Abstract
Astragalus sikokianus is a rare Japanese perennial of the seashore that was reported to be extinct in the wild. The small seed size and deep dormancy of A. sikokianus make it difficult for direct seeding restoration in aspects of seed handling, transport, planting, and seedling establishment. For the large-scale economic restoration of dormant small-seeded species, seed pelleting combined with the breaking of dormancy was studied. Physiological (prechilling and plant hormones) and physical (hot water, hydrochloric acid, and sulfuric acid) seed dormancy break treatments were evaluated. The dormant broken seeds were used for pelleting. The effects of the substrate, pellet sizes, and their interactions on germination were measured. The scarification of five rubs of seeds placed between sandpapers completely broke the physical dormancy of A. sikokianus. Seed coat impermeability inhibited germination. Pelleted seeds ranging from 2.0 to 4.0 mm in diameter showed more than 90% germination on filter paper. The germination of the pelleted seeds was measured in commercial, field, and sand soil conditions. The highest germination was shown in sand (70–74% GP), regardless of the pellet size, whereas unpelleted scarified seeds germinated only 48%. These results suggest that small-seeded species with physical dormancy can be used for seed-based restoration after seed pelleting.
1. Introduction
Astragalus L. is the largest genus in the Fabaceae family and is considered one of the most diverse genera in angiosperms, with approximately 3270 species grouped into 100 subgenera [1,2]. There are 99 vulnerable (G3), 61 imperiled (G2), and 26 critically endangered (G1) species among the 625 Astragalus species listed in the NatureServe database [3]. Astragalus sikokianus, a rare perennial plant distributed along the coast of Japan, was reported to be extinct in the wild until several individuals were found in southern parts of South Korea [4]. A. koraiensis, which was reported to be an endangered species on the red list based on the IUCN evaluation in 2008, has similar morphological characteristics to A. sikokianus and was classified as the same species as A. sikokianus in 2015 [5,6,7]. In the genus Astragalus distributed in South Korea, there are seven native species and two varieties of Astragalus. Among them, one vulnerable and one critically endangered species were listed in the Korea National Arboretum [5].
Astragalus species have a hard seed coat and seed dormancy that often require scarification or stratification to break dormancy [8,9,10]. In particular, low germination has been observed for the endangered species of A. contortuplicatus [11], A. nitidiflorus [12], and A. bibullatus [13]. On the other hand, this seed dormancy inhibits ex situ or in situ conservation efforts to conserve species that are rapidly vanishing from native habitats. Various dormancy breakage treatments, such as heat, stratification, physical scarification, sulfuric acid, and smoke water, have been attempted for Astragalus species [8,9,10,13,14,15,16]. Although A. sikokianus is extinct in the wild, there is little information available in the literature on the germination characteristics of the seed and the techniques for breaking dormancy. Moreover, in the case of Astragalus, the germination characteristics vary from species to species, and even for collections within species [8,13,15].
Seed coating technology improves the field delivery by modifying the seed size and shape. Pelleting is a type of seed coating for small, expensive, or uneven shaped seeds that use inert materials, such as bentonite, calcium carbonate, talc, diatomaceous earth, and sand, to apply a thicker cover (filler) with a binder [17]. Binders and fillers must be compatible with active compounds, and not adversely impact germination and growth. Depending on the type of pelleting and filler, water can be used instead of a binder [18].
In previous studies, seed pellets have been differently named in restoration depending on how the pelletized seeds were distributed and what the purpose of the study was, such as “compressed or extruded pellets or seed pods”, “seed coating”, “seed bombs”, “seed balls”, and “encrusted seed” [19]. Seed pelleting has been used in agriculture since ancient times. The use of seed pelleting started in the 1940s in large-scale restoration in Arizona, USA. It generally corresponded to the mechanization of restoration seeding [19]. So far, its application to native seeds has been limited to experimental trials [18]. Because of the high cost of seed pelleting, the application of pelleting to wild plant species for ecological restoration has been rarely studied [17]. However, seeds are the most cost-effective option for ecological restoration compared with planting seedlings, particularly on larger scales or in highly biodiverse ecosystems [20]. Seed-pelleting approaches designed specifically for restoration are essential [17,19,21]. The high failure rate of seed-based restoration has been attributed to physiological, logistical, and ecological environmental factors, including low seed viability, dormancy, difficulties in handling and delivery due to variations in the seed size and morphology, and variability in the environmental conditions across the restoration sites [17]. In particular, small seeds in large-scale restoration have difficulties in handling and sowing efficiency. Therefore, small-seeded species were either excluded from restoration or planted with low germination [22,23]. Seed pelleting improves the flowability and broadcast delivery of a small-seeded species in Artemisia tridentate. Seed conglomeration that was made with seed coater in small (<1 mm) and low-purity seeds could broadcast 2.2 times further and increase germination in the laboratory, but not field trials, in Utah, USA. Improved broadcasting of conglomerated seeds can save time and reduce labor costs and costs associated with seeding equipment [23]. On the other hand, no studies have been conducted on seed pelleting of small dormant seeds in wild species.
