Abstract
Rice (Oryza sativa L.) is one of the most significant food crops worldwide. Flooding can significantly impact the survival and emergence of rice seedlings in the direct planting form of rice, leading to a loss of production. This work investigated the critical phase of rice seed germination to the seedling establishment under submergence stress. It also explored the underlying physiological and molecular processes of shoot and root submergence tolerance. Physiological and transcriptomic analysis of flood-tolerant varieties (LS273) and non-flood-tolerant varieties (ZZ39) were performed. Under submergence stress, LS273 showed longer roots, taller shoots than ZZ39, and lower levels of malondialdehyde and GSSG, but a greater level of GSH/GSSG. In response to stress induced by submersion, LS273 produces more auxin than ZZ39. Within shoots, 4285 DEGs specific to LS273 and 4389 DEGs specifics to ZZ39 were found using the RNA-seq technique. There were 3983 specific DEGs in LS273 and 3292 specific DEGs in ZZ39 in the roots. Antioxidants and plant growth hormones were primarily mentioned in the annotations for these DEGs. Overall, our research provides a thorough foundation for investigating the molecular network underlying shoots’ and roots’ resistance to submergence stress in rice seedlings, leading us to hypothesize that the resistance of flood-tolerant rice may be attributed to high levels of oxidation resistance and auxin accumulation.
1. Introduction
Abiotic stress has a significant impact on plant growth. Flood stress has become a significant abiotic stress affecting plant growth due to global warming [1]. Under floods, plants partially or wholly submerged in water experience a reduction in oxygen diffusion, preventing plants from respiring oxygen. Rice (Oryza sativa L.) seedlings have weak resistance to stress. Submergence stress in the seedling stage will lead to the decline of rice seedling emergence rate and seedling quality, thus affecting rice yield. Therefore, choosing rice varieties that are flood-tolerant at the seedling stage and investigating the flooding tolerance mechanism is vital.
Under the pressure of flooding, rice is in an anoxic environment. Carbohydrate catabolism changes from aerobic to anaerobic [2]. In this way, ATP (adenosine triphosphate) production efficiency is low, affecting rice’s normal growth. The submergence tolerance of different rice varieties at the germination stage is widely variable [3]. The survival and emergence of rice under flood stress in the living environment can be ensured by cultivating flood-resistant varieties that exhibit vigorous shoot growth. Rice has developed several physiological and morphological processes to adapt to underwater germination [4]. The growth escape mechanism is one of the most effective flood-resistant systems. Due to the fast mobilization of nutrients, the shoots spread quickly and break free from the water’s surface in need of oxygen. It was discovered that SNORKEL might control the production of auxin and gibberellin caused by ethylene accumulation [5]. Amylase is activated by ethylene, which causes seeds to germinate and release stored energy [6]. Under hypoxia, there was a high expression of Ramy3D in the coleoptiles and radicles of rice seedlings. Such genes are controlled by feedback from starch breakdown products, such as the monosaccharide glucose in the endosperm [7]. The locus associated with hypoxia tolerance in rice is OssTPP7, which encodes trehalose-6-phosphatase and is responsible for increasing the conversion of starch to glucose. Efficient utilization of sugars promotes seedling and coleoptile elongation [8]. Under hypoxic conditions, pyruvate decarboxylase (PDC1 and PDC2) and alcohol dehydrogenase (ADH1 and ADH2) [9] genes stimulate hypoxia and stress regulation upon activation. Additionally, rice calcineurin B-like protein 10 (oscbl10) influences Ca2+ tide current and α-amylase activity, enhancing rice’s ability to withstand submersion under anaerobic conditions [10].
Reactive oxygen species (ROS) are produced when plants are exposed to oxidative stress after ablation, causing damage to cell membranes [11]. Malondialdehyde (MDA) is one of the lipid peroxidation products formed by the lipid peroxidation reaction between ROS and macromolecular substances such as the side chains of polyunsaturated fatty acids and nucleic acids related to phospholipids, enzymes, and membrane receptors of biological membranes [12]. The homeostasis of ROS during rice recovery after submergence stress is because of the high levels of ascorbic acid and glutathione reductase activity, ensuring better functioning of the ascorbic acid-glutathione cycle and more efficient detoxification of H2O2 [13]. Recent research suggests that flood-tolerant genotypes may contain larger levels of soluble sugar, starch, and chlorophyll, which may contribute to their increased ability to store energy during submersion stress and lessen damage during submersion stress due to stable membrane integrity [14].
Direct seeding has long been recognized as a resource- and energy-efficient and climate-resilient rice crop establishment method. However, removing herbicides is more challenging because weeds and rice grow simultaneously using the direct seeding method [15]. When a field is submerged after sowing, anaerobic conditions are created that can significantly reduce weed germination and growth. It helps manage weeds in the early stages of rice growth but also reduces germination in rice. In addition, submergence will cause soil gleyization and enrich reducing substances such as Fe2+, Mn2+, H2S, etc., affecting nutrient uptake by plant roots and resulting in root damage or even death [16]. These problems can be resolved by selecting submergence-tolerant rice varieties at the germination and seedling phases [17], making direct sowing the preferred method by growers and making submergence weed control more controllable.
This study aimed to determine the submergence tolerance of rice cultivars after germination. Physiological assays and transcriptome approaches were utilized to explore the shoots and roots of submergence-tolerant and sensitive cultivars and tissue sites under flood stress to understand the underlying molecular mechanisms of various resistance cultivars. It also offers a theoretical foundation for the model of weeding through submersion in the rice field using direct sowing.
2. Results
2.1. Morphological and Physiological Analysis of LS273 and ZZ39 Shoots and Roots in Response to Submergence Stress
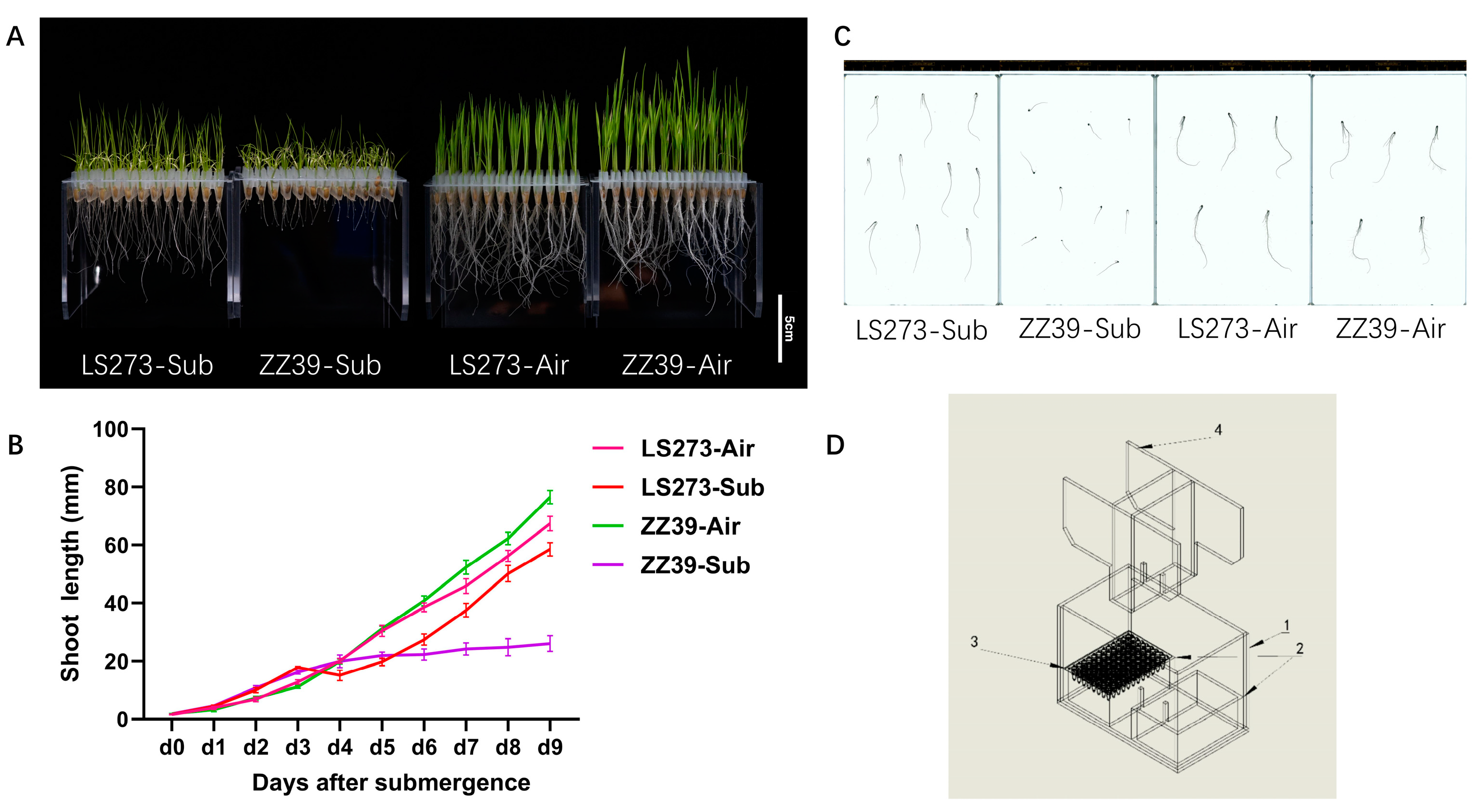
Growth under submergence stress varied significantly between ZZ39 and LS273 varieties (Figure 1A). There is almost no significant difference between these varieties with blank control. Three days before submergence, the coleoptiles of the two cultivars were rapidly elongated due to submergence stress. From day 5, the shoot height was different under submergence conditions (Figure 1B). However, under blank control, there was no significant difference in the growth curves between ZZ39 and LS273 shoots. After being subjected to submergence stress for 9 days, compared with the blank control, the shoot height and root length of the LS273 variety decreased by 10.49 and 53.70%, and the fresh weight decreased by 25.96%. The root surface area, volume, tips, and new weight decreased by 49.81, 57.14, 86.26, and 38.63%, respectively. The shoot height and root length of the ZZ39 cultivar decreased by 67.47 and 86.40%, respectively, and the root surface area, root volume, and root tips decreased by 81.42, 81.08, and 95.26%, respectively (Figure 1C). Under submergence stress, the shoot height and root length of the LS273 cultivar were 142.65 and 250.00% higher than ZZ39. The root surface area, root volume, root tips, and fresh weight increased by 64.39, 53.33, 78.10, and 16.70%, respectively. The results demonstrate that submergence stress can significantly affect the growth of rice, precisely that of the roots (Table 1). Compared with ZZ39, LS273 could particularly resist the hypoxic stress brought about by submergence via rapid growth.
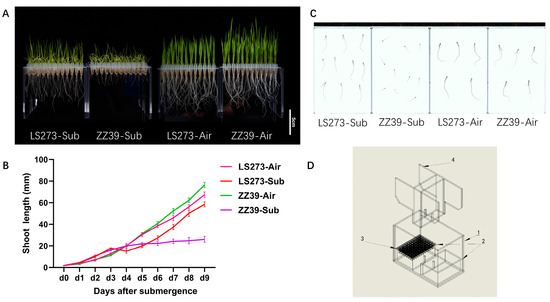
Figure 1.
Morphological characteristics of the LS273 and ZZ39 cultivars during shoots growth under normal and flooded conditions. (A) Phenotypic difference between LS273 and ZZ39 on 9 days. (B) Growth dynamics of seedling height in LS273 and ZZ39. (C) Root scanning diagram of ZZ39 and LS273 (resolution 300 ppi). (D) Schematic diagram of flooding identification equipment: (1) Flooded box, (2) root shading box, (3) open-well PCR plate, (4) upper platen. LS273-Sub: LS273 flooding treatment; ZZ39-Sub: LS273 flooding treatment; LS273-Air: LS273 blank control; ZZ39-Air: ZZ39 blank control.

