BFroots ranged from 10.7 to 13.3 and TF ranged from 0.31 to 0.45. At the same time, the grasses differed significantly in terms of adaptation to Zn stress, as expressed by the variation in TI.
The highest tolerance index (TI = 70) on Zn-contaminated soil was shown by D. caespitosa. This fact proves the lowest loss of biomass of this wild grass, and thus its best adaptation to the excess of Zn among the 4 tested cultivated grasses. Few studies by other authors comparing the behavior of native plant species from metal-contaminated sites with non-native species confirm the greater tolerance of native species [42].
Of the grasses tested, D. caespitosa also showed the lowest Zn concentration in the shoots and the lowest BFshoot and TF. This implies that the defense strategy of D. caespitosa relied mainly on the adequate distribution of this metal in the plant. Zn taken from the soil was mainly accumulated in the roots and, to a small extent, transferred to the shoots, thus protecting the photosynthetic apparatus from damage.
L. perenne and F. rubra showed the highest tolerance among the cultivated grasses. These grasses had similar TIs (60 and 55), so the decrease in biomass under excess Zn was similar for them. However, the grasses differed in the concentration of Zn in the shoots and roots, and thus in BFshoot and BFroot. Although both grasses had a very similar Zn tolerance, L. perenne accumulated more Zn in the tissues compared to F. rubra. The BFshoot for L. perenne was 5.4 and for F. rubra it was 4.7. The BFroot was 12.0 and 10.7, respectively. At the same time, L. perenne had the highest translocation factor (TF = 0.45) of all grasses. Nevertheless, L. perenne grew best under Zn soil contamination compared to other cultivated grasses. This implies that it activated other additional defense mechanisms than reducing Zn uptake by the roots and limiting the transfer of this metal to the shoots. In a study by Elekes [45], L. perenne showed the highest Zn accumulation capacity among several grasses tested and was selected for a subsequent phytoremediation experiment near a metallurgical plant.
The next two grasses, F. pratensis and P. pratensis, showed the lowest tolerance to excess Zn among the four cultivated grasses tested. TI values for them were only 48 and 43, respectively. At the same time, F. pratensis accumulated the most Zn in the shoot tissues (BFshoot = 5.9) and roots (BFroot = 13.3) among all grasses. Its defense mechanism against Zn stress was probably weaker than that of other grasses. In contrast, P. pratensis had the lowest BFshoot (4.3) and TF (0.36) among the cultivated grasses, indicating a reduction in Zn transport to the shoots and accumulation in the roots. In view of the low tolerance of this grass to excess Zn in the soil, it seems that this mechanism was not sufficient to defend against Zn stress. The high BFshoot and BFroot for F. pratensis and P. pratensis are favorable for the use of these grasses for phytostabilization. However, too low of a TI indicates their poor adaptation to stress conditions and prevents their use for phytoremediation. Additionally, in a study by Atabayeva [43], Poa pratensis was the least tolerant grass to the presence of Zn in the soil.
The tolerance of grasses used for phytoremediation of Zn-contaminated soils could be improved by modifying soil properties that affect the immobilization of metals and thus limit metal uptake by plants [50]. In recent years, many studies have confirmed the increase in plant tolerance to HM as a result of the exogenous supply of SA [51,52].
5. Summary
Of the five grasses tested, the highest phytoremediation potential was shown by the wild grass D. caespitosa, the seed of which came from zinc-contaminated areas. This grass had a significantly higher tolerance to Zn and a lower transfer of Zn from the roots to shoots than the other cultivated grasses tested.
All four tested cultivated grasses accumulated Zn mainly in the roots and limited its translocation to the shoots (BFroot > 1 and TF < 1), which could make them useful for phytostabilization. However, considering the tolerance of grasses to excess zinc in the soil, L. perenne and F. rubra were shown to be more suitable for phytostabilization than F. pratensis and P. pratensis. F. pratensis and P. pratensis showed the lowest tolerance to Zn stress among all the grasses tested.
Author Contributions
Conceptualization, J.K. and E.S.-G.; investigation, J.K. and E.S.-G.; methodology, J.K. and E.S.-G.; writing—original draft, J.K. and E.S.-G.; writing—review and editing, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge funding from the Polish Ministry of Agriculture and Rural Development under the 4.12 Scientific Research Program of the Institute of Soil Science and Plant Cultivation in Pulawy.
