Detection of ‘Candidatus Phythoplasma prunorum’ in Apricot Trees and its Associated Psyllid Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Samples Collection
2.2. Insect Sample Collection
2.3. Nucleic Acid Extraction
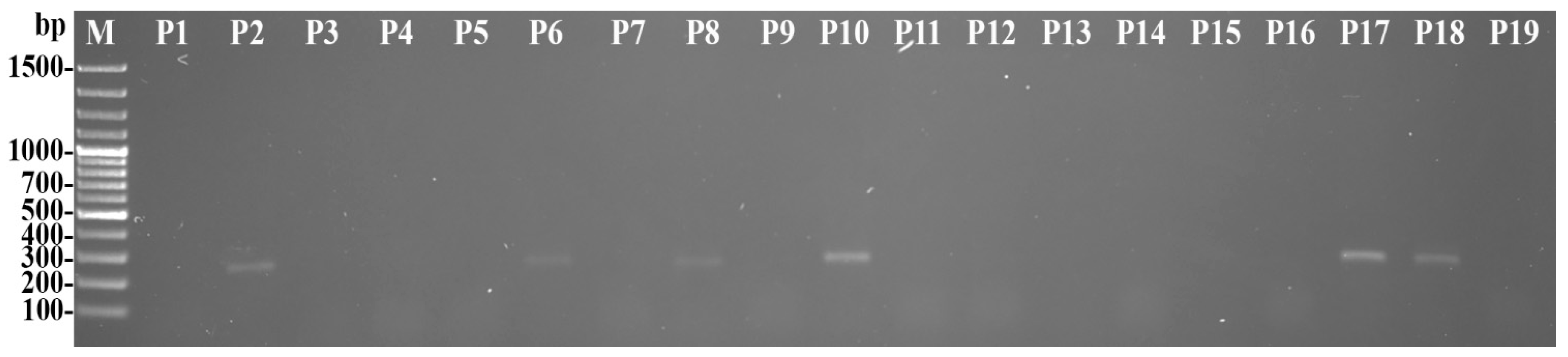
2.4. Pathogen Identification
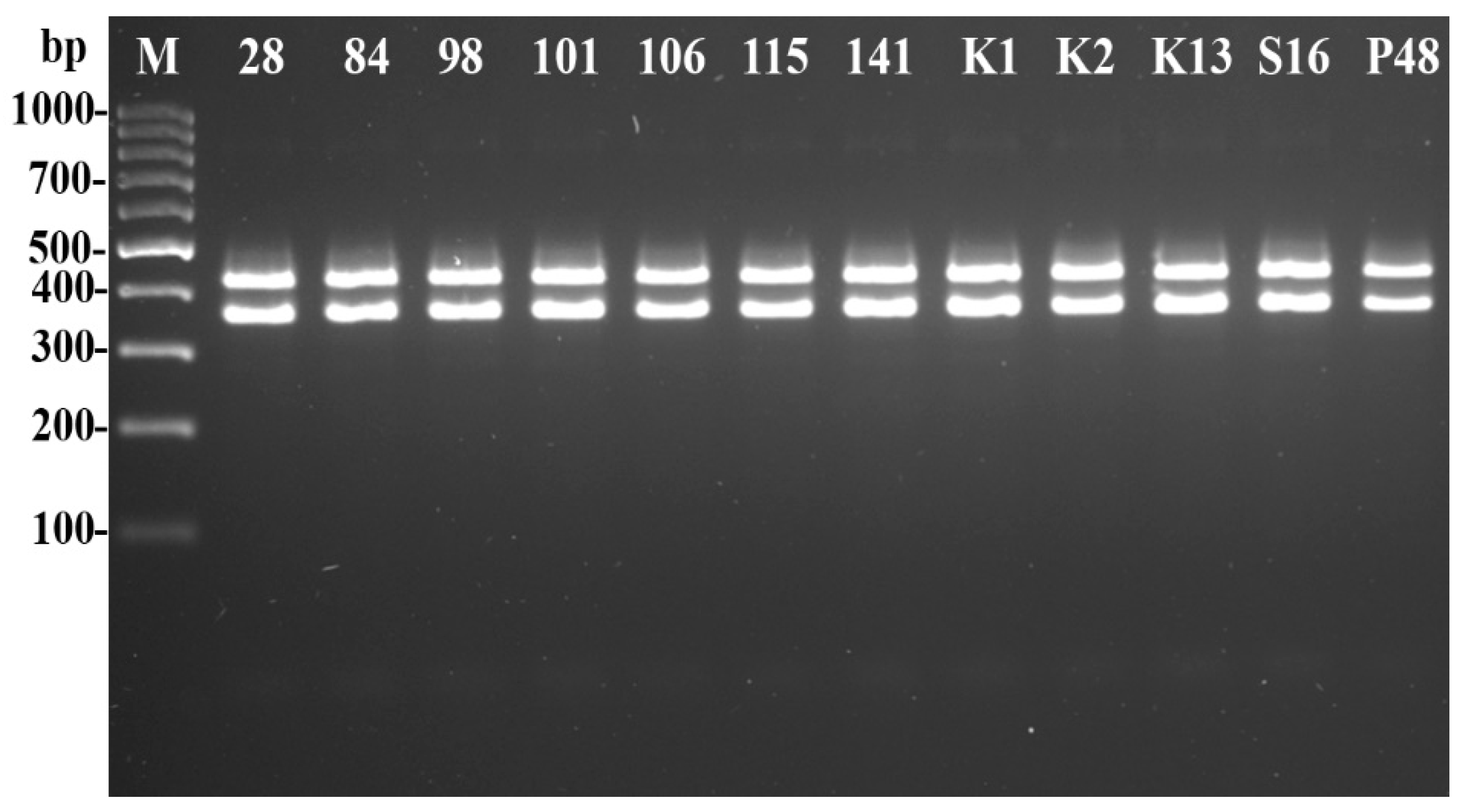
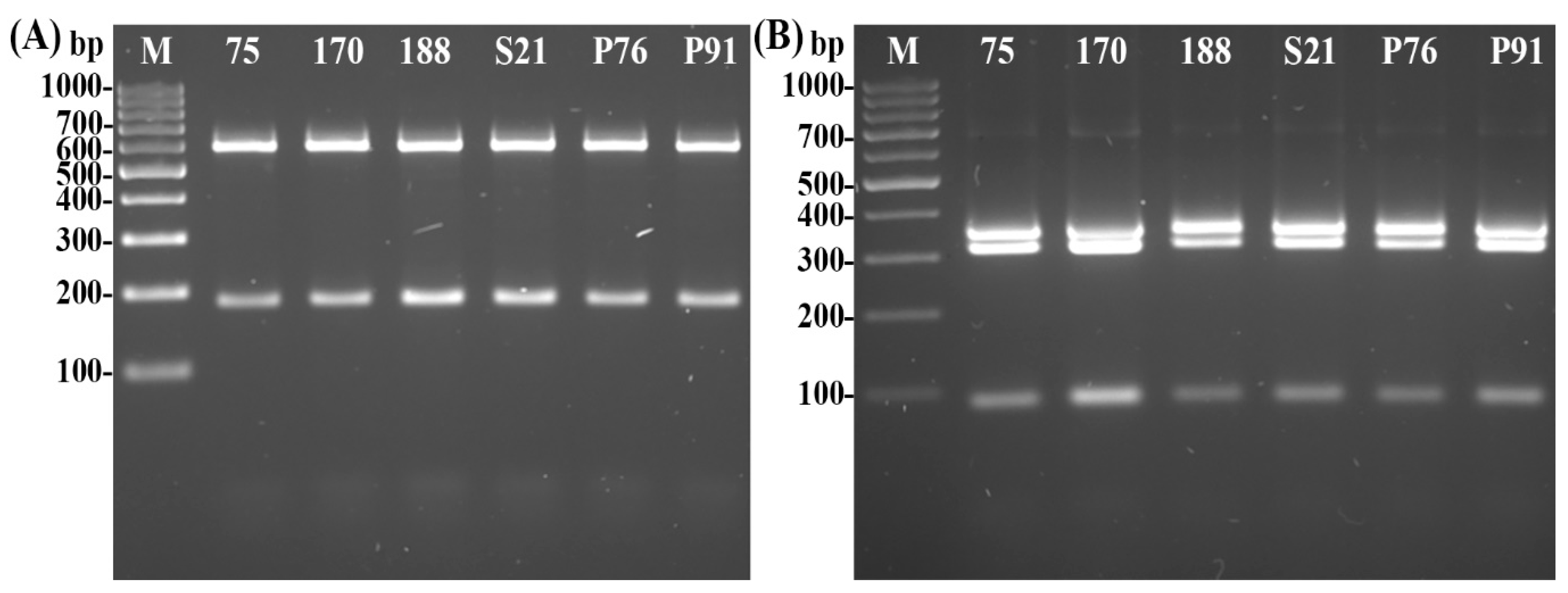
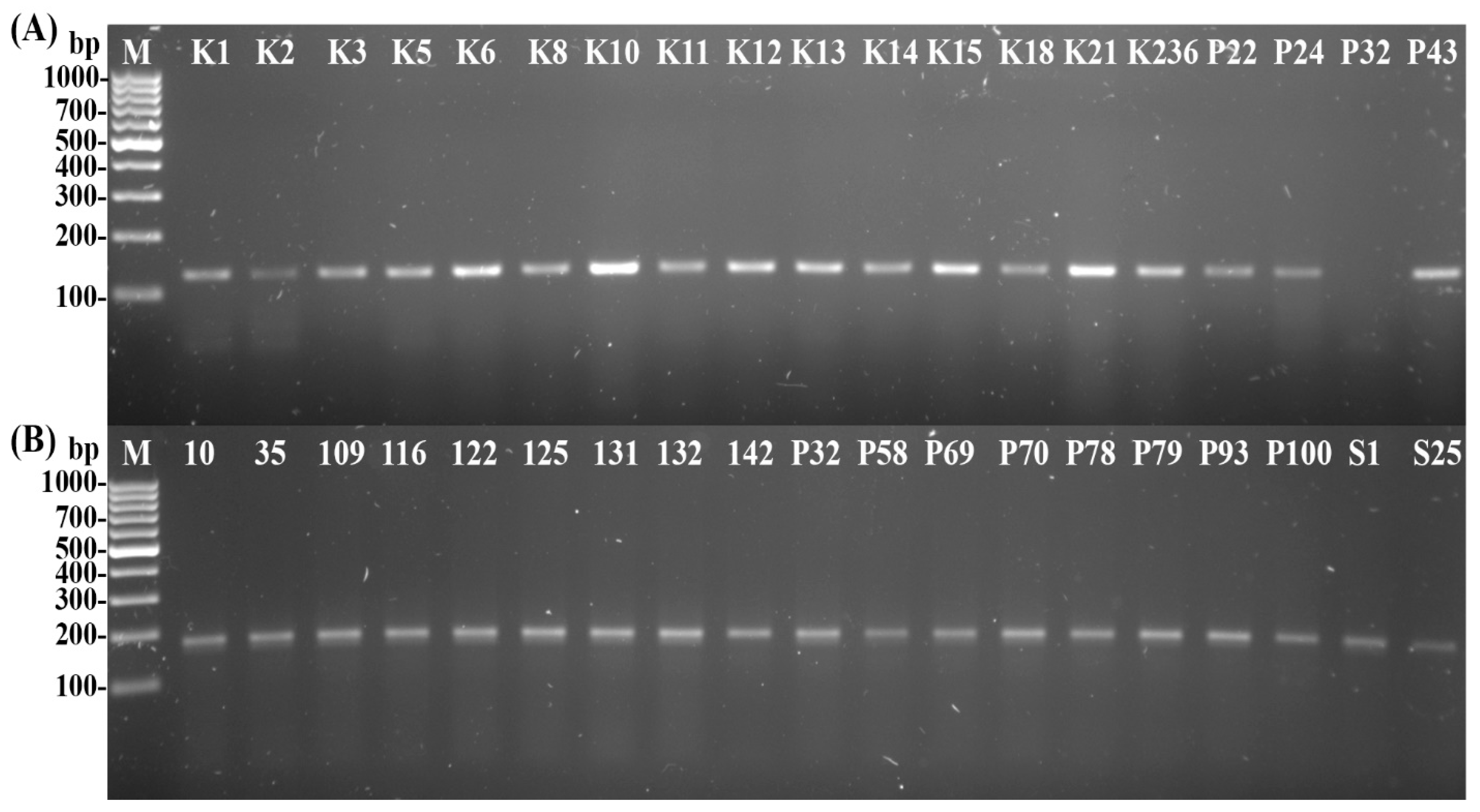
2.5. Psyllid Identification
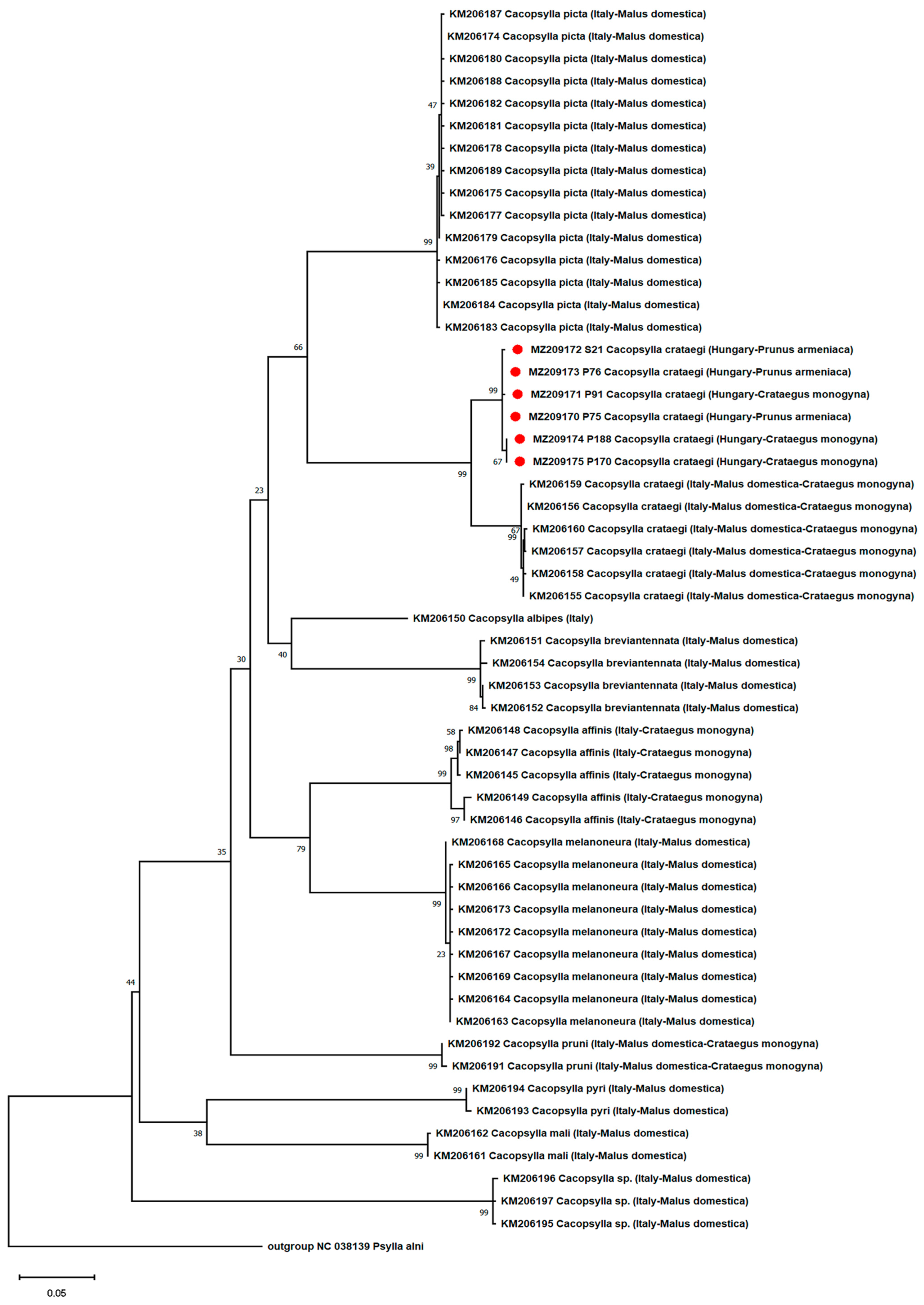
2.6. Phylogenetic and Recombination Analysis of Psyllids
2.7. Statistical Analysis of Abundance and Phytoplasma Presence of Psyllids
3. Results
3.1. Disease Occurrence in Plants
3.2. Insect Samples
- Classical Identification of Psyllids
- Psyllids Collected on Apricot
- Psyllids Collected on Non-Apricot Hosts
- ‘Candidatus Phytoplasma prunorum’ Incidence in Psyllids
- Molecular Identification of Psyllids
- Phylogenetic and Recombination Analysis of the Psyllids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcone, C.; Jarausch, B.; Jarausch, W. Candidatus Phytoplasma prunorum, the causal agent of European stone fruit yellows: An overview. J. Plant Pathol. 2010, 92, 19–34. [Google Scholar]