Table 1.
Submergence stress affects the phenotype of rice of different resistances.
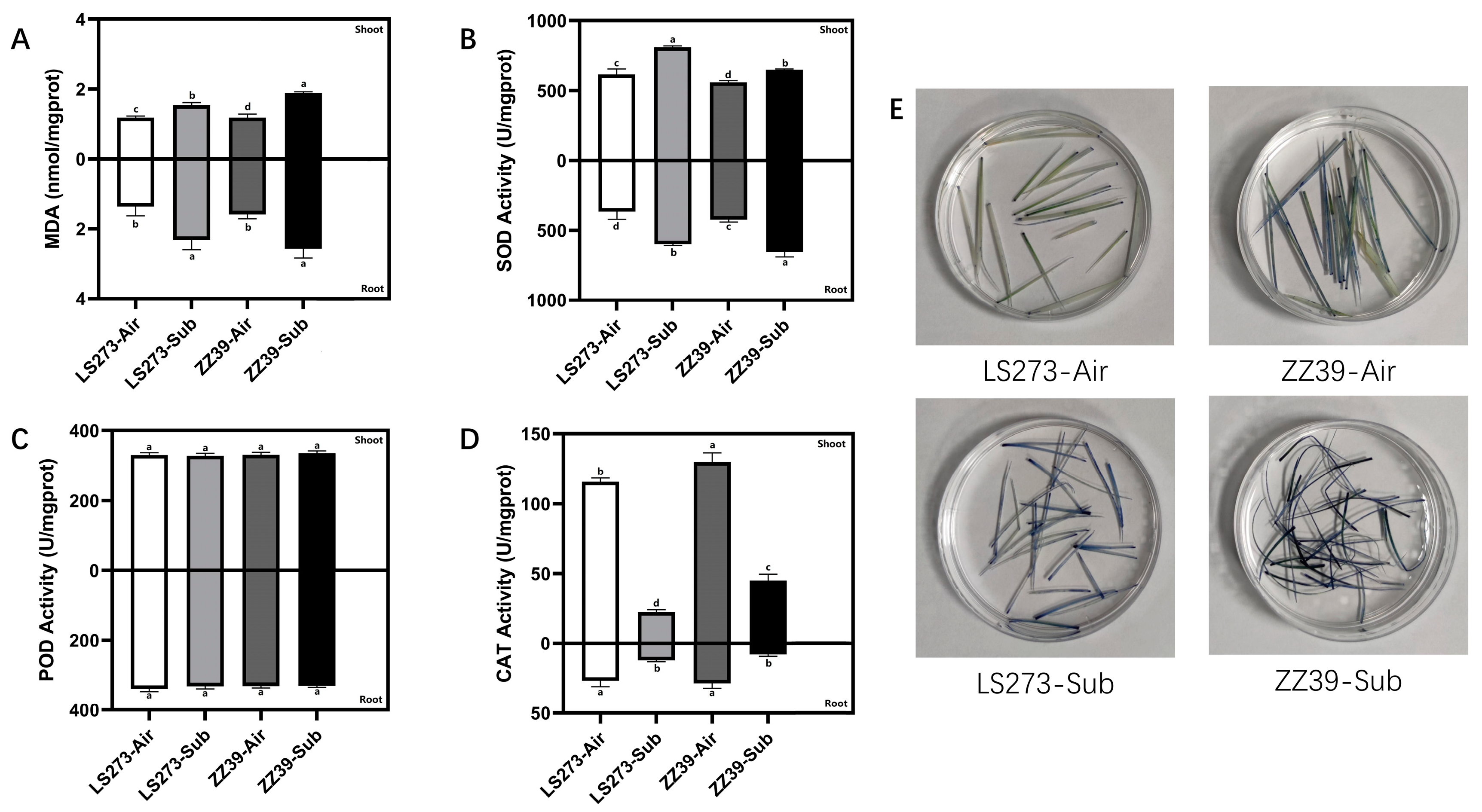
MDA is an important product of membrane lipid peroxidation. Rice injury under submergence stress is closely related to membrane lipid peroxidation induced by reactive oxygen species accumulation. We found that the MDA content of LS273 and ZZ39 increased significantly after submergence stress. The MDA content of the two cultivars showed no significant difference between shoots and roots when not under submergence stress (Figure 2A). The MDA content of ZZ39 was significantly different from LS273 and higher by 18.09% in shoots subjected to submergence stress. The MDA content of ZZ39 in roots was 9.73% higher than LS273. Shoots and roots antioxidant enzyme activity were measured, and it was found that both shoots and root superoxide dismutase (SOD) activity were significantly increased when subjected to submergence stress, with the highest SOD activity observed in LS273 shoots (Figure 2B). The SOD activity of ZZ39 under submergence stress was 19.84% higher than the blank control and 9.55% higher than that of the LS273. Peroxidase (POD) activity was not significantly different between treatments (Figure 2C). CAT activity was significantly reduced in both shoots and roots of the two cultivars under submergence stress and much higher in the shoots than in the roots (Figure 2D). Toluidine blue (TB) staining of the shoots showed that the superoxide anion radical (O2−) content of ZZ39 was higher than that of the LS273 cultivar under both the blank control and the submergence stress (Figure 2E).
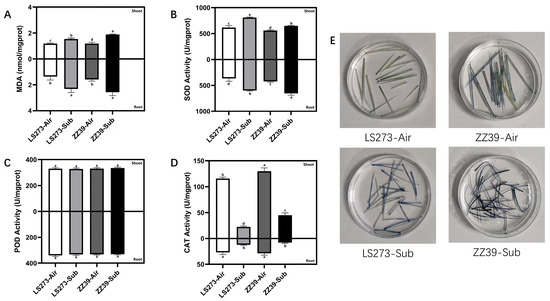
Figure 2.
Results of antioxidative enzymes and TB staining. (A) MDA content; (B) SOD activity; (C) POD activity; (D) CAT activity; (E) TB staining results. Columns with the same letter are not significantly different at p < 0.05, one-way ANOVA, followed by Tukey’s honest significant difference and other tests.
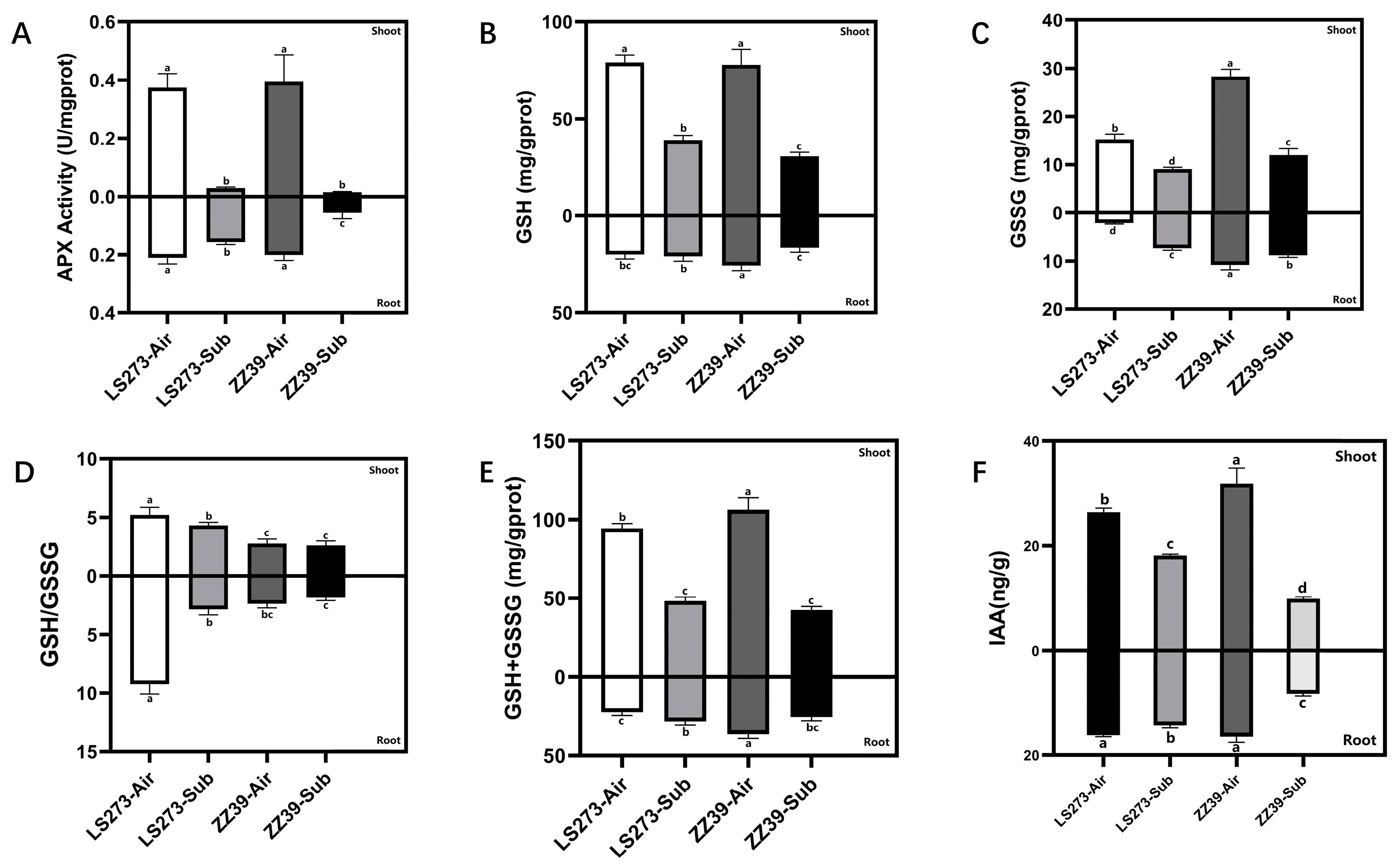
Under blank control, ascorbate peroxidase (APX) activity was significantly higher in shoots than in the roots, and there was no significant difference in APX activity in the same parts of the two varieties (Figure 3A). Under submergence stress, APX activity was significantly reduced in the shoots and roots of LS273 by 91.89 versus 23.81% and the shoots and roots of ZZ39 by 94.87 versus 70%, respectively.
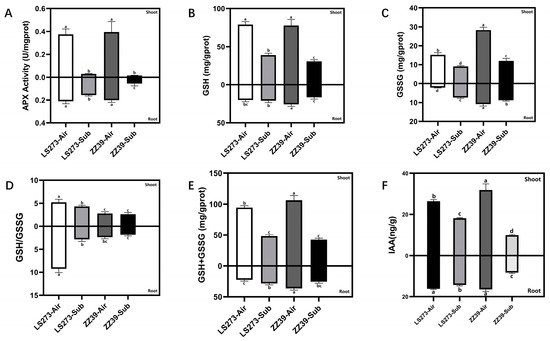
Figure 3.
Glutathione cycle and growth hormone measurement. (A) APX activity; (B) GSH content; (C) GSSG content; (D) GSH/GSSG; (E) GSH + GSSG content; (F) IAA content. Columns with the same letter are not significantly different at p < 0.05, one-way ANOVA, followed by Tukey’s honest significant difference tests.