The objectives of this study were to (i) determine the optimal dormancy-breaking method for A. sikokianus and (ii) to evaluate the applicability of seed pelleting for dormant small seed species for large-scale ecological restoration use. For this purpose, the germination of pelleted seeds of various sizes combined with seed dormancy-breaking pretreatments was evaluated under various soil conditions.
2. Materials and Methods
2.1. Seed Materials
Seeds of A. sikokianus for the seed dormancy experiment were provided by the National Institute of Biological Resources (ID no. NIBRGR0000384852). Seed propagation was performed to produce a sufficient seed quantity for the seed pelleting experiments at the Research Farm of Dong-A University (35°6′53.7″ N, 128°58′6.15″ E). The fruit of A. sikokianus is an indehiscent legume containing 34 ± 2.5 seeds. The shape of the seeds is compressed, reniform-broad, and very small (1.944 g per 1000 seeds, length 1.96 ± 0.07 mm, width 1.58 ± 0.10 mm). The seeds of A. sikokianus were collected on August 2021. After adjusting the seed moisture content to 7–8% or less, the seeds were sealed in a plastic bag and stored at 4 °C until use.
2.2. Prechilling, GA3, and Ethephon Treatments
The seeds were placed on a moistened paper towel for 30, 60, and 90 days at 4 °C for the prechilling treatment. For the plant hormone treatment, 110 seeds were immersed in a petri dish (60 × 15 mm) of 500 mg·L−1 ethephon or 1000 mg·L−1 GA3 solution (8 mL) for 24 h on an orbital shaker (150 rpm) in the dark at 20 °C.
2.3. Hot Water and Acid Scarification
The seeds were soaked in hot distilled water at 70 °C for 5, 10, 20, and 30 min, respectively. For acid scarification, the seeds were treated with hydrochloric acid (Sigma, 320331) and sulfuric acid solutions (Junsei, 83010S1250). The seeds were soaked in a 37% hydrochloric acid solution for 5, 10, and 20 min and a 95% sulfuric acid solution for 10, 20, 30, and 60 min. After the scarification treatments, the seeds were rinsed thoroughly with running tap water and dried at room temperature.
2.4. Mechanical Scarification
The mechanical treatments were applied by rubbing the seeds with 80-grit sandpaper to weaken the seed coat. The seeds were placed on sandpaper and rubbed one, three, and five times with a spatula attached to the sandpaper (Supplementary Materials, Figure S1). The sandpaper-treated and control seeds were immersed in water for 12, 18, and 24 h at 20 °C. The seed moisture content (SMC) was measured according to ISTA rules [24].
2.5. Seed Pelleting
The pelleting formula consisted of pelleting materials (fillers), including diatomite, talc, and kaolin (mixtures produced by NOROO Holdings Co., LTD, Seoul, Republic of Korea), and water instead of binders. Owing to the limited amount of seeds, two types of equipment were mixed, as follows: a mini pan coater (Ecoco Co., Ltd., Hwaseong, Republic of Korea) and a rotary coater (Shinnong Co. Ltd., Hwaseong, Republic of Korea). In the first step, the seed sizes were increased into pellet seeds, 1 mm in size, using a pan coater with a rotation speed of 20 to 120 rpm. In the second step, the seeds pre-pelleted in the first step were transferred to a rotary coater and made into a circle at a rotation speed of 500 to 1200 rpm, and the hardness was increased. To attach the pellet filling, water was sprayed on the seed surface instead of the binder while the seeds spun in the coater. In the third step, after adding the pellet filler and water, the coating pan was rotated continuously for 30 min, and the surface was dried using an air dryer (50 °C). Pelleted seeds were further dried at 8% SMC in a seed dryer. The pelleted seeds were passed through sieves with round holes of 2.5, 3.0, 3.5, and 4.0 mm and divided into four groups. Dried pellet seeds were sealed in a plastic bag and stored at 4 °C until use. Unpelleted naked seeds and unpelleted sandpaper-treated seeds were used as a control. To evaluate the effectiveness of seed pelleting for restoration, the effect of the pellet seed size (2.0–2.5, 2.5–3.0, 3.0–3.5, and 3.5–4.0 mm diameter) and the soil type (commercial soil, sandy loam field soil, and sand) on seed germination were observed.
2.6. Germination Test
After the seed treatments, 100 seeds were placed on two layers of filter paper in 100 mm Petri dishes (four replicates of 25 seeds each) moistened with 5 mL of distilled water. The seeds were incubated at 25 °C under 24 h of light for 14 days. Germination was scored daily for 14 days. The seed was considered to be germinated when the radicle length exceeded 2 mm. The effect of the pelleting on seedling emergence was evaluated by burying four replicates of 25 seeds in the soils and counting radicle emergence daily for 28 days. The germination percentage (GP), mean germination time (MGT), germination rate (GR), germination uniformity (GU), and healthy seedling percentage (HS) were calculated according to the following formulae [25]:
where D is the number of days after planting, N is number of final germinated seeds, n is number of germinated seeds on day D, and S is number of planted seeds.