- Lorenz, K.; Dosba, F.; Poggi-Pollini, C.; Llacer, G.; Seemüller, E. Phytoplasma diseases of Prunus species in Europe are caused by genetically similar organisms. J. Plant Dis. Prot. 1994, 101, 567–575. [Google Scholar]
- Steffek, R.; Follak, S.; Sauvion, N.; Labonne, G.; MacLeod, A. Distribution of ‘Candidatus Phytoplasma prunorum’ and its vector Cacopsylla pruni in European fruit-growing areas: A review. EPPO Bull. 2012, 42, 191–202. [Google Scholar] [CrossRef]
- Cieślińska, M.; Morgaś, H. Detection and identification of ‘Candidatus Phytoplasma prunorum’,‘Candidatus Phytoplasma mali’ and ‘Candidatus Phytoplasma pyri’in stone fruit trees in Poland. J. Phytopathol. 2011, 159, 217–222. [Google Scholar] [CrossRef]
- Ivić, D.; Plavec, J.; Ivančan, G. The occurrence of ‘Candidatus Phytoplasma prunorum’in apricot orchards in Baranja. Pomol. Croat. Glas. Hrvat. Agron. Društva 2017, 21, 101–106. [Google Scholar]
- Riedle-Bauer, M.; Paleskić, C.; Schwanzer, J.; Kölber, M.; Bachinger, K.; Antonielli, L.; Schönhuber, C.; Elek, R.; Stradinger, J.; Emberger, M.; et al. Epidemiological and molecular study on ‘Candidatus Phytoplasma prunorum’in Austria and Hungary. Ann. Appl. Biol. 2019, 175, 400–414. [Google Scholar] [CrossRef]