The glutathione (GSH) measurement versus L-Glutathione oxidized (GSSG) content demonstrated that the GSH content of the shoots was significantly higher than that of the roots. Furthermore, there was no significant difference in the GSH content between LS273 and ZZ39 shoots under blank control (Figure 3B). Compared with the blank control, the GSH content of LS273 and ZZ39 decreased by 50.64 and 60.78%, respectively. The content of GSH in the roots was significantly higher under the blank control in ZZ39 than in LS273. Comparing the content of GSH in roots under submergence stress and blank control, we found no significant difference in LS273, while ZZ39 decreased by 35.42%. The GSSG content of the shoots in both cultivars was significantly higher than that of the roots under blank control (Figure 3C). Still, it was quite different between LS273 and ZZ39 shoots under the blank control. The GSSG content was 46.13% higher in the ZZ39 than in the LS273. Compared with the blank control, the GSSG content of LS273 and ZZ39 shoots under submergence stress decreased by 50.52 and 57.86%, respectively. Under a blank control, there was a considerable difference between the GSSG contents of LS273 and ZZ39 roots, with ZZ39 having 75.40% more than LS273. Under submersion stress, the GSSG of LS273 grew by 70.56% compared to the blank control, whereas that of ZZ39 declined by 18.18%.
Under submergence stress, the GSH/GSSG level of the LS273 shoot and root dropped by 17.62 and 69.16%, respectively, compared to the blank control (Figure 3D). Compared with the blank control, no significant changes in the GSH/GSSG were observed between the ZZ39 shoots and roots under submergence stress. GSH/GSSG in LS273 was 47.13 and 39.77% higher in shoots and 74.13 and 34.39% higher in roots than ZZ39, under both the blank control and submergence stress, respectively. The content of GSH + GSSG was higher in the shoots than in the roots of both cultivars (Figure 3E). Under submergence stress, the GSH + GSSG level in the shoots of LS273 and ZZ39 declined significantly compared to the blank control, while it increased by 21.30% in the roots of LS273 and reduced by 30.30% in the roots of ZZ39.
The IAA content in LS273 and ZZ39 shoots and roots under blank control was significantly higher than under submergence stress and markedly lower in ZZ39 shoots and roots than in LS273 (Figure 3F). Compared with the blank control, the IAA content of LS273 shoots and roots decreased by 31.46 and 11.67%, and ZZ39 shoots and roots decreased by 69.00 and 49.65%.
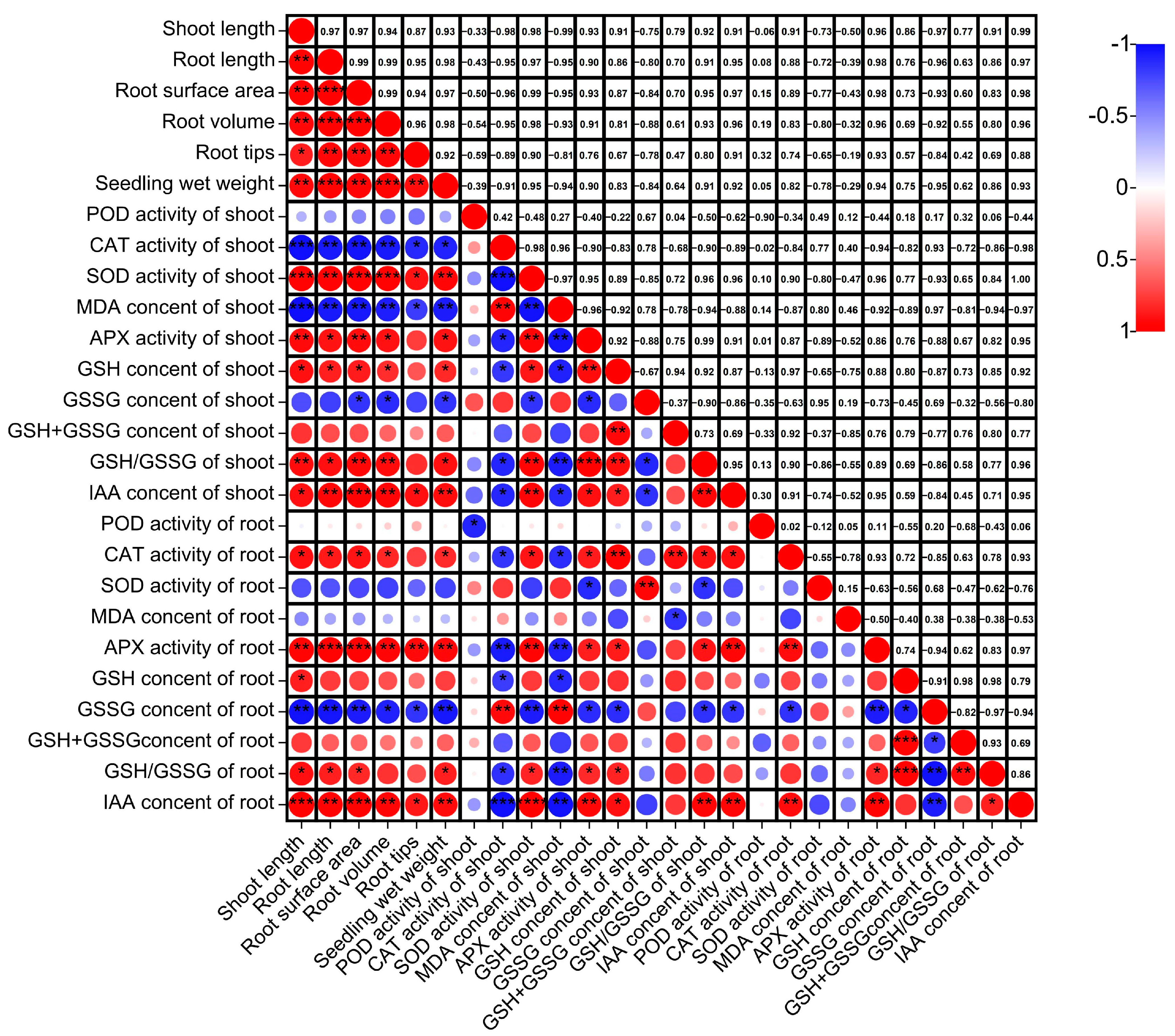
Correlation analysis (Figure 4) demonstrated that shoot height was highly and significantly correlated with SOD activity, APX activity, and GSH/GSSG (p < 0.01), positively correlated with IAA content of shoots (p < 0.05), and negatively correlated with CAT activity and MDA content of shoots (p < 0.01). Root length in the roots system was highly and significantly correlated with IAA content and APX activity (p < 0.01), CAT activity and GSH/GSSG (p < 0.05), and GSSG content (p < 0.01). The root surface area, root volume, root tips, and shoot wet weight were similar to the root length.
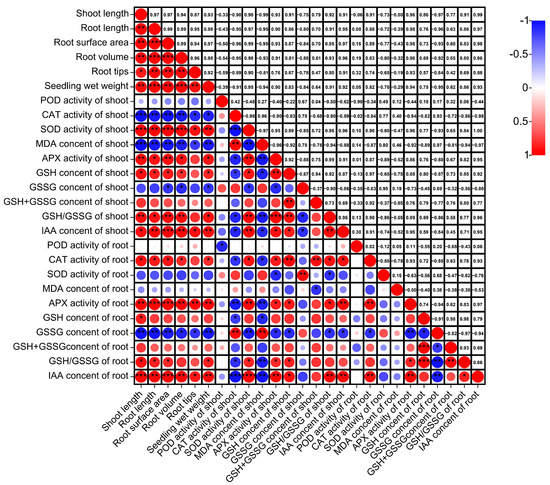
Figure 4.
Correlation analysis of shoot growth and physiological indicators under submergence stress. ****: significant at the 0.0001 probability level (p < 0.0001); ***: significant at the 0.001 probability level (p < 0.001); **: significant at the 0.01 probability level (p < 0.01); *: significant at the 0.05 probability level (p < 0.05).
2.2. RNA Sequencing (RNA-Seq) Analysis and Identification of Differentially Expressed Genes (DEGs)
RNA was extracted from the shoots and roots of the LS273 and ZZ39 grown under submergence stress and blank control. The shoots and roots were collected 7 days after sowing. On average, the RNA-Seq analysis resulted in 42.86–73.62 million raw reads with 92.47% clean reads (Table S1). Clean reads with high-quality scores amounted to over 94.20% at the Q30 level (Table S1). Refer to the genome download link at http://www.mbkbase.org/R498/R498_Chr.fasta.gz. Take note of the download link for the information: http://www.mbkbase.org/R498/R498_IGDBv3_coreset.tar.gz. The degree of gene expression was determined using the fragments per kilobase of transcript per million fragments mapped (FPKM) process, and the abundance of gene expression was analyzed using Cufflink’s software. Individual samples were analyzed using between-sample correlation analysis with PCA principal effect component analysis (Figure S1). The PCA results show that the biological repeats of each treatment were significantly aggregated, indicating the credibility of the transcriptome analysis conclusion. The largest source of variation in PC1 (65.6%) was tissue differences. PCA2 (18.92%) was caused by differences, indicating clear transcriptome variations between tissues. The closer spacing between samples of the same variety’s roots and shoots suggests that the transcriptome change was larger in the shoots than in the roots.
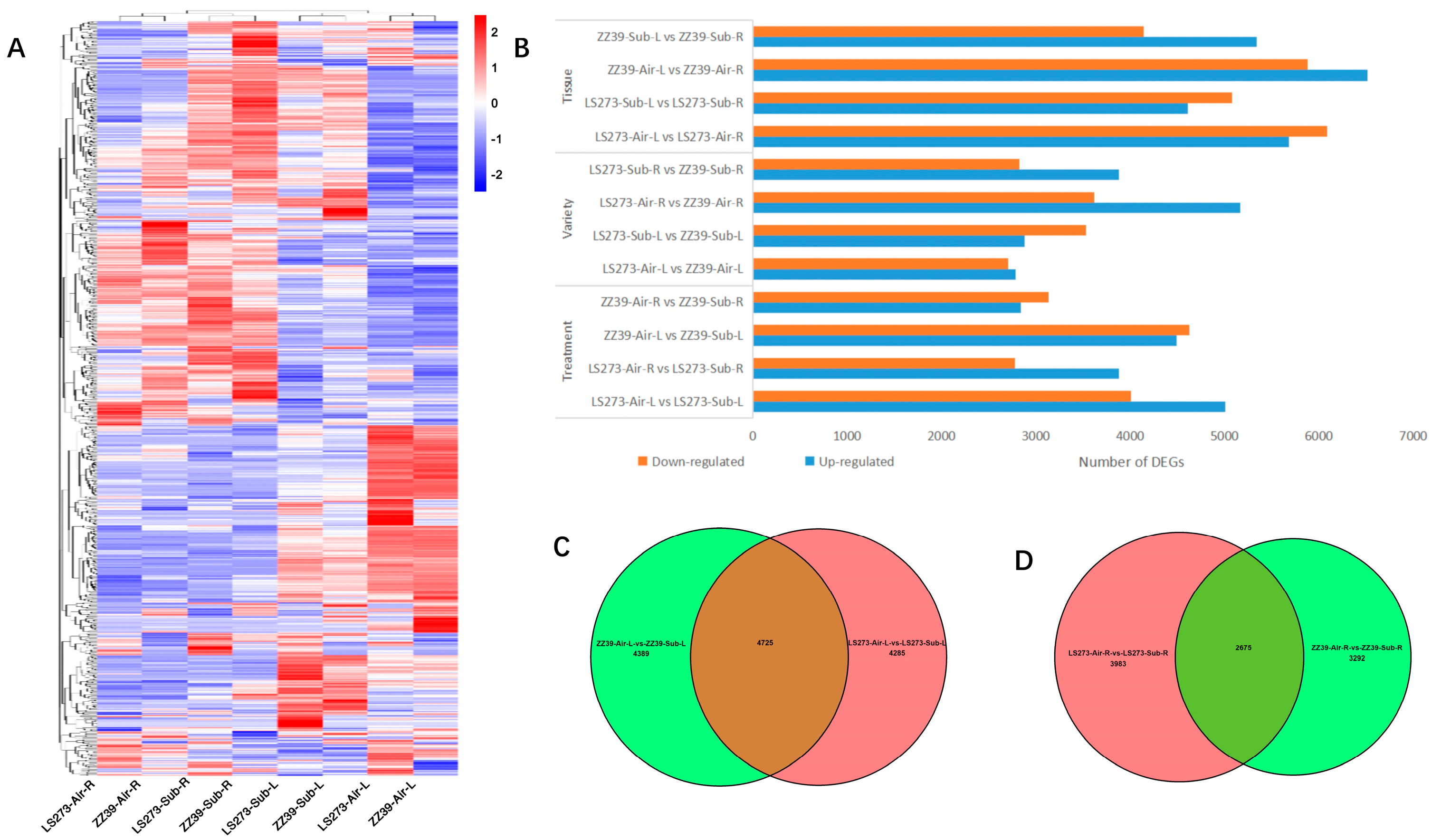
In addition, we selected 758 rice genes related to submergence stress reported by previous studies and the expression of the corresponding genes in the samples for cluster analysis (Figure 5A). Cluster analysis identified different expression levels among treatments, varieties, and tissues. The DEGs were divided into tissue, variety, and treatment categories (Figure 5B). ZZ39 underwent submersion stress with 9485 DEGs (5341 upregulated, 4144 downregulated) in the tissue dimension. ZZ39 has 12,386 DEGs in blank control, of which 6511 were upregulated and 5875 were downregulated. After the stress from submergence, LS2732 had 9687 DEGs (4580 downregulated and 4607 upregulated). In blank control, LS273 has 11,759 DEGs (5677 upregulated and 6082 downregulated). Under submergence stress, there are 6703 DEGs in roots (3879 upregulated and 2824 downregulated) and 6415 DEGs in shoots (2887 upregulated and 3533 downregulated) in the various dimensions. Moreover, there are 8786 DEGs (5166 upregulated and 3620 downregulated) in roots and 5493 DEGs (2786 upregulated and 2707 downregulated) in shoots under blank control. In the treatment dimension, ZZ39 had 5967 DEGs (2836 upregulated and 3131 downregulated) in roots and 9114 DEGs (4488 upregulated and 4626 downregulated) in shoots. LS273 had 6658 DESs (3879 upregulated and 2779 downregulated) in roots and 9010 DEGs (5003 upregulated and 4007 downregulated) in shoots. The number of DEGs demonstrated differences in the dimensions of treatment, variety, and tissue, among which the differences between different tissues were the most significant and the difference between different varieties was the least important.