Germination percentage (%) = N/S × 100
Mean germination time (day) = ∑(Dx × nx)/N
Germination rate (% day−1) =100 × ∑(nx/Dx)
Germination uniformity = ∑[(∑(Dx × nx)/N) − Dx)2 × nx]/(N − 1)
Healthy seedling percentage (%) = Number of healthy seedlings/S × 100
2.7. Statistical Analysis
All data were analyzed statistically using the analysis of variance (ANOVA). One-way ANOVA was performed to examine the dormancy-breaking effects of seed treatments on germination, and two-way ANOVA analyzed the effects of the soil type and pellet size and their interactions on germination. The means were compared using the Duncan multiple range test (DMRT) at a 5% level. Statistical analyses were conducted using SAS System for Windows, Version 8.01 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Effects of Seed Dormancy Breaking Treatments on Germination Characteristics
Untreated intact seeds of A. sikokianus did not germinate. The moist chilled seeds placed on a moistened paper towel at 4 °C for 30, 60, or 90 days did not germinate. The seeds soaked in a 500 mg L−1 ethephon or 1000 mg L−1 GA3 solution for 24 h did not germinate (data not shown). The physiological dormancy-breaking methods were unsuitable for A. sikokianus seeds.
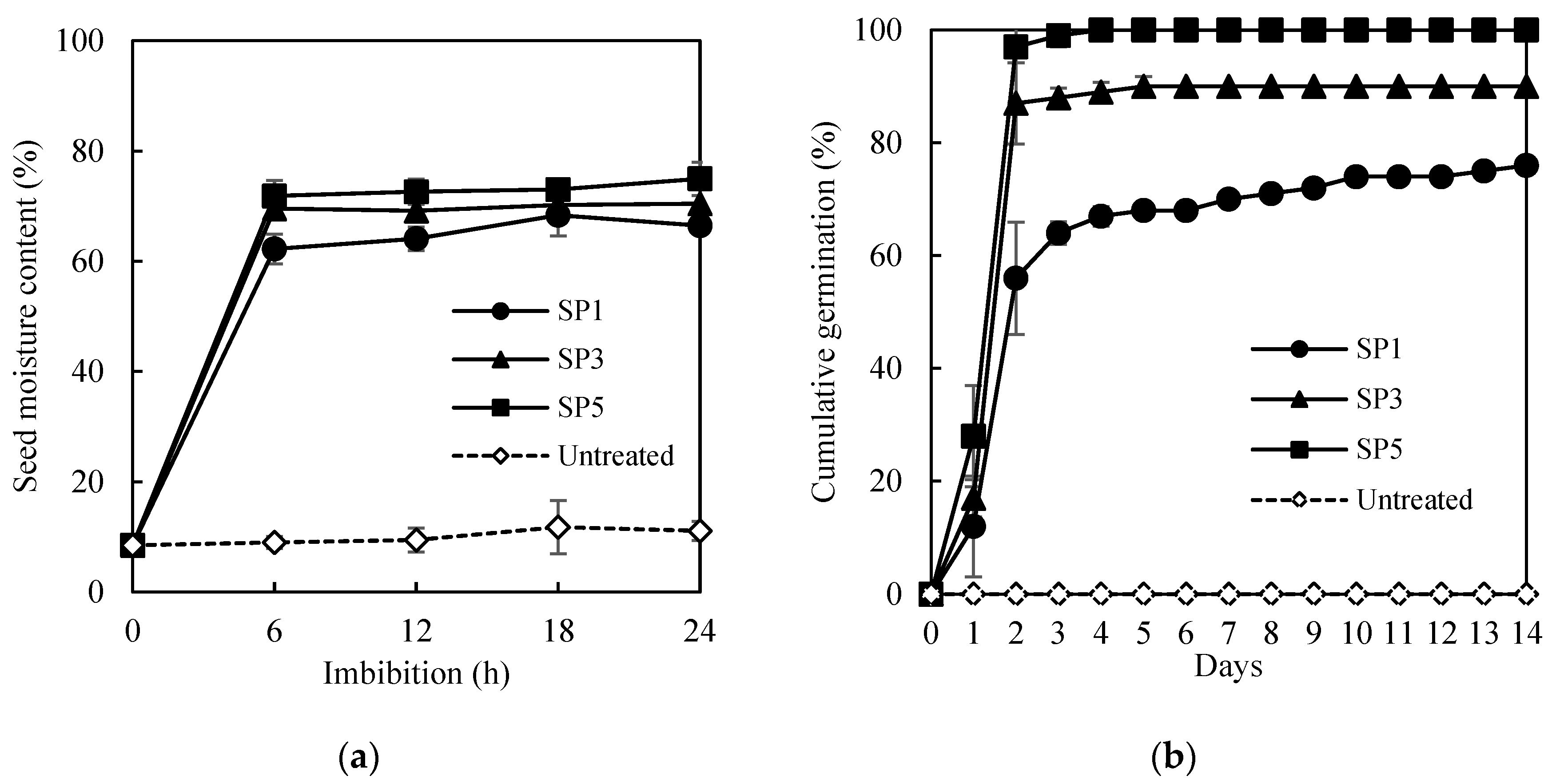
The germination after using hot water, hydrochloric acid, and sulfuric acid-treated seeds was measured (Table 1). The seeds soaked in 70 °C hot water (5, 10, 20, and 30 min) and 37% hydrochloric acid solution (5, 10, and 20 min) showed a less than 4% GP. The seeds treated in a 95% sulfuric acid solution for 60 min showed 9% GP, but the germinated seeds could not develop into healthy seedlings (HS, 0%). As a result, the hot water, hydrochloric acid, and sulfuric acid treatment did not have a positive effect on the germination of A. sikokianus seeds. Significant effects of sandpaper (SP) rubbing were detected, as shown in Figure 1. Untreated seeds showed an 8.5% seed moisture content (SMC) before imbibition. After 6, 12, 18, and 24 h of imbibition, the SMC of the untreated seeds was 9.2, 9.4, 11.8, and 11.1%, respectively (Figure 1a, Supplementary Materials, Table S1). The SMC of the sandpaper-treated seeds increased by more than 62% after 6 h of imbibition. The SMC increased with the increased rubbing with sandpaper. The highest SMC was achieved in the SP5-treated seeds (74.9% SMC). The SMC of the SP3- and SP1-treated seeds also increased to 70.5% and 66.5% after 24 h of imbibition, respectively.