Data Availability Statement
Presented data in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pinto, A.P.; Mota, A.D.; De Varennes, A.; Pinto, F.C. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci. Total Environ. 2004, 326, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Karczewska, A.; Lewińska, K.; Gałka, B. Arsenic extractability and uptake by velvetgrass Holcus lanatus and ryegrass Lolium perenne in variously treated soils polluted by tailing spills. J. Hazard. Mater. 2013, 262, 1014–1102. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanislawska-Glubiak, E.; Igras, J. Applicability of energy crops for metal phytostabilization of soils moderately contaminated with copper, nickel and zinc. J. Food Agric. Environ. 2011, 9, 693–697. [Google Scholar]
- Korzeniowska, J.; Stanisławska-Glubiak, E. Phytoremediation potential of Phalaris arundinacea, Salix viminalis and Zea mays for nickel-contaminated soils. Int. J. Environ. Sci. Technol. 2019, 16, 1999–2008. [Google Scholar] [CrossRef]
- Stanislawska-Glubiak, E.; Korzeniowska, J.; Kocon, A. Effect of the reclamation of heavy metal-contaminated soil on growth of energy willow. Pol. J. Environ. Stud. 2012, 21, 187–192. [Google Scholar]
- Manara, A. Plant responses to heavy metal toxicity. In Plants and Heavy Metals; Springer: Dordrecht, The Netherlands, 2012; pp. 27–53. [Google Scholar]
- Pinto, A.P.; Simoes, I.; Mota, A.M. Cadmium impact on root exudates of sorghum and maize plants: A speciation study. J. Plant. Nutr. 2008, 31, 1746–1755. [Google Scholar] [CrossRef]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Tech. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Repka, V.; Fiala, R.; Čiamporová, M.; Pavlovkin, J. Effects of ZnCl2 on ROS generation, plasma membrane properties, and changes in protein expression in grapevine root explants. Biologia 2016, 71, 528–537. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 1–37. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Ghotbi, F. Effects of Salicylic Acid, Jasmonic Acid, and Calcium Chloride on Reducing Chilling Injury of Pomegranate (Punica granatum L.). Fruit. J. Agric. Sci. Technol. 2014, 16, 163–173. [Google Scholar]
- Kushwaha, A.; Rani, R.; Kumar, S.; Gautam, A. Heavy metal detoxification and tolerance mechanisms in plants: Implications for phytoremediation. Environ. Rev. 2016, 24, 39–51. [Google Scholar] [CrossRef]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Chaney, R.L. Zinc phytotoxicity. In Zinc in Soils and Plants; Springer: Dordrecht, The Netherlands, 1993; pp. 135–150. [Google Scholar]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Differences in the concentration of micronutrients in young shoots of numerous cultivars of wheat, maize and oilseed rape. Agronomy 2022, 12, 2639. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Abaga, N.O.Z.; Dousset, S.; Mbengue, S.; Munier-Lamy, C. Is vetiver grass of interest for the remediation of Cu and Cd to protect marketing gardens in Burkina Faso? Chemosphere 2014, 113, 2–47. [Google Scholar]
- Xia, H.P. Ecological rehabilitation and phytoremediation with four grasses in oil shale mined land. Chemosphere 2004, 54, 345–353. [Google Scholar] [CrossRef]
- Aibibu, N.; Liu, Y.; Zeng, G.; Wang, X.; Chen, B.; Song, H.; Xu, L. Cadmium accumulation in Vetiveria zizanioides and its effects on growth, physiological and biochemical characters. Bioresource Technol. 2010, 101, 6297–6303. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Z. Physiological mechanism of hypertolerance of cadmium in Kentucky bluegrass and tall fescue: Chemical forms and tissue distribution. Environ. Exp. Bot. 2013, 96, 35–42. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Deng, L.; Gong, G.; Jia, Y.; Xu, X.; Li, T.; Li, Y.; Chen, H. Cadmium tolerance and accumulation characteristic Siegesbeckia orientalis L. Ecol. Eng. 2013, 51, 133–139. [Google Scholar] [CrossRef]
- Maric, M.; Antonijevic, M.; Alagic, S. The investigation of the possibility for using some wild and cultivated plants as hyperaccumulators of heavy metals from contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Theologides, C.P.; Costa, C.; Kalavrouziotis, J.K.; Varnavas, S.P. Assessment of toxic heavy metals concentrations in soils and wild and cultivated plant species in Limni abandoned copper mining site, Cyprus. J. Geoch. Explor. 2017, 178, 16–22. [Google Scholar] [CrossRef]
- PN-EN ISO/IEC 17025:2018-02; General Requirements for the Competence of Testing and Calibration Laboratories. Polish Committee for Standardization: Warsaw, Poland, 2018. (In Polish)
- PN-ISO 11047:2001; Soil Quality—Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc in Aqua Regia Extracts of Soil—Flame and Electrothermal Atomic Absorption Spectrometric Methods. Polish Committee for Standardization: Warsaw, Poland, 2013. (In Polish)
- PN-ISO 14235:2003; Soil Quality—Determination of Organic Carbon in Soil by Sulfochromic Oxidation. Polish Committee for Standardization: Warsaw, Poland, 2003. (In Polish)