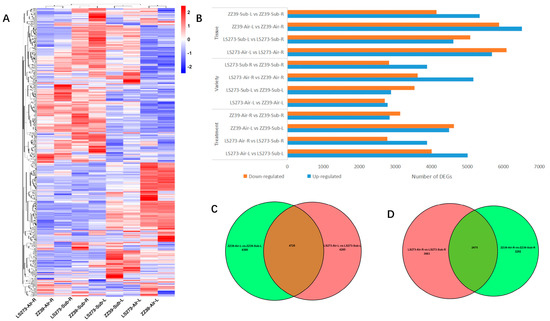
Figure 5.
DEGs under flooded conditions. (A) Heatmap of DEGs between treatments; (B) number of DEGs in different tissues, varieties, and treatments; (C) unique and shared DEGs in shoots of LS273 and ZZ39 under flooded conditions; (D) unique and shared DEGs in roots of LS273 and ZZ39 under flooded conditions.
2.3. GO and KEGG Pathway Enrichment Analysis in Shoots and Roots
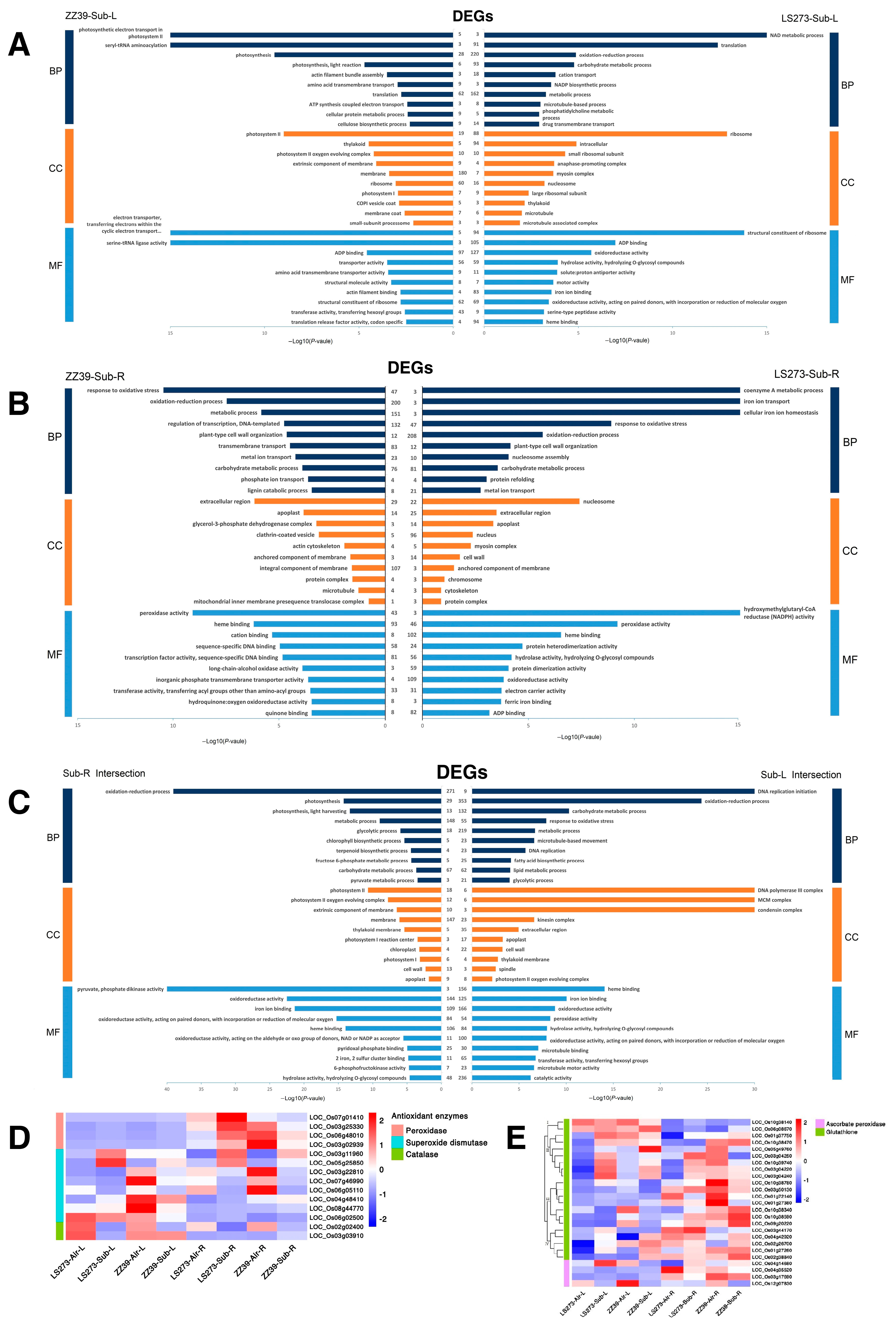
Through the collective analysis of DEGs of LS273-Air-L vs. LS273-Sub-L and ZZ39-Air-L vs. ZZ39-Sub-L, the similarities and differences of the expressed genes of the shoots of the two rice varieties under submergence conditions were obtained (Figure 5C). Among them, there were 4285 unique DEGs for LS273 and 4389 unique DEGs for ZZ39, with 4725 common DEGs. In order to understand the function of these special response genes in the shoots during submersion stress, GO and KEGG analyses were also carried out on DEGs.
GO analysis identified that the NAD metabolic process (GO:0019674) and carbohydrate metabolic process (GO:0005975) in the LS273 shoot was seriously affected (Figure 6A). In addition, the oxidoreductase activity (GO:0016491) and iron ion binding (GO:0005506) were also highly enriched in LS273. In the shoots of the ZZ39, photosynthetic electron transport in photosystem II (GO:0009772), photosystem II (GO:0009523) and photosystem I (GO:0009522) were primarily affected. In addition, seryl-tRNA aminoacylation (GO:0006434) was also highly enriched in ZZ39. The shoots of the two varieties are involved in the oxidation-reduction process (GO:0055114) and carbohydrate metabolic process (GO:0005975). In addition, the fatty acid biosynthetic process (GO:0006633), lipid metabolic process (GO:0006629), iron ion binding (GO:0005506), cell wall (GO:0005618), and glycolytic process (GO:0006096) were significantly enriched (Figure 6C). KEGG pathway analysis identified that LS273 was mainly improved in the peroxisome (dosa04146), plant hormone signal transduction (dosa04075), fatty acid metabolism (dosa01212), and flavone and flavanol biosynthesis processes (dosa00944) (Figure S2). ZZ39 was mainly enriched in the biosynthesis of secondary metabolites (dosa01110), aminoacyl-tRNA biosynthesis (dosa00970), and piperidine and pyridine alkaloid biosynthesis (dosa00960). The shoots of both cultivars were enriched in carbon metabolism (dosa01200), biosynthesis of secondary metabolites (dosa01110), metabolic pathways (dosa01100), and phenylpropanoid biosynthesis (dosa00940).
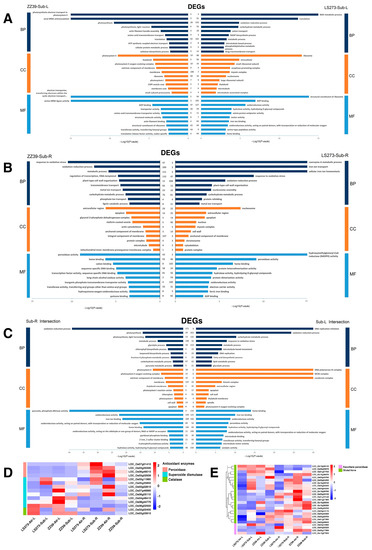
Figure 6.
GO enrichment under submergence stress and expression of DEGs under the antioxidant system. (A) The enrichment results of DEGs in shoots; (B) the enrichment results of DEGs in roots; (C) enrichment results of shared DEGs in shoots and roots; (D) expression of DEGs in antioxidant enzyme system; (E) expression of DEGs in glutathione cycle. BP: Biological Process; CC: Cell Component; MF: Molecular Function; I, II, II and IV: Cluster analysis type.
The difference of root expression genes between LS273-Air-R vs. LS273-Sub-R and ZZ39-Air-R vs. ZZ39-Sub-R under submergence conditions was obtained using aggregate analysis of DEGs of LS273-Air-R vs. LS273-Sub-R and ZZ39-Air-R vs. ZZ39-Sub-R (Figure 5D). Among them were 3983 unique DEGs in LS273, 3292 unique DEGs in ZZ39, and 2675 common DEGs. GO and KEGG analyses were conducted to clarify the role of the response genes in roots under submergence stress.