Table 1.
Effects of 95% H2SO4, 37% HCl, and hot water (70 °C) treatment on the germination percentage (GP), mean germination time (MGT), germination rate (GR), germination uniformity (GU), and healthy seedlings percentage (HS) of A. sikokianus seeds.
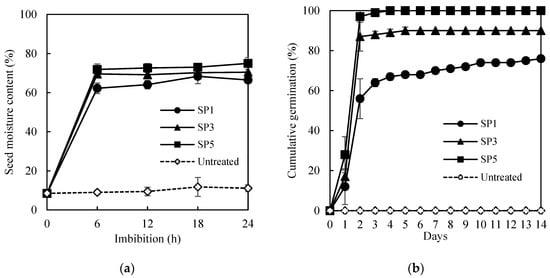
Figure 1.
Effects of scarification with sandpaper (SP) on the increase in seed moisture content (a) and germination percentage (b) of A. sikokianus. Seeds were mechanically scarified by sandpaper (SP) 1 (SP1), 3 (SP3), and 5 (SP5) times. Data represent mean of four replicates ± SD.
Scarified seeds with SP rubbing for one (SP1), three (SP3), and five (SP5) times were compared in germination (Figure 1b, Supplementary Materials, Figure S2). The GP increased increasing the number of rubbing steps. Scarified seeds started to germinate one day after imbibition, and germination was almost completed within 4–6 days. The seeds placed between coarse sandpaper and rubbed three or five times showed significantly higher germination (90 and 100%, respectively) than the seeds rubbed once (76%), which were significantly higher than those not scarified (0%). The SP treatment was the most appropriate dormancy-breaking method for A. sikokianus seeds that had a hard seed coat. The scarified seeds rubbed five times with sandpaper (SP5), which showed the highest germination (100%), were used for the pelleting treatment.
3.2. Effect of Seed Pelleting of Physical Dormant Seeds on Germination
Pelleted seeds with sandpaper-scarified seeds had a significantly higher GP of 90–100% than the untreated seeds (3% GP). The smaller pelleted seeds showed increased GP, GR, and HS with a shortened MGT. The 2.0–2.5 mm size, which had the highest and fastest germination rate, showed the same germination, of 100%, as that of the GP of unpelleted sandpaper scarified seeds. In contrast, the unpelleted untreated control showed only 3% germination (Table 2, Supplementary Materials, Figure S3).

Table 2.
Effect of pelleting and size of pelleted seeds on germination percentage (GP), mean germination time (MGT), germination rate (GR), germination uniformity (GU), and healthy seedling percentage (HS) of A. sikokianus seeds.
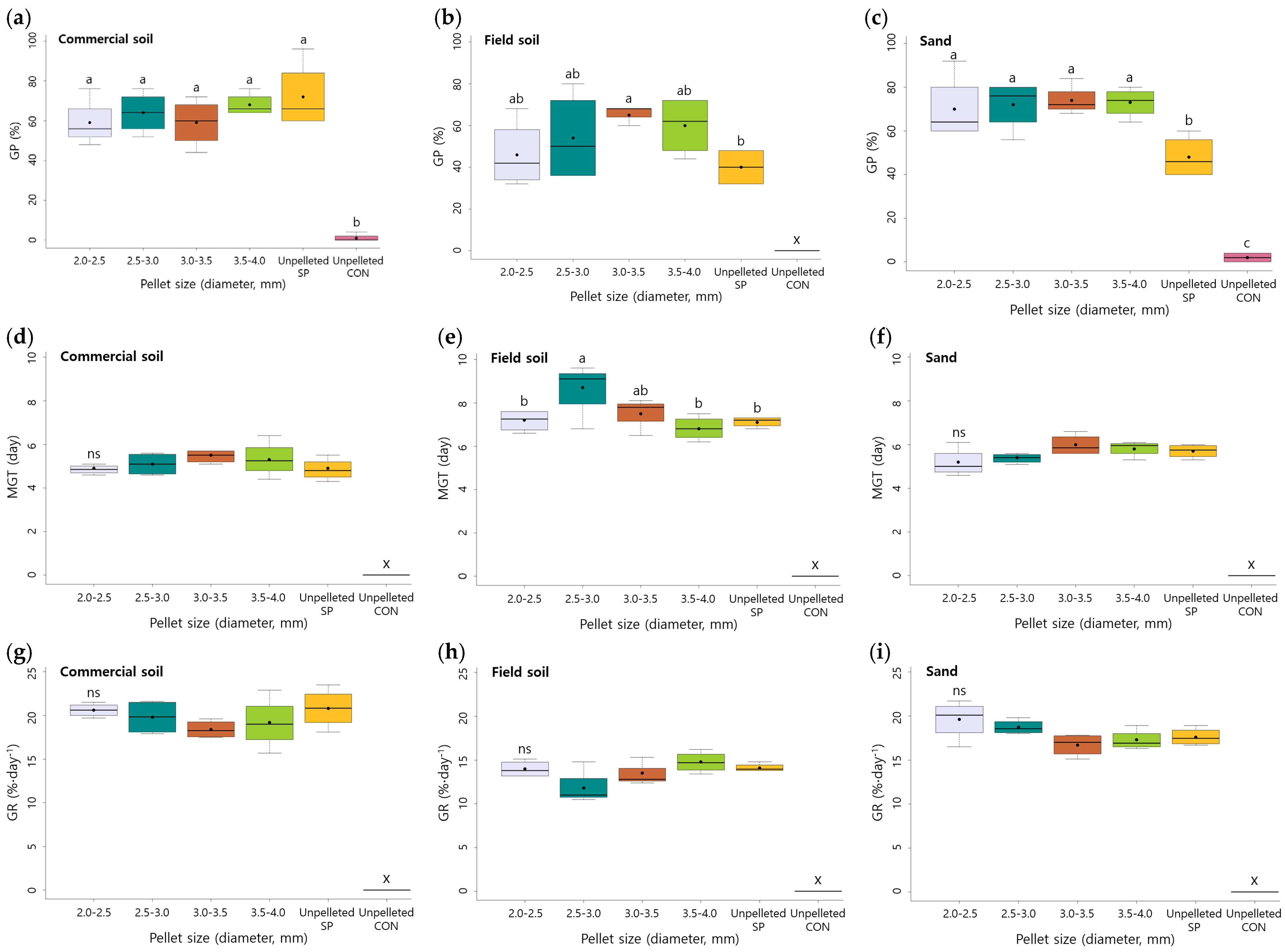