- Ludvikova, H.; Franova, J.; Sucha, J. Phytoplasmas in apricot, peach and sour cherry orchards in East Bohemia, Czech Republic. Bull. Insectol. 2011, 64, S67–S68. [Google Scholar]
- Nečas, T.; Ondrášek, I.; Krška, B. ‘Candidatus Phytoplasma prunorum’-a pathogen spreading uncontrollably in apricot orchards in the Czech Republic. Acta Hortic. 2015, 1105, 131–136. [Google Scholar] [CrossRef]
- Jarausch, W.; Lansac, M.; Saillard, C.; Broquaire, J.; Dosba, F. PCR assay for specific detection of European stone fruit yellows phytoplasmas and its use for epidemiological studies in France. Eur. J. Plant Pathol. 1998, 104, 17–27. [Google Scholar] [CrossRef]
- Jarausch, B.; Mühlenz, I.; Beck, A.; Lampe, I.; Harzer, U.; Jarausch, W. Epidemiology of European Stone Fruit Yellows in Germany. Acta Hortic. 2008, 781, 417–422. [Google Scholar] [CrossRef]
- Jarausch, W.; Jarausch, B.; Fritz, M.; Runne, M.; Etropolska, A.; Pfeilstetter, E. Epidemiology of European stone fruit yellows in Germany: The role of wild Prunus spinosa. Eur. J. Plant Pathol. 2019, 154, 463–476. [Google Scholar] [CrossRef]
- Morvan, G. Apricot chlorotic leaf roll. EPPO Bull. 1977, 7, 37–55. [Google Scholar] [CrossRef]
- Žežlina, I.; Rot, M.; Kač, M.; Trdan, S. Causal agents of stone fruit diseases in Slovenia and the potential for diminishing their economic impact–a review. Plant Prot. Sci. 2016, 52, 149–157. [Google Scholar]
- Kison, H.; Seemüller, E. Differences in strain virulence of the European stone fruit yellows phytoplasma and susceptibility of stone fruit trees on various rootstocks to this pathogen. J. Phytopathol. 2001, 149, 533–541. [Google Scholar] [CrossRef]
- Gazel, M.; Caglayan, K.; Serce, C.U.; Son, L. Evaluations of apricot trees infected by ‘Candidatus Phytoplasma prunorum’for horticultural characteristics. Rom. Biotechnol. Lett. 2009, 14, 4123–4129. [Google Scholar]
- Nečas, T.; Kiss, T.; Eichmeier, A.; Nečasova, J.; Ondrašek, I. The effect of phytoplasma disease caused by ‘Candidatus Phytoplasma prunorum’on the phenological and pomological traits in apricot trees. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 107–114. [Google Scholar] [CrossRef]
- Carraro, L.; Osler, R.; Loi, N.; Ermacora, P.; Refatti, E. Transmission of European stone fruit yellows phytoplasma by Cacopsylla pruni. J. Plant Pathol. 1998, 80, 233–239. [Google Scholar]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Ouvrard, D. Psyl’list—The World Psylloidea Database. Available online: http://www.hemiptera-databases.com/psyllist (accessed on 2 February 2022).