In the GO analysis, DEGs of the LS273 cultivar were largely enriched in redox-related functions, including response to oxidative stress (GO:0006979) and peroxidase activity (GO:0004601), as well as carbohydrate metabolic process (GO:0005975) and metal ion transport (GO:0030001) (Figure 6B). The DEGs of ZZ37 were also largely enriched in redox functions, including response to oxidative stress (GO:0006979) and peroxidase activity (GO:0004601), metabolic processes (GO:0008152), and carbohydrate metabolic process (GO:0005975). Both materials were enriched in pyruvate, phosphate dikinase activity (GO:0050242), fructose 6-phosphate metabolic process (GO:0006002), oxidation-reduction processes (GO:0055114), and oxidoreductase activities (GO:0016491) (Figure 6C). The KEGG pathway analysis revealed that LS273 is predominantly enriched for plant hormone signal transmission (dosa04075), biosynthesis of secondary metabolites (dosa01110), metabolic pathways (dosa01100), and phenylpropanoid biosynthesis (dosa00940) (Figure S2). ZZ39 was primarily enriched in the biosynthesis of secondary metals (dosa01110), metabolic pathways (dosa01100), phenylpropanoid biosynthesis (dosa00940), and starch and sucrose metabolism (dosa00500). The DEGs shared by both varieties were highly enriched in carbon metabolism (dosa01200), biosynthesis of secondary metabolites (dosa01110), metabolic pathways (dosa01100), diterpenoid biosynthesis (dosa00904), glyoxylate and dicarboxylate metabolism (dosa00630), and tyrosine metabolism (dosa00350).
2.4. Shoot and Root DEGs in the Reactive Oxygen Species (ROS) Removal System
The production of ROS can have severe effects on plant growth. A primary mechanism for plants to alleviate the stressful environment is to scavenge the excessive superoxide ion accumulation formed by the ROS reaction. Since SOD, CAT, and POD are the primary antioxidant enzymes, KEGG analysis suggests that submersion stress significantly impacts the redox system of rice shoots. As a result, 18 DEGs connected to the method of antioxidant enzymes were found (Table S2). The expression of genes related to oxidases (LOC_Os07g01410, LOC_Os03g25330, LOC_Os06g48010, and LOC_Os03g02939) was higher in shoots than in roots. These genes were upregulated in both shoots and roots of LS273 following submergence stress. It is noteworthy that ZZ39 was upregulated in the shoot but downregulated in the roots following submergence stress (Figure 6D).
Rice contains numerous SODs, including four CuZn-SODs, two chloroplastic Fe-SODs, and one mitochondrial Mn-SOD. Differences in the expression of different species of SOD were identified in response to submergence stress at various sites. The genes LOC_Os03g22810, LOC_Os08g44770, LOC_Os03g11960, LOC_Os04g48410, and LOC_Os07g46990 belong to CuZn-SODs. Under submergence stress, LOC_Os03g11960 expression in the LS273 shoots and roots was upregulated, but no significant changes in its expression were observed in the ZZ39 shoots. LOC_Os03g22810 and LOC_Os07g46990 were downregulated. LOC_Os04g48410 expression in LS273 and ZZ39 shoots showed the opposite trend. LOC_Os08g44770 was significantly downregulated in ZZ39 shoots after submergence stress. LOC_Os06g05110 and LOC_Os06g02500 belong to the chloroplast Fe-SOD. LOC_Os06g05110 was downregulated in all parts following submergence stress. LOC_Os06g02500 was downregulated in the shoots and upregulated in the roots of both varieties. LOC_Os05g25850 belongs to the mitochondrial Mn-SOD, which is upregulated in all parts after submergence stress. The expression of catalase-related genes LOC_Os02g02400 (OsCATA) and LOC_Os03g03910 (OsCATC) in the shoots was higher than in the roots. Under submergence stress, the expression of LOC Os02g02400 was decreased relative to the blank control. The expression of LOC_Os03g03910 was downregulated in the LS273 shoots and upregulated in roots under submergence conditions.
APX is one of the most important antioxidant enzymes in plant active oxygen metabolism, particularly the key enzyme in the chloroplast for scavenging H2O2. It is also the primary enzyme in ascorbic acid (ASA) metabolism. ASA and GSH combine to eliminate H2O2 via the ascorbic acid-glutathione cycle (AsA-GSH). Four DEGs related to APX were screened (Table S3). LOC_Os04g14680 (OsAPx3), LOC_Os04g35520 (OsAPx7), LOC_Os03g17690 (OsAPx1) and LOC_Os12g07830 (OsAPx5), and these genes are expressed differently in different varieties and tissues (Figure 6E). Under submergence stress, glutathione can scavenge the increased hydrogen peroxide via the AsA-GSH cycle. Twenty-one DEGs related to glutathione were identified (Table S4). Following submergence stress, cluster analysis revealed significant changes in the expression of numerous genes (Figure 6E), including up- or downregulated glutathione-s transferase-related genes. The first DEG class was expressed more in the shoots than in the roots. Following submersion stress, the second kind of DEGs was markedly elevated in LS273 shoots. While the fourth kind of DEGs was promoted in LS273 and ZZ39 shoots following submergence stress, the third type of DEGs was higher than that in the shoot.
2.5. Auxin Regulation and Resistance to Submergence Stress in Rice
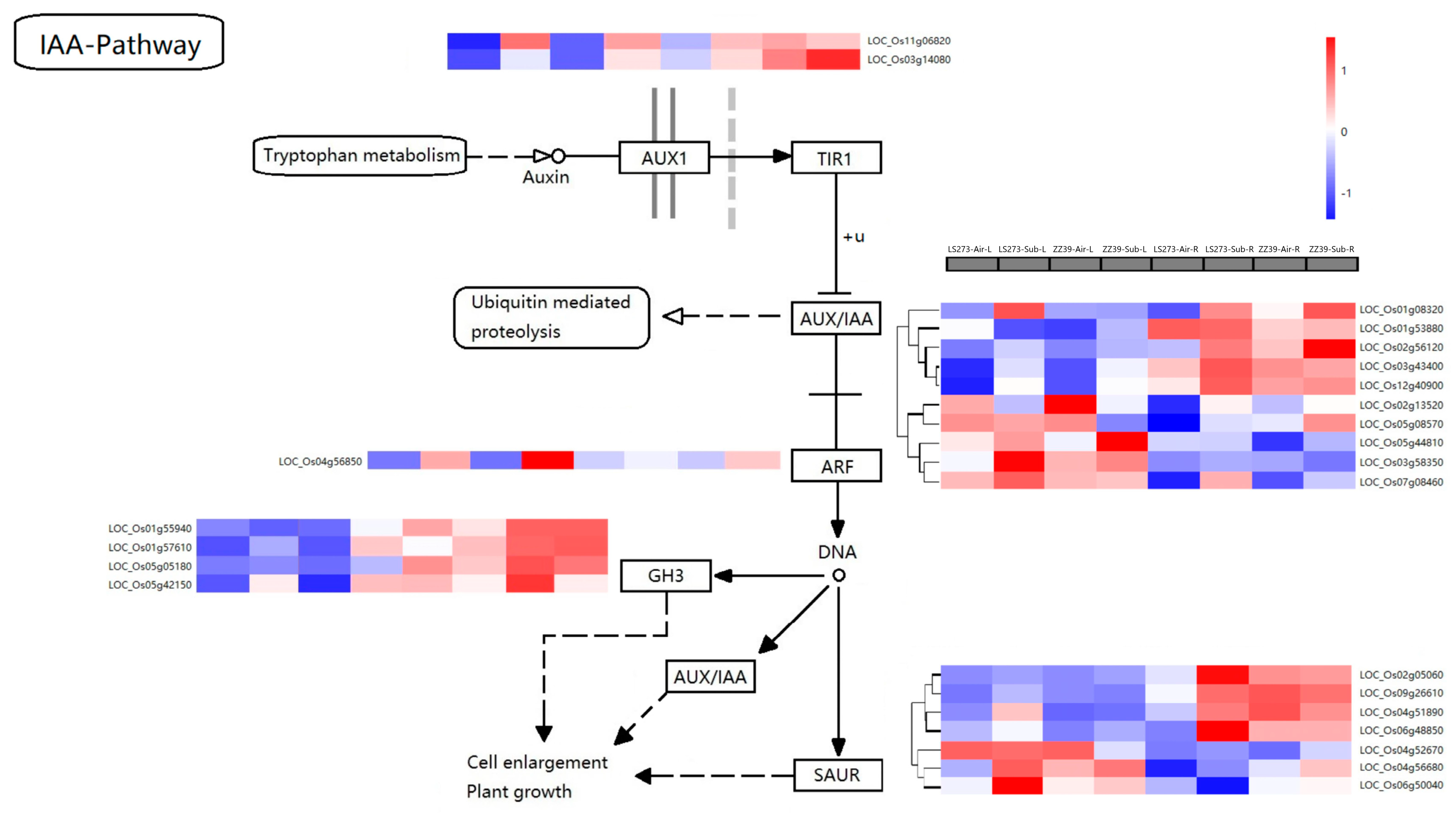
Auxin is one of the first hormones that was discovered and subsequently studied. Auxin promotes plant growth by expanding cell size. As determined by KEGG analysis, plant hormone signal transmission (dosa04075) was significantly enriched. Through differential gene screening, 24 DEGs related to auxin were found (Table S5).
The DEGs in auxin metabolism were analyzed using a KEGG amp (Figure 7). Among the genes involved in the process, AUX1-related genes LOC_Os11g06820 and LOC_Os03g1408 were upregulated under submergence stress. The upregulation of the shoot was much more significant than roots, but LOC_Os11g06820 was downregulated under submergence stress in ZZ39 roots. Auxin early response genes include AUX/IAA, GH3, and SAUR gene families. The expression of these genes was different under submergence stress. Among the AUX/IAA family genes, ten were classified into four categories via cluster analysis. LOC_Os01g08320 belonged to the first category of genes. In response to submergence stress, this gene was increased in the shoots and roots of LS273 and the roots of ZZ39 but not in the shoots. Under submergence stress, the expression of the second gene type (LOC_Os01g53880, LOC_Os02g56120, LOC_Os03g43400, and LOC_Os12g40900) was significantly lower in the shoots than in the roots and upregulated in both LS273 and ZZ39 shoots and roots. LOC_Os02g13520 and LOC_Os05g08570 belonged to the third category of genes, distinguished by their stress-induced downregulation in the shoots and upregulation in the roots.
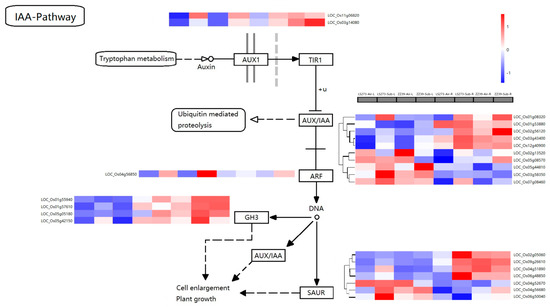
Figure 7.
Expression of DEGs in the IAA pathway.
LOC_Os05g44810, LOC_Os03g58350, and LOC_Os07g08460 belonged to the fourth group of genes, which were upregulated in LS273 shoots under submergence stress. ARF, as an auxin-responsive factor, can activate the expression of downstream genes, and LOC_Os04g56850, as an auxin-response factor-related gene, was upregulated in both the shoots and roots under submergence stress compared with the blank control. The upregulation rate of the shoot was significantly higher than that of the roots. LOC_Os01g55940, LOC_Os01g57610, LOC_Os05g05180, and LOC_Os05g42150 were associated with GH3-responsive genes. The expression of these DEGs in roots was much higher than in shoots. LOC_Os01g55940 was downregulated in LS273 shoots and upregulated in ZZ39 shoots. According to differences in gene expression, the SAUR gene family can be subdivided into four gene types. The expression of LOC_Os02g05060 and LOC_Os09g26610 in the shoots was lower than in the roots. The expression in LS273 was significantly upregulated under submergence stress but not significantly changed in ZZ39. Compared with the blank control, LOC_Os04g51890 and LOC_Os09g26610 expression in the shoots and roots were upregulated under submergence stress, and the upregulation in LS273 was significantly higher than in ZZ39. LOC_Os04g52670 expression in the shoot was higher than in the roots. When LOC_Os04g56680 and LOC_Os06g50040 were subjected to submergence stress, the variation of LS273 gene expression was greater than that of ZZ39.