The various sizes (2.0–2.5, 2.5–3.0, 3.0–3.5, and 3.5–4.0) of the pelleted seeds were sown in commercial soil, field soil, and sand (Table 3, Supplementary Materials, Figures S4–S6). Significant effects of the substrate and sizes of the pelleted seeds were detected in GP, MGT, and GR, as shown in Table 3 (p < 0.001). However, their interactions were not observed. The unpelleted CON (unpelleted non-scarified control) seeds showed only 0–2% germination under all of the three substrates. In commercial soil, unpelleted SP seeds showed 72% GP, while the germination of pelleted seeds was slightly decreased to 59–68% GP depending on the size of the pelleted seeds. The highest germination characteristic was observed in the 3.5–4.0 mm sized pelleted seeds in commercial soil (68% GP). The germination of A. sikokianus seeds decreased in the field soil compared with other substrates. The unpelleted CON and SP seeds germinated only 0 and 40% in field soil, respectively, whereas all of the pelleted seeds showed higher germination rates than the unpelleted seeds. Pelleted seeds, 3.0–3.5 mm in size, showed the highest germination (65% GP) among the different sizes of pelleted seeds in field soil. Pelleted seeds sown in sand showed the highest germination (70–74%), regardless of the pellet size. The best germination was achieved in sand and commercial soil with a 2.5–4.0 mm pellet size (Figure 2).

Table 3.
Results of two-way ANOVA for the effect of the soil substrate and size of pelleted seeds on the germination percentage (GP), mean germination time (MGT), and germination rate (GR).
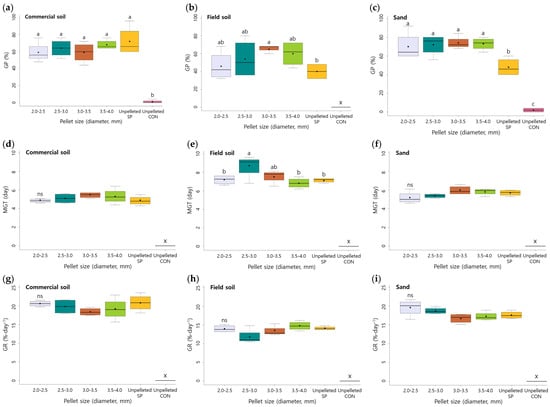
Figure 2.
Effect of the soil substrate and size of pelleted seeds on the (a–c) germination percentage, (d–f) mean germination time, and (g–i) germination rate (GR) of A. sikokianus seeds. Box plot showing germination traits in different sized pelleted seeds. Different letters indicated significant differences within a pelleting size at p < 0.05, according to Duncan’s multiple test, ns indicates non-significance. The box plots illustrate the results from four replicates. The bottom and top of the boxes show the 25 and 75 percentiles, respectively. The median is indicated by the black horizontal line, and the ends of the whiskers represent the minimum and maximum values. x: values are not available because of less than 2% GP.