- Sauvion, N.; Peccoud, J.; Meynard, C.N.; Ouvrard, D. Occurrence data for the two cryptic species of Cacopsylla pruni (Hemiptera: Psylloidea). Biodivers. Data J. 2021, 9, e68860. [Google Scholar] [CrossRef]
- Kiss, E.; Mergenthaler, E.; Kiss, B.; Viczián, O. A csonthéjasok európai sárgulása (ESFY) magyarországi terjedésének hátterében álló okok. In Proceedings of the 61. Növényvédelmi Tudományos Napok, Budapest, Hungary, 21 February 2015; p. 60. [Google Scholar]
- Mergenthaler, E.; Kiss, B.; Kiss, E.; Viczián, O. Survey on the occurrence and infection status of Cacopsylla pruni, vector of European stone fruit yellows in Hungary. Bull. Insectol. 2017, 70, 171–176. [Google Scholar]
- Viczián, O.; Kiss, B.; Kiss, E.; Orosz, S.; Juhász, A.L.; Mergenthaler, E. Mit tudunk a ‘Ca. Phytoplasma prunorum’ fitoplasma terjedéséről ma és mit gondolunk ugyanerről? [The significance of phytoplasma (‘Ca. Phytoplasma prunorum’) disease of stone fruits-what do we know and what is need to be done?]. Növényvédelem 2017, 78, 525–531. [Google Scholar]
- Sauvion, N.; Lachenaud, O.; Genson, G.; Rasplus, J.Y.; Labonne, G. Are there several biotypes of Cacopsylla pruni ? Bull. Insectol. 2007, 60, 185–186. [Google Scholar]
- Peccoud, J.; Labonne, G.; Sauvion, N. Molecular test to assign individuals within the Cacopsylla pruni complex. PLoS ONE 2013, 8, e72454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarausch, B.; Weintraub, P.; Sauvion, N.; Maixner, M.; Foissac, X. Diseases and insect vectors. In Phytoplasma and Phytoplasma Disease Management: How to Reduce their Economic Impact; Bertaccini, A., Ed.; International Phytoplasmologist Working Group (IPWG): Bologna, Italy, 2014; pp. 111–121. [Google Scholar]
- Marie-Jeanne, V.; Bonnot, F.; Thébaud, G.; Peccoud, J.; Labonne, G.; Sauvion, N. Multi-scale spatial genetic structure of the vector-borne pathogen ‘Candidatus Phytoplasma prunorum’ in orchards and in wild habitats. Sci. Rep. 2020, 10, 5002. [Google Scholar] [CrossRef] [Green Version]
- Etropolska, A.; Jarausch, W.; Jarausch, B.; Trenchev, G. Molecular typing of Bulgarian specimen of the phytoplasma vectors Cacopsylla pruni Scopoli and Cacopsylla melanoneura (Foerster). Bulg. J. Agric. Sci. 2016, 22, 98–102. [Google Scholar]
- Lepres, L.A.; Mergenthaler, E.; Viczián, O.; Tóth, F. A szilva levélbolha (Cacopsylla pruni Scopoli, 1763) jelenlétének felmérése és „Candidatus Phytoplasma prunorum′′ kórokozóval való fertőzöttségének vizsgálata egy heves megyei kajszibarack ültetvényben. Növényvédelem 2018, 54, 197–203. [Google Scholar]
- Oettl, S.; Schlink, K. Molecular Identification of Two Vector Species, Cacopsylla melanoneura and Cacopsylla picta (Hemiptera: Psyllidae), of Apple Proliferation Disease and Further Common Psyllids of Northern Italy. J. Econ. Entomol. 2015, 108, 2174–2183. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): A global synthesis. J. Nat. Hist. 2009, 43, 65–179. [Google Scholar] [CrossRef]
- Ossiannilsson, F. The Psylloidea (Homoptera) of Fennoscandia and Denmark. In Fauna Entomologica Scandinavica; E. J. Brill: Leiden, The Netherlands, 1992; Volume 26, p. 346. [Google Scholar]
- Ripka, G. Levélbolhák 1; Agroinform Kiadó: Budapest, Hungary, 2010. [Google Scholar]
- Thébaud, G.; Yvon, M.; Alary, R.; Sauvion, N.; Labonne, G. Efficient transmission of ‘Candidatus Phytoplasma prunorum’ is delayed by eight months due to a long latency in its host-alternating vector. Phytopathology 2009, 99, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Labonne, G.; Lichou, J. Data on the life cycle of Cacopsylla pruni, Psyllidae vector of European stone fruit yellows (ESFY) phytoplasma, in France. Acta Hortic. 2004, 657, 465–470. [Google Scholar] [CrossRef]