2.6. Validation of qPCR Results
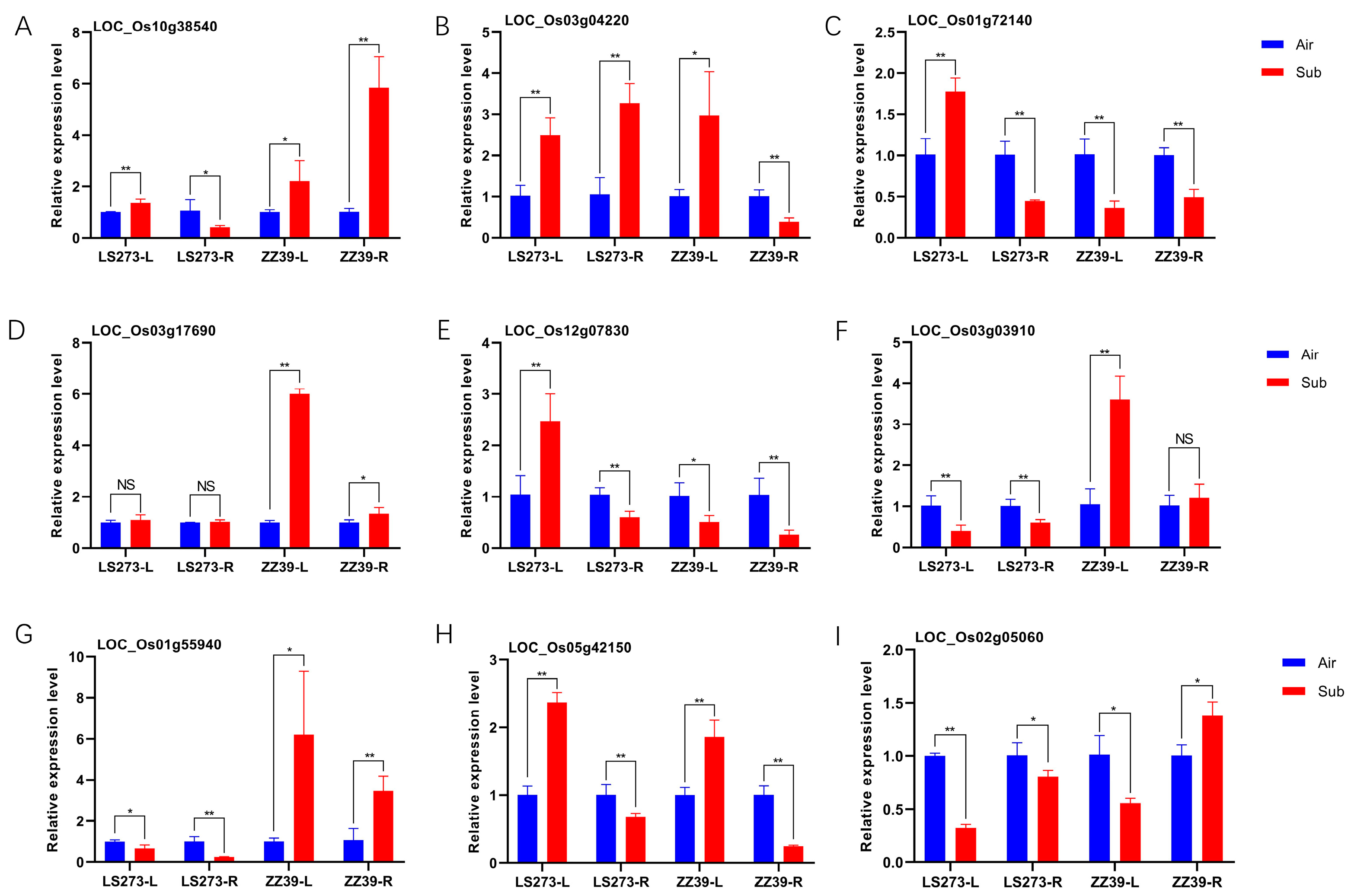
To verify the effect of the antioxidant system and auxin on rice under submergence stress, we detected the expression of related genes in different parts of different cultivars (Figure 8). We selected three glutathione S-transferase-related genes for detection. LOC_Os01g72140 was significantly upregulated in LS273 shoots but downregulated in ZZ39 shoots; LOC_Os10g38540 was significantly downregulated in LS273 roots but upregulated considerably in ZZ39 roots; LOC_Os03g04220 was significantly upregulated in LS273 roots but significantly downregulated in ZZ39 roots. The expression of LOC_Os03g17690 (OsAPX1) was not different in LS273 but was upregulated in ZZ39. Although it was downregulated in ZZ39 shoots, LOC_Os12g07830 (OsAPX5) was upregulated in LS273 shoots. In the shoots and roots of LS273 but upregulated in the shoots of ZZ39, LOC_Os03g03910 (OsCATC) was differentially expressed. The expression of LOC_Os01g55940 (OsGH3-2) was upregulated in ZZ39 and downregulated in LS273. After the stress from submersion, LOC_Os05g42150 (OsGH3-4) was upregulated in shoots and downregulated in roots. The expression of LOC_Os02g05060 of the SAUR gene family was higher in roots than in shoots after submergence stress.
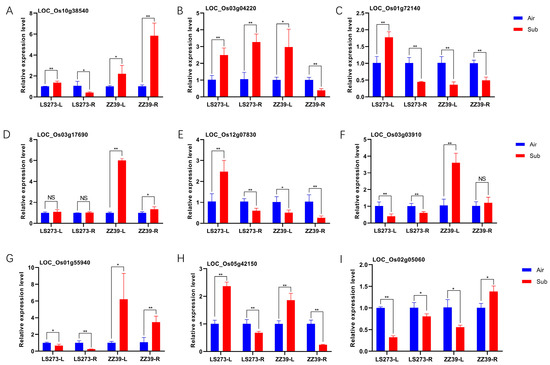
Figure 8.
Quantitative real-time PCR (q-PCR) determination of antioxidant-related genes in different parts of varieties (A–F). Quantitative real-time PCR (q-PCR) to determine auxin-related genes in different parts of various varieties (G–I). **: significant at the 0.01 probability level (p < 0.01); *: significant at the 0.05 probability level (p < 0.05); NS: no significant difference.
3. Discussion
The phenotypic and physiological indices identified differences in the responses of resistant and susceptible varieties under submergence stress. The phenotypic difference was primarily reflected in that the phenotypic indexes of ZZ39 were significantly lower than LS273 after submergence stress. The dynamic examination of shoot height showed that 4 days after seeding, the growth rate of LS273 shoots dramatically increased. LS273′s main root length was the same as under the blank control, but submergence stress impeded the growth of its lateral roots and root hairs. According to the phenotypic traits of the shoots and roots, the submergence-tolerant variety LS273 may withstand hypoxic stress brought on by submersion by growing quickly. This pattern of rapid growth and escape is similar to previous research conclusions. This flood adaptation is described as low O2 escape syndrome (LOES) [18].
ROS is a harmful byproduct of plant metabolism and is a general term for a class of molecules or ions with high oxidative activity. As a signaling molecule, ROS plays an essential role in mediating plant responses to abiotic stresses [19]. Mitochondria and chloroplasts are the key sites of ROS production, as excess ROS can cause damage to nucleic acids, proteins, and lipids. MDA is also an important indicator of cellular oxidative damage. Different resistant maize inbred lines showed significant differences in MDA content after drought stress [20]. This study showed that, after submergence stress, the MDA concentration of LS273 shoots was considerably lower than that of ZZ39 shoots. These findings are consistent with earlier findings about submergence stress in rice during the tillering stage, and they show that submergence stress may cause membrane lipid peroxidation in susceptible cultivars [21].
Both water stress [22] and potassium ion stress [23] will increase the activity of antioxidant enzymes, while low temperature will inhibit enzymatic activity [24]. This study found that compared with the blank control, the SOD enzyme activity of the roots and shoots was significantly increased, and the CAT enzyme activity was significantly decreased under the submergence stress treatment. The genes related to the ROS scavenging system were analyzed, and significant differences in the expression of genes related to different enzymes were identified. Compared to the roots, the expression levels of the four genes encoding members of the peroxidase family proteins were significantly lower in the shoots. The role of the superoxide dismutase-related gene ALM1, which is found in the chloroplast, is to prevent excessive ROS from disrupting plant development normally [25]. This study found that this gene was downregulated in both shoots and roots under submergence stress, and there were few differences in the expression levels between roots and shoots. Other genes related to superoxide dismutase can also have specific differences in expression. When rice shoots are subjected to drought stress, the expression of the catalase genes OsCATA and OsCATC are inhibited [26]. This study also found that the expression levels of OsCATA and OsCATC in LS273 shoots were higher than in roots. Notably, OsCATC was unchanged in shoots but upregulated in the roots of ZZ39 under submergence stress conditions.
In addition to the antioxidant enzyme system, the ascorbate-glutathione cycle (AsA-GSH) is also an essential antioxidant system. GSH and APX are responsible for scavenging drought and low-temperature-induced ROS detoxification, and their increased expression is correlated with germination rate, root length, and fresh weight accumulation [27]. This study demonstrated a significant reduction in APX activity in shoots and roots after submergence. OsAPX3, OsAPx7, OsAPX1, and OsAPx5 in the AsA-GSH cycle were differentially expressed in each variety and different tissues following submergence stress. These results are similar to previous studies under heat stress [28] and salt stress [29]. GSH is one of the most important antioxidants in the AsA-GSH cycle, and a higher GSH pool can maintain the structural stability of membrane proteins [30]. GSSG and GSH can be produced through the interaction of specific enzymes. We found that the decrease in GSH content in submergence-tolerant varieties was much lower than that in non-submergence-tolerant varieties. The changes in GSSG content in the roots of the two varieties were also opposite. Under submergence stress, the GSH concentration of LS273 was higher than that of ZZ39 in the shoots and roots. The GSSG content of LS273 was lower than that of ZZ39 in the shoots and roots. GSH/GSSG can effectively reflect the redox state of cells and is also one of the leading indicators of the glutathione redox cycle [31]. Under blank control conditions and submergence stress, the GSH/GSSG ratios in LS273 shoots and roots were higher than in ZZ39, suggesting that LS273 has a more vital anti-redox ability. TB staining also proves that LS273 contains less superoxide anion than ZZ39. The submergence tolerance of LS273 is hypothesized to be mostly attributable to its stable and effective antioxidant system, which ensures the steady state of cell function and maintains effective growth and development based on the measurement of physiological indicators and the results of TB staining.
With the discovery and verification of the Sub1 gene [32] and the cloning of SNORKEL1 and SNORKEL2 [33], many previous studies have shown that ethylene, ABA, and GA participate in the elongation of the coleoptile during the anaerobic germination of seeds in the early stages [34]. This study investigated auxin as the key factor in improving the submergence of rice following germination. Auxin is the first hormone to promote plant growth [35] and plays a vital role in plant growth and development. Auxin affects the elongation and division of plant cells, the growth of the main and lateral roots and hypocotyls, the development of vascular tissue, plant gravitropism and phototropism, and the formation of root hairs and flower organs. It is important for plants’ morphogenesis and early development [36]. This study discovered that both auxin content and auxin-related gene expression are impacted by submergence stress. The levels of auxin, the height of the shoots, and the length of the roots all showed a strong positive association. This suggests that rice’s capacity to emerge from water under submersion stress is influenced by the auxin levels.
AUX1 co-transporter is responsible for auxin transport from extracellular to intracellular [37]. According to KEGG pathway analysis, AUX1-related genes were significantly upregulated after submergence stress. There are three classes of auxin-responsive genes, and they are called Aux/IAA, SAUR, and GH3. The Aux/IAA family is crucial in controlling the expression of genes that respond to auxin. It has been found that the SAUR gene generates a category of highly labile transcripts. GH3 is responsible for the feedback regulation of auxin, thereby controlling the homeostasis of intracellular auxin. AUX/IAA plays a central role in regulating auxin-responsive gene expression. Studies on Arabidopsis mutants showed that the function of the AUX/IAA gene was specific, and the auxin response was tissue-dependent, which may have both promoting and inhibiting effects [38]. The ten DEGs associated with AUX/IAA proteins also showed this specificity.