- PN-EN ISO 10390:2022-09; Soil, Treated Biowaste and Sludge—Determination of pH (ISO 10390:2021). Polish Committee for Standardization: Warsaw, Poland, 2022. (In Polish)
- PN-R-04033:1998; Soil and Mineral Soil Materials—Particle Size Distribution on Soil Classes. Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish)
- PN-R-04023:1996; Agrochemical Soil Analyse–Determination of Assimilated Phosphorus Contents. Committee for Standardization, Warsaw, Poland, 1996. (In Polish)
- PN-R-04022:1996; Agrochemical Soil Analyse—Determination of Assimilated Potassium Content. Committee for Standardization: Warsaw, Poland, 1996. (In Polish)
- PN-R-04020:1994; Agrochemical Soil Analyse—Determination of Assimilated Magnesium Content. Committee for Standardization: Warsaw, Poland, 1994. (In Polish)
- PN-R-04014:1991; Agrochemical Plant Analyse—Methods of Mineralization of Plant Material for Determination Macro- and Microelements. Polish Committee for Standardization: Warsaw, Poland, 1991. (In Polish)
- Wilkins, D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Melo, E.E.C.; Costa, E.T.S.; Guilherme, L.R.G.; Faquin, V.; Nascimento, C.W.A. Accumulation of arsenic and nutrients by castor bean plants grown on an As-enriched nutrient solution. J. Hazard. Mater. 2009, 168, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Compton, H.R.; Prince, G.R.; Fredericks, S.C.; Gussman, C.D. Phytoremediation of dissolved phase organic compounds: Optimal site considerations relative to field case studies. Remediation 2003, 13, 21–37. [Google Scholar] [CrossRef]
- Favas, P.J.; Pratas, J.; Varun, M.; D’Souza, R.; Paul, M.S. Phytoremediation of soils contaminated with metals and metalloids at mining areas: Potential of native flora. In Environmental Risk Assessment of Soil Contamination; Maria, C., Hernandez, S., Eds.; IntechOpen: Shanghai, China, 2014. [Google Scholar]
- Fu, S.; Wei, C.; Xiao, Y.; Li, L.; Wu, D. Heavy Metals Uptake and Transport by Native Wild Plants: Implications for Phytoremediation and Restoration. Environ. Earth Sci. 2019, 78, 103. [Google Scholar] [CrossRef]
- Tordoff, G.M.; Baker, A.J.M.; Willis, A.J. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Heckenroth, A.; Rabier, J.; Dutoit, T.; Torre, F.; Prudent, P.; Laont-Schwob, I. Selection of native plants with phytoremediation potential for highly contaminated Mediterranean soil restoration: Tools for a non-destructive and integrative approach. J. Environ. Manag. 2016, 183, 850–863. [Google Scholar] [CrossRef]
- Lamb, D.T.; Ming, H.; Megharaj, M.; Naidu, R. Relative tolerance of a range of Australian native plant species and lettuce to copper, zinc, cadmium, and lead. Arch. Environ. Contam. Toxicol. 2010, 59, 424–432. [Google Scholar] [CrossRef]
- Atabayeva, S. Heavy metals accumulation ability of wild grass species from industrial areas of Kazakhstan. In Phytoremediation; Ansari, S.S., Gill, R., Gill, G.R., Lanza, L., Newman, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 157–208. [Google Scholar]
- De Conti, L.; Marques, A.C.; Ceretta, C.A.; Tarouco, C.P.; Nicoloso, F.T.; Ferreira, P.A.; Tiecher, T.L.; Tassinari, A.; Bicalho da Silva, I.C.; Brunetto, G. Tolerance and phytoremediation potential of grass species native to South American grasslands to copper-contaminated soils. Int. J. Phytoremediat. 2021, 23, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Elekes, C.C. Eco-technological solutions for the remediation of polluted soil and heavy metal recovery. In Environmental Risk Assessment of Soil Contamination; IHernández-Soriano, M.C., Ed.; TechOpen: London, UK, 2014; pp. 309–335. [Google Scholar]
- Guterres, J.; Rossato, L.; Doley, D.; Pudmenzky, A.; Bee, C.; Cobena, V. Assessing germination characteristics of Australian native plant species in metal/metalloid solution. J. Hazard. Mater. 2019, 364, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Cheraghi, M.; Lorestani, B.; Khorasani, N.; Yousef, N.; Karami, M. Findings on the phytoextraction and phytostabilization of soils contaminated with heavy metals. Biol. Trace Elem. Res. 2011, 144, 1133–1141. [Google Scholar] [CrossRef]
- Roccotiello, E.; Manfredi, A.; Drava, G.; Minganti, V.; Mariotti, M.; Berta, G.; Cornara, L. Zinc tolerance and accumulation in the ferns Polypodium cambricum L. and Pteris vittata L. Ecotoxicol. Environ. Saf. 2010, 73, 1264–1271. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of phytoremediation potential of Nerium indicum with inorganic modifier calcium carbonate and organic modifier mushroom residue to lead-zinc tailings. Int. J. Environ. Res. Public Health 2022, 19, 10353. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The role of salicylic acid in plants exposed to heavy metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef]
- Stanislawska-Glubiak, E.; Korzeniowska, J. Effect of salicylic acid foliar application on two wheat cultivars grown under zinc stress. Agronomy 2022, 12, 60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).