- Carraro, L.; Ferrini, F.; Ermacora, P.; Loi, N. Role of wild Prunus species in the epidemiology of European stone fruit yellows. Plant Pathol. 2002, 51, 513–517. [Google Scholar] [CrossRef]
- Jarausch, W.; Danet, J.L.; Labonne, G.; Dosba, F.; Broquaire, J.; Saillard, C.; Garnier, M. Mapping the spread of apricot chlorotic leaf roll (ACLR) in southern France and implication of Cacopsylla pruni as a vector of European stone fruit yellows (ESFY) phytoplasmas. Plant Pathol. 2001, 50, 782–790. [Google Scholar] [CrossRef]
- Yvon, M.; Labonne, G.; Thébaud, G. Survival of European stone fruit yellows phytoplasma outside fruit crop production areas: A case study in southeastern France. Acta Hortic. 2004, 657, 477–481. [Google Scholar] [CrossRef]
- Gallinger, J.; Jarausch, B.; Jarausch, W.; Gross, J. Host plant preferences and detection of host plant volatiles of the migrating psyllid species Cacopsylla pruni, the vector of European Stone Fruit Yellows. J. Pest Sci. 2020, 93, 461–475. [Google Scholar] [CrossRef]
- Carraro, L.; Loi, N.; Ermacora, P. Transmission characteristics of the European stone fruit yellows phytoplasma and its vector Cacopsylla pruni. Eur. J. Plant Pathol. 2001, 107, 695–700. [Google Scholar] [CrossRef]
- Etropolska, A.; Jarausch, W.; Jarausch, B.; Trenchev, G. Detection of European fruit tree phytoplasmas and their insect vectors in important fruit-growing regions in Bulgaria. Bulg. J. Agric. Sci. 2015, 21, 1248–1253. [Google Scholar]
- Warabieda, W.; Soika, G.; Cieślińska, M. Cacopsylla pruni in Poland and its significance as a vector of ‘Candidatus Phytoplasma prunorum’. Žemdirb. (Agric.) 2018, 105, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Serçe, Ç.U.; Yvon, M.; Kaya, K.; Gazel, M.; Cengız, F.C.; Çağlayan, K.; Sauvion, N. Survey on the presence of Cacopsylla pruni in Turkey: Preliminary results. Bull. Insectol. 2011, 64, S145–S146. [Google Scholar]
- Bodnár, D.; Szalai, B.; Tarcali, G.; Viczián, O.; Mergenthaler, E. Phytoplasma infection status survey in plum psyllid (Cacopsylla pruni) population. Acta Agrar. Debr. 2019, 2, 45–48. [Google Scholar] [CrossRef]
- Ermacora, P.; Ferrini, F.; Loi, N.; Martini, M.; Osler, R. Population dynamics of Cacopsylla pruni and ‘Candidatus Phytoplasma prunorum’infection in North-Eastern Italy. Bull. Insectol. 2011, 64, S143–S144. [Google Scholar]
- Burckhardt, D.; Sharma, A.; Raman, A. Checklist and comments on the jumping plant-lice (Hemiptera: Psylloidea) from the Indian subcontinent. Zootaxa 2018, 4457, 1–38. [Google Scholar] [CrossRef]
- Horváth, G. Ordo. Hemiptera. In A Magyar Birodalom Állatvilága (Fauna Regni Hungariae). III. Arthropoda. (Insecta. Hemiptera.); Királyi Magy; Természettudományi Társulat: Budapest, Hungary, 1897; p. 72. [Google Scholar]
- Kontschán, J.; Kiss, E.; Ripka, G. Új adatok a hazai levélbolhák (Insecta: Psylloidea) előfordulásaihoz. [New data to occurrences of the Hungarian jumping plant lice (Insecta: Psylloidea).]. Növényvédelem 2020, 81, 197–202. [Google Scholar]
- Kontschán, J.; Ripka, G. Új adatok a hazai levélbolhák (Insecta: Psylloidea) előfordulásaihoz II. [New data to occurrences of the Hungarian jumping plant lice (Insecta: Psylloidea) II.]. Növényvédelem 2021, 82, 336–341. [Google Scholar]
- Ripka, G. Újabb adatok a díszfa- és díszcserjefajok levélbolha-faunájának ismeretéhez (Homoptera, Psylloidea) [Recent data on the psyllid fauna of ornamental trees and shrubs of Hungary (Homoptera: Psylloidea)]. Növényvédelem 1997, 33, 269–273. [Google Scholar]
- Ripka, G.; Kiss, B. További adatok a hazai parlagfűállományokban előforduló levélbolha-fajok (Hemiptera: Psylloidea) ismeretéhez [Recent data to the knowledge on psyllid species (Hemiptera: Psylloidea) occurring on common ragweed in Hungary]. Növényvédelem 2008, 44, 257–261. [Google Scholar]