For example, LOC_Os03g58350 was significantly upregulated in shoots but not in roots under submergence stress, and LOC_Os07g08460 was significantly upregulated in both shoots and roots of LS273, but ZZ39 did not demonstrate any significant changes under submergence stress. SAUR is an auxin fast-response gene family consisting of 79 members in Arabidopsis, including SAUR19 and SAUR63, which are positive regulators of cell growth [39]. Seven genes related to the SAUR family were detected in this study, and gene expression differences were identified. For example, the expression level of LOC_Os02g05060 in roots was higher than in shoots and was upregulated in roots of LS273 but unchanged in ZZ39 roots after submergence stress. LOC_Os04g52670 showed greater expression in shoots than in roots. An auxin in situ response gene is the GH3 gene. Auxin amino acid synthase activity in the GH3 protein participates in salicylic and jasmonic acid to drive plant defense responses and dynamically regulate auxin balance [40]. The four GH3-related genes identified in this study had higher root expression levels than the shoots. Auxin enrichment increased the expression of LOC_Os05g42150 (GH3-4), and we found that the expression of GH3-4 in shoots was upregulated but decreased in roots, indicating that both rice species tried to increase leaf IAA content and reduce root IAA content after submergence. LOC_Os01g55940 was speculated to be related to GH3.2. We found that the expression levels of GH3.2 were opposite in different resistant materials, which showed that LS273 was downregulated and ZZ39 was upregulated. A previous study on Arabidopsis discovered that overexpression (OE) of GH3.2 results in dwarf plants with shorter taproots [41], which is consistent with the current findings. ARF auxin response factor is a unique family of plant transcription factors [42]. ARF induces a specific response to plant growth by interacting with AUX/IAA [43]. In this study, ARF auxin response factor-related genes were increased in both cultivars’ shoots and roots, with shoot expression varying more than roots. These findings suggest that submergence-tolerant rice varieties strive to improve auxin content in various tissues by changing the expression of genes associated with the auxin pathway to achieve the objectives of escaping water from shoot growth and protecting root growth so that it can absorb nutrients.
Based on the information mentioned above, we speculate that LS273, a submergence-tolerant cultivar, can maintain a more stable antioxidant system under flooding stress than ZZ39, maintaining the capacity of plants to thrive. Although this shift in antioxidant capacity already occurs during normal growth, immersion stress causes it to become more noticeable. We speculate that ASA-GSH may be primarily responsible for stabilizing this antioxidant system. By promoting the development of IAA, the submergence-tolerant variety LS273 can also ensure the long-term growth of stems and roots under submersion stress.
Previous studies on flooding stress at the rice bud stage mainly focused on seed germination and coleoptile formation [5,6,7]. Previous studies have looked at the ability of longer mesoblasts and coleoptiles to promote emergence versus resistance to adversity. Since flooding stress can significantly reduce the occurrence of weeds [16], we speculated that under the direct seeded mode of rice, selecting a flooding-tolerant cultivar paired with artificial flooding stress might be possible to protect rice growth while reducing weed numbers. This modality can significantly reduce the amount of herbicide and protect the ecological environment. Thus, our study focused on the outcome of continuous flooding for 9 days after seed germination. We found that the flooding tolerance of rice at this stage was not determined by the coleoptile length but rather by the combination of the antioxidant capacity and auxin of rice.
Meanwhile, we suggest that not only the ability of seedlings to escape from the water surface may guarantee rice growth during direct sowing, but also the ability of the root system to become immobilized in the soil is equally important. Therefore, we analyzed shoots and roots under flooding stress and found significant regulatory differences between shoots and roots at the transcriptome level. In conclusion, our study offers a foundation for the molecular network of several rice tissue types during submersion stress at the seedling stage. It presents theoretical backing for rice cultivation under direct sowing using a flooding-tolerant rice variety and a rice cultivation model under flooding stress.
4. Materials and Methods
4.1. Identification Methods and Material Selection
Based on summarizing the research methods so far [4,17,18], a device for identifying the submergence tolerance of rice seedlings was designed (Figure 1D). This device avoids root damage, more clearly displays the phenotypic traits of root and shoot under submersion stress, reduces interference between seeds and external factors, and increases the identification conclusion reliability and repeatability compared to previous identification methods using centrifuge tubes and potted plants. The identification procedure was as follows: rice seeds were sterilized with 1.5% (v/v) sodium hypochlorite for 20 min and rinsed with double-distilled water (ddH2O), soaked in water at 26 °C for 36 h, moistened, and germinated at 36 °C for 12–16 h until seeds germinated about 1 mm long. Rice seeds that met the criteria were placed within the bottom open cell PCR plate (with an opening radius of 1.2 mm). The seeded PCR plates were placed on a light shielding box inside the apparatus, to which a cover was subsequently added. After that, they were placed inside an artificial climate chamber (FH-1200, China). The temperature was set to 26 degrees Celsius, 50% humidity, 14 h of light, 10 h of darkness, and a light intensity of 6000 lx.
Water was added to the water level of 9 cm and changed every 3 days, and after the submergence, stress was maintained for 9 days. The blank control sowed the seeds on the same PCR plate and placed them in the submergence identification apparatus, adding water to 1/2 of the seeds. We screened more than 1000 materials, and through multiple screening and identification, we finally determined the submergence-resistant variety LS273 and the submergence-sensitive variety ZZ39. Multiple identifications were used by LS273 and ZZ39 to establish their submergence resistance specificity. The China Rice Research Institute developed the indica rice variety ZZ39. Indica variety LS273 is a high-generation inbred line of R513/R900. The examined rice seeds were collected in Changsha, China, between September and October 2020 and stored for three months before the experiment’s start.
4.2. Phenotype Determination and Physiological Index Determination
Seedling height was recorded daily using vernier calipers after sowing. The root was scanned using an LA-S Plant roots analyzer (Wanshen, China, Hangzhou) on the 9th day after sowing. Ten seedlings were sampled to determine the fresh weight after blotting off the surface moisture with paper towels on the 9th day after sowing. Plant samples were taken on the 9th day after sowing, divided into shoot and root (the seedling is divided into three parts: seed, shoot, and root. The shoot part does not take the coleoptile), and subsequently immediately placed in liquid nitrogen to snap freeze and placed in −80 °C ultra-low temperature freezer for determination of physiological indexes. Using biochemical index determination kits (Jiangsukeming, China, Jiangsu) for superoxide dismutase activity (SOD), catalase activity (POD), peroxidase activity (CAT), ascorbic acid peroxidase activity (APX), malondialdehyde (MDA) content, glutathione (GSH) content, and L-Glutathione oxidized (GSSG) content. For rapid visualization of damaged shoots, we used the toluidine blue test. The seedlings were taken on the 9th day after sowing, washed with pure water, and the excess water absorbed. The tissue parts were immersed in TB staining solution, vacuumed, and protected from light for 1 h. The samples were taken out, immersed in 95% ethanol at 80 °C for decolorization, and photographed for recording [44].
4.3. Total RNA Extraction, Library Preparation, and De Novo Sequencing
Samples were taken 7 days after sowing, and roots were separated from seedlings and immediately frozen in liquid nitrogen before being stored at −80 °C until used for transcriptome analysis. All samples were collected in three biological replicates. The total RNA was extracted from samples using Trizol Reagent (Invitrogen, Waltham, MA, USA), and then RNA degradation and contamination were monitored on 1% agarose gels. RNA purity was checked using the NanoPhotometer® spectrophotometer (IMPLEN, Westlake Village, CA, USA), and RNA concentration was measured using the Qubit® RNA Assay Kit in the Qubit® 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). A total of 1.5 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using the NEBNext® UltraTMRNA Library Prep Kit for Illumina® (NEB, Ipswich, MA, USA). Then, the library preparations were sequenced on an Illumina Hiseq 4000 (Illumina, San Diego, CA, USA), and 150 bp paired-end reads were generated.
4.4. Sequencing Analysis and Differential Expression Analysis
Clean reads were obtained by removing adaptor sequences, more than 10%Nbases and low-quality (Q ≤ 20) reads with more than 50% bases from each dataset to gain more reliable results. The reads were mapped onto the transcriptome assembly by TopHat 2 (V2.1.1) to map the reads to the Oryza sativa L. genome. Read counts per gene were expressed as the expected number of fragments per kilobase of transcripts per million mapped fragments (FPKM). Unigene abundance differences between the samples were calculated based on the ratio of the FPKM values and false discovery rate (FDR). Genes with FDR ≤ 0.05 and FPKM ≥ 10 were considered DEGs.
GO classification was performed via WEGO (http://wego.genomics.org.cn/cgi-bin/wego/index.pl=10/02/2021), and the GO distributions of DEGs were then obtained from three levels: biological process, cellular component, and molecular function [45]. For each Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway, the numbers of DEGs were compared to the entire reference gene set by hypergeometric tests to find out the pathways enriched with regulated genes [46]. KEGG enrichment analyses were adjusted using the Bonferroni correction, and a corrected p adjust ≤ 0.05 was chosen as the threshold value for determining significantly enriched GO terms and KEGG enrichment pathways.
4.5. Quantitative Real-Time PCR
A total of nine genes were differentially expressed and subjected to qRT-PCR verification. Total RNA was extracted using the RC411-01 RT-PCR reagent (Vazyme Biotech, Nanjing, China). The cDNA was synthesized according to the instructions of the MMLV reverse transcriptase Kit (28025013) (Thermo Fisher Scientific, Waltham, MA, USA). The qRT-PCR was FastStart Universal SYBR Green Master (Rox) superMIX reaction kit (4913850001) (Roche, Mannheim, Germany). The Actin gene was used as an internal reference. The primer pairs were designed using Primer Premier 5.0 software. The specific primers for genes involved in the hormone signaling pathway are listed in Table S6. Each biological sample was tested in triplicate, and the standard deviation (SD) values of the means were calculated using standard statistical methods. The expression of genes was analyzed using the DDCt data analysis method, and relative gene expression was calculated using the 2−∆∆Ct method [47].
4.6. Identification Methods and Material Selection
Endogenous hormone content was measured for samples of LS273 and ZZ39. Endogenous indoleacetic acid (IAA) was determined using an ultra-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UPLC-ESI-MS/MS) system [48].
4.7. Statistical Analysis
In this study, SPSS 20.0 statistical software was used to perform three repeated one-way analyses of variance (ANOVA), Duncan’s multiple comparisons, Pearson correlation analysis, principal component analysis, and stepwise regression analysis. For other data processing, Microsoft Excel 2019 software was used. Pictures were drawn using https://www.chiplot.online/ and Graphpad-Prism 8.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13010171/s1, Figure S1: The PCA results; Figure S2: KEGG pathway results; Table S1: RNA-Seq analysis resulted; Table S2: DEGs connected to the method of antioxidant enzymes; Table S3: DEGs related to APX; Table S4: DEGs related to glutathione; Table S5: DEGs related to auxin; Table S6: The specific primers for genes involved in the hormone signaling pathway.