4. Discussion
Seeds of the Astragalus genus have a hard seed coat and physical dormancy, resulting in difficult germination, and enhancement methods are not well developed. Many scarification treatments for various Astragalus species have been evaluated [8,9,10,14,15,26,27]. A. sikokianus, extinct in the wild in Japan, also has deep seed dormancy and an appropriate seed propagation method has not been determined. In this study, untreated seeds, moist chilled seeds for up to 90 days, and plant hormone (ethephon or GA3)-treated seeds were not germinated (Table 1). Similar to the present data, up to 16 weeks of cold treatment on moist sand was almost completely ineffective at breaking the dormancy in A. arpilobus. After 12 months of burial (and on the surface), only 2–4% of A. arpilobus germinated and remained not imbibed [10]. A. sikokianus have hard seed coats and physical dormancy, similar to most temperate Astragalus species [9,10,15].
Seed-coat-imposed dormancy has been confirmed in several species of the Astragalus genus. Various mechanical scarifications have been applied. The dormancy of A. hamosus, A. sinicus, and A. arpilobus, which are annual species, was broken in 94–100% of seeds exposed to 25–60 min of 70–97% sulfuric acid [10,14,27]. Sulfuric acid scarification (98%, 20 min) of cicer milkvetch, a perennial legume, showed significant variation among 10 polycross progenies tested for the reduction of hard seed; the germination rates ranged from 58 to 99% [8]. On the other hand, Statwick [9] reported that 98% sulfuric-acid-treated cicer milkvetch seeds treated for five minutes resulted in ~30% germination. In the present study, acid scarification with sulfuric acid (95%) or HCl (37%) for 5–60 min did not show a dormancy-breaking effect in A. sikokianus (Table 1). Hot water and dry heat treatments also effectively broke the seed dormancy in A. hamosus and A. sinicus [14,27]. Nevertheless, hot water (70 °C) immersion for 5–30 min in the present study showed 0–4% germination. Long et al. [10] also reported that dry heat produced only ≤5% of the seeds germinated, and wet-heated seeds showed the highest germination (31%) in 70–100 °C water (soaking time 5–240 min) in A. arpilobus. These differences in germination rate within the genus or species could result from different species, maternal effects, or collection conditions [8,13,15,28].
In this study, the highest germination (100%) was obtained within two days after sowing by mechanical seed treatments with sandpaper. Increasing the number of sandpaper rubbing steps significantly influenced the seed permeability and germinability (Figure 1a,b). Carleton et al. [29] reported that mechanically scarified A. cicer seeds showed increased germination, reaching 86%. Miklas et al. [8] reported no structural difference in the seed coat anatomy among 10 polycross progenies with 12 to 77% for hard seeds. The progenies of heavier seeds responded better to scarification than the lighter seeds. The variation was attributed to the volume/thickness and length/weight effects. Larger seeds were easier to scarify because of their greater surface area. The thinner seeds had less physical evidence of scarification than the thicker seeds. Seed roundness, rather than seed weight, affects the efficacy of scarification [15]. Similar to the present results, physical scarification tends to be reliable for Astragalus. Mechanical scarification is much safer to use because of the unsafe and impractical sulfuric acid to break dormancy [26]. The major disadvantage of using physical scarification, namely the labor-intensive nature of individually damaging the seed coats with sandpaper, a razor blade, or nail clippers, can be overcome using batch scarification methods [9,26]. In the case of a severely limited number of seeds available from rare species, which may have only dozens or hundreds of reproductive individuals, the time required to scarify individually is minimal, whereas the higher seed loss with batch scarification equipment would be unacceptable. This paper suggests additional optimization trials with specific simple, safe, and rapid methods by placing seeds between sandpaper and rubbing using sandpaper attached to a spatula.
Seeds of A. sikokianus have a water-impermeable seed coat because untreated seeds showed an 8.5% moisture content after 24 h of soaking, whereas sandpaper scarified seeds showed a 75% moisture content (Figure 1a) as reported for 33 species of Astragalus [10]. 99% or 100% of scarified seeds germinated at water potentials of 0 to −0.30 MPa, but germination declined to 86% at −0.51 MPa in A. arpilobus [10], and approximately 30% of the scarified seeds of A. australis var. olympicus germinated at −1.5 MPa [30]. The critical seed moisture content for germination was higher in proteinous seeds, such as soybean, and lower in starchy seeds, like rice. The lowest moisture content for the germination of soybean was 49.3%. Soybean showed the highest value of the least seed moisture content required for germination among various vegetable and crop seeds and began germination under −0.19 MPa of moist condition. There was a positive relationship between the lowest moisture content of the seed and soil required for germination [31]. Therefore, imbibition of 75% SMC are mandatory conditions for germination in A. sikokianus of Fabaceae family.