- Križanac, I.; Mikec, I.; Budinščak, Ž.; Musić, M.Š.; Škorić, D. Diversity of phytoplasmas infecting fruit trees and their vectors in Croatia. J. Plant Dis. Prot. 2010, 117, 206–213. [Google Scholar] [CrossRef]
- Tedeschi, R.; Lauterer, P.; Brusetti, L.; Tota, F.; Alma, A. Composition, abundance and phytoplasma infection in the hawthorn psyllid fauna of northwestern Italy. Eur. J. Plant Pathol. 2009, 123, 301–310. [Google Scholar] [CrossRef]
- Miñarro, M.; Somoano, A.; Moreno, A.; García, R.R. Candidate insect vectors of apple proliferation in Northwest Spain. SpringerPlus 2016, 5, 1240. [Google Scholar] [CrossRef] [Green Version]
- Fischnaller, S.; Parth, M.; Messner, M.; Stocker, R.; Kerschbamer, C.; Reyes-Dominguez, Y.; Janik, K. Occurrence of different Cacopsylla species in apple orchards in South Tyrol (Italy) and detection of apple proliferation phytoplasma in Cacopsylla melanoneura and Cacopsylla picta. Cicadina 2017, 17, 37–51. [Google Scholar]
- Daire, X.; Clair, D.; Reinert, W.; Boudon-Padieu, E. Detection and differentiation of grapevine yellows phytoplasmas belonging to the elm yellows group and to the stolbur subgroup by PCR amplification of non-ribosomal DNA. Eur. J. Plant Pathol. 1997, 103, 507–514. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissues. Focirs 1990, 12, 13–15. [Google Scholar]
- Marzachì, C.; Veratti, F.; Bosco, D. Direct PCR detection of phytoplasmas in experimentally infected insects. Annals of Applied Biology 1998, 133, 45–54. [Google Scholar] [CrossRef]
- Mergenthaler, E. Fitoplazmás Betegségek Magyarországon: Korszerű Diagnosztikai Módszerek Fejlesztése [Phytoplasma Diseases in Hungary: Development of Improved Diagnostical Methods]. Ph.D. Thesis, Budapesti Közgazdaságtudományi és Államigazgatási Egyetem, Budapest, Hungary, 2004. [Google Scholar]
- Burckhardt, D. Identification Key for the Central European Cacopsylla Species. Available online: https://www.dlr.rlp.de/Internet/global/Themen.nsf/b81d6f06b181d7e7c1256e920051ac19/5f722b729e24cfccc12584430022b32e/$FILE/cacopsylla_pruni.pdf (accessed on 15 January 2015).
- Burckhardt, D. Identification Key for the Central European Cacopsylla Species. Available online: https://www.dlr.rlp.de/Internet/global/Themen.nsf/7683c11d82324367c1256ea600533a09/7e0b2d87f5d7df3bc1258443001fed2f/$FILE/cacopsylla_crataegi.pdf (accessed on 15 January 2015).
- Sanger, F.; Coulson, A.R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J. Mol. Biol. 1975, 94, 441–448. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; p. 352. [Google Scholar]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.P.; Murrell, B.; Khoosal, A.; Muhire, B. Detecting and analyzing genetic recombination using RDP4. In Bioinform; Keith, J.M., Ed.; Springer: New York, NY, USA, 2017; Volume 1525, pp. 433–460. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing [Software Package], 4.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hasegawa, M.; Kishino, H.; Yano, T.-A. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef] [PubMed]
| Plant Material | N 1 | I 2 (%) | S 3 (%) | |
|---|---|---|---|---|
| Apricot | Scion | 81 | 25.9 | 61.9 |
| Rootstock | 14 | 28.6 | 0 | |
| Graft | 81 | 29.6 | 62.5 | |
| Wild plants | Blackthorn | 15 | 26.7 | 0 |
| Hawthorn | 11 | 27.3 | 0 | |
| Examined Plants | Species | N 1 | Comparative Ratios (%) | 95% Confidence Interval | Z 5 | p 4 | |
|---|---|---|---|---|---|---|---|
| LCI 2 | UCI 3 | ||||||
| All examined plants | C. pruni | 183 | 44.2 | 0.39 | 0.49 | 2.31 | 0.021 |
| C. crataegi | 231 | 55.8 | 0.51 | 0.61 | |||