Author Contributions
Y.Z. and H.L. contributed to the conception of the study; H.L., M.W., W.L., S.L. and Z.C. performed the experiments; Z.Y. and Y.Z. contributed significantly to analysis and manuscript preparation; H.L. performed the data analyses and wrote the manuscript; S.L. helped perform the analysis with constructive discussions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Hunan Agricultural Science and Technology Innovation Fund Project (2020CX06) and the National Key R&D Program (2021YFD1401100).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Hunan Hybrid Rice Research Center for the scientific research conditions which enabled us to complete the content of this research successfully. A special thanks to Xiang Lu for helping us with the preliminary preparations for the test.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Setter, T.L.; Waters, I. Review of Prospects for Germplasm Improvement for Waterlogging Tolerance in Wheat, Barley and Oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Gibbs, J.; Greenway, H. Mechanisms of Anoxia Tolerance in Plants. I. Growth, Survival and Anaerobic Catabolism. Funct. Plant Biol. 2003, 30, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Kaspary, T.E.; Roma-Burgos, N.; Merotto, A. Snorkeling Strategy: Tolerance to Flooding in Rice and Potential Application for Weed Management. Genes 2020, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Wu, Y.S.; Chen, C.T.; Lai, M.H.; Yen, H.M.; Yang, C.Y. Physiological and Molecular Responses of Seedlings of an Upland Rice (‘Tung Lu 3′) to Total Submergence Compared to Those of a Submergence-Tolerant Lowland Rice (‘FR13A’). Rice 2017, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Watanabe, K.; Fukazawa, A.; Mori, H.; Abe, F.; Kawaguchi, K.; Oyanagi, A.; Nakazono, M. Ethylene and Reactive Oxygen Species Are Involved in Root Aerenchyma Formation and Adaptation of Wheat Seedlings to Oxygen-Deficient Conditions. J. Exp. Bot. 2014, 65, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A Variable Cluster of Ethylene Response Factor-like Genes Regulates Metabolic and Developmental Acclimation Responses to Submergence in Rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Yim, H.K.; Park, H.G.; Lim, J.; Kim, S.H.; Hwang, Y.S. Interference with Oxidative Phosphorylation Enhances Anoxic Expression of Rice α-Amylase Genes through Abolishing Sugar Regulation. J. Exp. Bot. 2010, 61, 3235–3244. [Google Scholar] [CrossRef]
- Kretzschmar, T.; Pelayo, M.A.F.; Trijatmiko, K.; Gabunada, L.F.M.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.; Sreenivasulu, N.; Bailey-Serres, J.; et al. A Trehalose-6-Phosphate Phosphatase Enhances Anaerobic Germination Tolerance in Rice. Nat. Plants 2015, 1, 15124. [Google Scholar] [CrossRef]
- Kaspary, T.E.; Cutti, L.; Rafaeli, R.S.; Delatorre, C.A.; Merotto, A. Genes Related to Flooding Tolerance during Germination and Early Growth of Weedy Rice. Weed Res. 2020, 60, 435–449. [Google Scholar] [CrossRef]
- Ye, N.H.; Wang, F.Z.; Shi, L.; Chen, M.X.; Cao, Y.Y.; Zhu, F.Y.; Wu, Y.Z.; Xie, L.J.; Liu, T.Y.; Su, Z.Z.; et al. Natural Variation in the Promoter of Rice Calcineurin B-like Protein10 (OsCBL10) Affects Flooding Tolerance during Seed Germination among Rice Subspecies. Plant J. 2018, 94, 612–625. [Google Scholar] [CrossRef]
- Rawyler, A.; Arpagaus, S.; Braendle, R. Impact of Oxygen Stress and Energy Availability on Membrane Stability of Plant Cells. Ann. Bot. 2002, 90, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S.; et al. Effects of Melatonin on Anti-Oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Ella, E.S.; Kawano, N.; Ito, O. Importance of Active Oxygen-Scavenging System in the Recovery of Rice Seedlings after Submergence. Plant Sci. 2003, 165, 85–93. [Google Scholar] [CrossRef]
- Bui, L.T.; Ella, E.S.; Dionisio-Sese, M.L.; Ismail, A.M. Morpho-Physiological Changes in Roots of Rice Seedling upon Submergence. Rice Sci. 2019, 26, 167–177. [Google Scholar] [CrossRef]
- Rao, A.N.; Johnson, D.E.; Sivaprasad, B.; Ladha, J.K.; Mortimer, A.M. Weed Management in Direct-Seeded Rice; Elsevier Masson SAS: Îledefrance, France, 2007; Volume 93. [Google Scholar]
- Chauhan, B.S. Weedy Rice (Oryza sativa) II. Response of Weedy Rice to Seed Burial and Flooding Depth. Weed Sci. 2012, 60, 385–388. [Google Scholar] [CrossRef]
- Chamara, B.S.; Marambe, B.; Kumar, V.; Ismail, A.M.; Septiningsih, E.M.; Chauhan, B.S. Optimizing Sowing and Flooding Depth for Anaerobic Germination-Tolerant Genotypes to Enhance Crop Establishment, Early Growth, and Weed Management in Dry-Seeded Rice (Oryza sativa L.). Front. Plant Sci. 2018, 9, 1654. [Google Scholar] [CrossRef]
- Ismail, A.M.; Ella, E.S.; Vergara, G.V.; Mackill, D.J. Mechanisms Associated with Tolerance to Flooding during Germination and Early Seedling Growth in Rice (Oryza sativa). Ann. Bot. 2009, 103, 197–209. [Google Scholar] [CrossRef]
- Taheri, P.; Kakooee, T. Reactive Oxygen Species Accumulation and Homeostasis Are Involved in Plant Immunity to an Opportunistic Fungal Pathogen. J. Plant Physiol. 2017, 216, 152–163. [Google Scholar] [CrossRef]
- Waititu, J.K.; Zhang, X.; Chen, T.; Zhang, C.; Zhao, Y.; Wang, H. Transcriptome Analysis of Tolerant and Susceptible Maize Genotypes Reveals Novel Insights about the Molecular Mechanisms Underlying Drought Responses in Leaves. Int. J. Mol. Sci. 2021, 22, 6980. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, Y.; Ella, E.S.; Ismail, A.M. Mechanisms Associated with Tiller Suppression under Stagnant Flooding in Rice. J. Agron. Crop Sci. 2019, 205, 235–247. [Google Scholar] [CrossRef]
- Li, T.; Wang, R.; Zhao, D.; Tao, J. Effects of Drought Stress on Physiological Responses and Gene Expression Changes in Herbaceous Peony (Paeonia lactiflora Pall.). Plant Signal. Behav. 2020, 15, 1746034. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lv, X.; Yang, J.; Chen, B.; Zhao, W.; Meng, Y.; Wang, Y.; Zhou, Z.; Oosterhuis, D.M. Effects of Potassium Deficiency on Antioxidant Metabolism Related to Leaf Senescence in Cotton (Gossypium hirsutum L.). F. Crops Res. 2016, 191, 139–149. [Google Scholar] [CrossRef]
- Han, B.; Ma, X.; Cui, D.; Wang, Y.; Geng, L.; Cao, G.; Zhang, H.; Han, L. Comprehensive Evaluation and Analysis of the Mechanism of Cold Tolerance Based on the Transcriptome of Weedy Rice Seedlings. Rice 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, C.; Ai, P.; Cui, X.; Zhang, Z. ALM1, Encoding a Fe-Superoxide Dismutase, Is Critical for Rice Chloroplast Biogenesis and Drought Stress Response. Crop J. 2021, 9, 1018–1029. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Li, Y.; Zhang, J. ABA Controls H2O2 Accumulation through the Induction of OsCATB in Rice Leaves under Water Stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef]
- Chou, T.S.; Chao, Y.Y.; Kao, C.H. Involvement of Hydrogen Peroxide in Heat Shock- and Cadmium-Induced Expression of Ascorbate Peroxidase and Glutathione Reductase in Leaves of Rice Seedlings. J. Plant Physiol. 2012, 169, 478–486. [Google Scholar] [CrossRef]
- Hong, C.Y.; Hsu, Y.T.; Tsai, Y.C.; Kao, C.H. Expression of ASCORBATE PEROXIDASE 8 in Roots of Rice (Oryza sativa L.) Seedlings in Response to NaCl. J. Exp. Bot. 2007, 58, 3273–3283. [Google Scholar] [CrossRef]
- Shan, C.; Wang, B.; Sun, H.; Gao, S.; Li, H. H2S Induces NO in the Regulation of AsA-GSH Cycle in Wheat Seedlings by Water Stress. Protoplasma 2020, 257, 1487–1493. [Google Scholar] [CrossRef]
- Anjum, N.A.; Umar, S.; Chan, M.T. Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Mackill, D.J.; Ismail, A.M.; Singh, U.S.; Labios, R.V.; Paris, T.R. Development and Rapid Adoption of Submergence-Tolerant (Sub1) Rice Varieties, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 115. [Google Scholar]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The Ethylene Response Factors SNORKEL1 and SNORKEL2 Allow Rice to Adapt to Deep Water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef]
- Van Veen, H.; Mustroph, A.; Barding, G.A.; Vergeer-van Eijk, M.; Welschen-Evertman, R.A.M.; Pedersen, O.; Visser, E.J.W.; Larive, C.K.; Pierik, R.; Bailey-Serres, J.; et al. Two Rumex Species from Contrasting Hydrological Niches Regulate Flooding Tolerance through Distinct Mechanisms. Plant Cell 2013, 25, 4691–4707. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; Friml, J. Auxin: A Trigger for Change in Plant Development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Mockaitis, K.; Estelle, M. Auxin Receptors and Plant Development: A New Signaling Paradigm. Annu. Rev. Cell Dev. Biol. 2008, 24, 55–80. [Google Scholar] [CrossRef]
- Yang, Y.; Hammes, U.Z.; Taylor, C.G.; Schachtman, D.P.; Nielsen, E. High-Affinity Auxin Transport by the AUX1 Influx Carrier Protein. Curr. Biol. 2006, 16, 1160. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Wang, X.; Hagen, G.; Guilfoyle, T.J.; Tiwari, S.B.; Wang, X.; Hagen, G.; Guilfoyle, T.J. AUX/IAA Proteins Are Active Repressors, and Their Stability and Activity Are Modulated by Auxin Published by: American Society of Plant Biologists (ASPB) Linked References Are Available on JSTOR for This Article: AUX/IAA Proteins Are Active Repre. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef]
- Farquharson, K.L. SAUR19 Links Auxin and Plasma Membrane H+-ATPases in Cell Expansion SAUR19 Links Auxin and Plasma Membrane H+-ATPases in Cell Expansion. Plant Cell 2014, 26, 1835. [Google Scholar] [CrossRef]
- Jain, M.; Kaur, N.; Tyagi, A.K.; Khurana, J.P. The Auxin-Responsive GH3 Gene Family in Rice (Oryza sativa). Funct. Integr. Genom. 2006, 6, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Guilfoyle, T. Auxin-Responsive Gene Expression: Genes, Promoters and Regulatory Factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.; Luan, S.; Li, J. Auxin Controls Seed Dormancy through Stimulation of Abscisic Acid Signaling by Inducing ARF-Mediated ABI3 Activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef]
- Piya, S.; Shrestha, S.K.; Binder, B.; Neal Stewart, C.; Hewezi, T. Protein-Protein Interaction and Gene Co-Expression Maps of ARFs and Aux/IAAs in Arabidopsis. Front. Plant Sci. 2014, 5, 744. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.R.; Zhang, H.; Gao, J.; Shan, J.X.; Ye, W.W.; Lin, H.X. TT2 Controls Rice Thermotolerance through SCT1-Dependent Alteration of Wax Biosynthesis. Nat. Plants 2022, 8, 53–67. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for Linking Genomes to Life and the Environment. Nucleic Acids Res. 2008, 36 (Suppl. 1), 480–484. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, W.; Zhao, G.; Fan, X.; Long, H.; Fan, Y.; Shi, M.; Tan, X.; Zhang, L. New Insights of Salicylic Acid into Stamen Abortion of Female Flowers in Tung Tree (Vernicia fordii). Front. Genet. 2019, 10, 316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).