In this study, seed enhancement technologies that break seed dormancy through mechanical scarification combined with seed pelleting were used for small-seeded species restoration. In the laboratory, the differences in germination according to the size of the pelleted seeds were not significant and germinated >90% in the filter paper. On the other hand, as the size increased, the MGT increased from 3.3 to 4.4 days in 3.0–3.5 and 3.5–4.0 mm pelleted seeds (unpelleted seeds 2.9 days MGT) (Table 2). A decrease in MGT means fast and uniform germination. Seed pelleting involves covering a seed coat with a layer of a pelleting material that causes an increase in the mass, size, and shape of the seed [17]. Tomato seed coating increased the mean time to germination and decreased germination percentage compared with the non-coated seeds. Seed coating acted as a barrier that delayed the emergence of tomato seedlings [32]. Fast germination provides important establishment benefits of faster field germination in order to outcompete weed species [33].
In the soil substrate, germination of unpelleted SP seeds in commercial soil, field soil, and sand decreased to 72%, 40%, and 48% compared with the filter paper (100% GP), respectively. In the sand, pellets improved seedling emergence compared with the unpelleted SP by 54% (Figure 2). Depending on the species and seed size, seedling emergence can be curtailed due to improper seed placement in the soil [34]. Overall, these results indicate that seed pellets may improve the seeding efforts of A. sikokianus and may aid in the emergence of other small-seeded species. Extruded pellets (aggregate seeds) improved the seedling emergence of small-seeded species of Artemisia tridentata Nutt. spp. [35]. Sugar beet seeds showed a significant difference between the germination percent in different sizes of seeds, and the extra usage of covering material for seed pelleting decreased the viability and germination uniformity of seeds [36]. Increasing the seed pelleting thickness reduced the seedling emergence, yield, and product quality. The thickest pellet seeds (4.5–4.75 mm) were unsuitable in similar and semi-arid climatic conditions. A pellet size of 3.5–4.25 mm (coated thickness 0.25–0.75 mm) was the appropriate thickness to obtain the optimal results [37]. In the present study, the thick pelleted seeds (3.0–3.5 mm) showed the highest germination in the field soil. Considering the naked seeds, the ratio of pellet thickness was much higher in A. sikokianus than in sugar beet.
The pellet properties can affect the respiration rate under excess water conditions in filter paper. The differences in the swelling ability of a pellet are caused by the use of materials of different types and qualities for pelleting [38]. The high water absorbency of the materials used in the pellet causes the pellet to swell, which pushes seeds to the surface and produces small voids or conduits for the emerging seedlings to follow [34]. The selection of pelleting material has a significant impact on germination. Diatomite, a water-distributing material, exhibited higher levels of germination under most water availabilities than the water-absorbing material, calcium bentonite [39]. In the present study, pellet materials mixed with diatomite, talc, and kaolin in a special ratio were used. The selection of pelleting material is crucial because it directly affects the germination speed and subsequent establishment rates [39,40].
5. Conclusions
This paper is the first to report the seed pelleting of dormant seeds, especially in small-seeded wild species. The seed pelleting combined with dormancy-breaking techniques described in this study have great potential in A. sikokianus restoration or reintroduction. On the other hand, the response to particular dormancy-breaking treatments, pelleted methods (materials and size), and germination environments (substrate) vary significantly among plant species. Thus, considering the climatic conditions after sowing, proper irrigation scheduling would benefit field soil conditions without sufficient moisture. These results indicate that small dormant seeds also can be applied to pelleting for restoration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13010206/s1, Table S1. Seed moisture content (SMC) of sandpaper (SP) treated seeds after 6, 12, 18, and 24 h of imbibition in water; Figure S1. Light micrograph of sandpaper (SP) treated seeds; Figure S2. Photos of germination of sandpaper (SP) treated seeds; Figure S3. Photos of germination of pelleted seeds on filter papers; Figure S4. Photos of germination of pelleted seeds in commercial soil; Figure S5. Photos of germination of pelleted seeds in field soil; Figure S6. Photos of germination of pelleted seeds in sand.
Author Contributions
Conceptualization, D.-H.K., Y.-J.J., S.-Y.K. and S.-H.C.; methodology, D.-H.K., Y.-J.J., H.-J.J. and J.-H.L.; validation, S.-Y.K., S.-H.C. and D.-H.K.; investigation, Y.-J.J. and H.-J.J.; data curation, Y.-J.J. and H.-J.J.; writing—original draft preparation, D.-H.K. and Y.-J.J.; writing—review and editing, D.-H.K. and J.-H.L., supervision, D.-H.K.; project administration, D.-H.K., S.-Y.K. and S.-H.C.; funding acquisition, D.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Project No. 2019R1A2C1089863) and the National Institute of Biological Resources (Project No. NIBR 202215101) and Green Fusion Technology Program funded by the Ministry of Environment.
Data Availability Statement
The data presented in this study are available within the article of supplementary material or request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chaudhary, L.B.; Rana, T.S.; Anand, K.K. Current status of the systematics of Astragalus L.(Fabaceae) with special reference to the Himalayan species in India. Taiwania 2008, 53, 338–355. [Google Scholar] [CrossRef]
- Watrous, K.M.; Cane, J.H. Breeding biology of the threadstalk milkvetch, Astragalus filipes (Fabaceae), with a review of the genus. Am. Midl. Nat. 2011, 165, 225–240. [Google Scholar] [CrossRef]
- NatureServe Explorer: An Online Encyclopedia of Life. Available online: http://explorer.natureserve.org (accessed on 20 October 2022).