| P. armeniaca | C. pruni | 133 | 59.9 | 0.53 | 0.66 | 2.89 | 0.004 |
| C. crataegi | 89 | 40.1 | 0.34 | 0.47 | |||
| P. domestica | C. pruni | 26 | 86.7 | 0.68 | 0.96 | 3.83 | <0.001 |
| C. crataegi | 4 | 13.3 | 0.04 | 0.32 | |||
| P. spinosa | C. pruni | 16 | 61.5 | 0.41 | 0.79 | 0.98 | 0.327 |
| C. crataegi | 10 | 38.5 | 0.21 | 0.59 | |||
| P. cerasifera | C. pruni | 4 | 100 | 0.40 | 1.00 | 1.5 | 0.13 |
| C. crataegi | 0 | 0 | 0.00 | 0.60 | |||
| C. monogyna | C. pruni | 4 | 3.0 | 0.01 | 0.08 | 10.71 | <0.001 |
| C. crataegi | 128 | 97.0 | 0.92 | 0.99 | |||
| C. pruni | C. crataegi | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Examined Plants | Sex 1 | N 2 | Proportion (%) | 95% Confidence Interval | Z 5 | p6 | N 2 | Proportion (%) | 95% Confidence Interval | Z 5 | p 6 | ||
| LCI 3 | UCI 4 | LCI 3 | UCI 4 | ||||||||||
| All examined plants | ♂ | 40 | 21.9 | 0.16 | 0.29 | 7.54 | <0.001 | 93 | 40.3 | 0.34 | 0.47 | 2.89 | 0.004 |
| ♀ | 143 | 78.1 | 0.71 | 0.84 | 138 | 59.7 | 0.53 | 0.66 | |||||
| P. armeniaca | ♂ | 27 | 20.3 | 0.16 | 0.29 | 6.76 | <0.001 | 35 | 39.3 | 0.29 | 0.50 | 1.91 | 0.056 |
| ♀ | 106 | 79.7 | 0.72 | 0.86 | 54 | 60.7 | 0.50 | 0.71 | |||||
| P. domestica | ♂ | 3 | 11.5 | 0.03 | 0.31 | 3.73 | <0.001 | 1 | 25.0 | 0.01 | 0.78 | 0.50 | 0.62 |
| ♀ | 23 | 88.5 | 0.69 | 0.97 | 3 | 75.0 | 0.22 | 0.99 | |||||
| P. spinosa | ♂ | 6 | 37.5 | 0.16 | 0.64 | 0.75 | 0.453 | 5 | 50.0 | 0.24 | 0.76 | 0.00 | 1.00 |
| ♀ | 10 | 62.5 | 0.36 | 0.84 | 5 | 50.0 | 0.24 | 0.76 | |||||
| P. cerasifera | ♂ | 1 | 25.0 | 0.01 | 0.78 | 0.50 | 0.62 | 0 | |||||
| ♀ | 3 | 75.0 | 0.22 | 0.99 | 0 | ||||||||
| C. monogyna | ♂ | 2 | 50.0 | 0.15 | 0.85 | 0.00 | 1.00 | 53 | 41.4 | 0.33 | 0.50 | 1.86 | 0.063 |
| ♀ | 2 | 50.0 | 0.15 | 0.85 | 75 | 58.6 | 0.50 | 0.67 | |||||
| Pathogen Incidence | Sex 1 | Statistical Analysis | |||
|---|---|---|---|---|---|
| ♂ | ♀ | ||||
| C. pruni | N 2 | Infected | 1 | 11 | |
| Non-infected | 38 | 133 | |||
| Ratio of individuals within males/females (%) | Infected | 2.6 | 7.6 | Odds ratio = 0.32 (CI 3: 0.007;2.330) Relative risk = 0.34 Fisher test p 4 = 0.47 | |
| Non-infected | 97.4 | 92.4 | |||
| C. crataegi | N2 | Infected | 3 | 3 | |
| Non-infected | 91 | 134 | |||
| Ratio of individuals within males/females (%) | Infected | 3.2 | 2.2 | Odds ratio = 1.47 (CI 3: 0.193;11.218) Relative risk = 1.46 Fisher test p 4 = 0.69 | |
| Non-infected | 96.8 | 97.8 | |||
| Pathogen Incidence | Species | Statistical Analysis | ||
|---|---|---|---|---|
| C. pruni | C. crataegi | |||
| N 1 | Infected | 12 | 6 | |
| Non-infected | 171 | 225 | ||
| Ratio of individuals within species (%) | Infected | 6.6 | 2.6 | Odds ratio = 2.63 (CI 2: 0.890;8.705) Relative risk = 2.52 Fisher test p 3 = 0.06 |
| Non-infected | 93.4 | 97.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koncz, L.S.; Petróczy, M.; Pénzes, B.; Ladányi, M.; Palkovics, L.; Gyócsi, P.; Nagy, G.; Ágoston, J.; Fail, J. Detection of ‘Candidatus Phythoplasma prunorum’ in Apricot Trees and its Associated Psyllid Samples. Agronomy 2023, 13, 199. https://doi.org/10.3390/agronomy13010199
Koncz LS, Petróczy M, Pénzes B, Ladányi M, Palkovics L, Gyócsi P, Nagy G, Ágoston J, Fail J. Detection of ‘Candidatus Phythoplasma prunorum’ in Apricot Trees and its Associated Psyllid Samples. Agronomy. 2023; 13(1):199. https://doi.org/10.3390/agronomy13010199
Chicago/Turabian StyleKoncz, László Sándor, Marietta Petróczy, Béla Pénzes, Márta Ladányi, László Palkovics, Piroska Gyócsi, Géza Nagy, János Ágoston, and József Fail. 2023. "Detection of ‘Candidatus Phythoplasma prunorum’ in Apricot Trees and its Associated Psyllid Samples" Agronomy 13, no. 1: 199. https://doi.org/10.3390/agronomy13010199