- Whitton, I.; Sharrock, S. Conservation of Threatened Japanese Plants in UK Gardens; Botanic Gardens Conservation International: Richmond, UK, 2011; p. 64. [Google Scholar]
- Checklist of Vascular Plants in Korea (Alien Plants). Available online: http//www.Nature.go.kr/kpni/index.do (accessed on 28 October 2022).
- Choi, I.; Kim, S.; Choi, B. A taxonomic revision of Astragalus L.(Fabaceae) in Korea. Korean J. Plant Taxon. 2015, 45, 227–238. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 28 October 2022).
- Miklas, P.N.; Townsend, C.E.; Ladd, S.L. Seed Coat Anatomy and the Scarification of Cicer Milkvetch Seed. Crop Sci. 1987, 27, 766–772. [Google Scholar] [CrossRef]
- Statwick, J.M. Germination pretreatments to break hard-seed dormancy in Astragalus cicer L. (Fabaceae). PeerJ 2016, 4, e2621. [Google Scholar] [CrossRef]
- Long, Y.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Seed dormancy and germination characteristics of Astragalus arpilobus (Fabaceae, subfamily Papilionoideae), a central Asian desert annual ephemeral. S. Afr. J. Bot. 2012, 83, 68–77. [Google Scholar] [CrossRef]
- Molnár, V.A.; Sonkoly, J.; Lovas-Kiss, Á.; Fekete, R.; Takacs, A.; Somlyay, L.; Toeroek, P. Seed of the threatened annual legume, Astragalus contortuplicatus, can survive over 130 years of dry storage. Preslia 2015, 87, 319–328. [Google Scholar]
- Vicente, M.J.; Segura, F.; Aguado, M.; Migliaro, D.; Franco, J.A.; Martínez-Sánchez, J.J. Genetic diversity of Astragalus nitidiflorus, a critically endangered endemic of SE Spain, and implications for its conservation. Biochem. Syst. Ecol. 2011, 39, 175–182. [Google Scholar] [CrossRef]
- Albrecht, M.A. Seed germination ecology of three imperiled plants of rock outcrops in the southeastern United States. J. Torrey Bot. Soc. 2012, 139, 86–95. [Google Scholar] [CrossRef]
- Kim, S.; Oh, S.; Hwang, W.; Kim, S.; Choi, K.; Kang, H. Physical dormancy in seeds of Chinese milk vetch (Astragalus sinicus L.) from Korea. Korean J. Crop Sci. 2008, 53, 421–426. [Google Scholar]
- Acharya, S.N.; Kastelic, J.P.; Beauchemin, K.A.; Messenger, D.F. A review of research progress on cicer milkvetch (Astragalus cicer L.). Can. J. Plant Sci. 2006, 86, 49–62. [Google Scholar] [CrossRef]
- Chou, Y.; Cox, R.D.; Wester, D.B. Smoke water and heat shock influence germination of shortgrass prairie species. Rangel. Ecol. Manag. 2012, 65, 260–267. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: Science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed enhancement: Getting seeds restoration-ready. Restor. Ecol. 2020, 28, S266–S275. [Google Scholar] [CrossRef]
- Gornish, E.; Arnold, H.; Fehmi, J. Review of seed pelletizing strategies for arid land restoration. Restor. Ecol. 2019, 27, 1206–1211. [Google Scholar] [CrossRef]
- Pérez, D.R.; González, F.; Ceballos, C.; Oneto, M.E.; Aronson, J. Direct seeding and outplantings in drylands of Argentinean Patagonia: Estimated costs, and prospects for large-scale restoration and rehabilitation. Restor. Ecol. 2019, 27, 1105–1116. [Google Scholar] [CrossRef]
- Killough, J.R. Reseeding the Range by Airplane. Rangel. Ecol. Manag. 1950, 3, 33–41. [Google Scholar] [CrossRef]
- Ott, J.E.; Cox, R.D.; Shaw, N.L. Comparison of postfire seeding practices for Wyoming big sagebrush. Rangel. Ecol. Manag. 2017, 70, 625–632. [Google Scholar] [CrossRef]
- Hoose, B.W.; Call, R.S.; Bates, T.H.; Anderson, R.M.; Roundy, B.A.; Madsen, M.D. Seed conglomeration: A disruptive innovation to address restoration challenges associated with small-seeded species. Restor. Ecol. 2019, 27, 959–965. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2019. [Google Scholar]
- Ranal, M.A.; Santana, D.G. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Townsend, C.E.; McGinnies, W.J. Mechanical Scarification of Cicer Milkvetch (Astragalus Cicer L.) Seed. Crop Sci. 1972, 12, 392–394. [Google Scholar] [CrossRef]
- Patanè, C.; Gresta, F. Germination of Astragalus hamosus and Medicago orbicularis as affected by seed-coat dormancy breaking techniques. J. Arid Environ. 2006, 67, 165–173. [Google Scholar] [CrossRef]
- Bhatt, A.; Carón, M.M.; Souza-Filho, P.R.; Gallacher, D.J. Maternal source affects seed germination of a rare Arabian deert species (Astragalus sieberi). Botany 2021, 99, 293–301. [Google Scholar] [CrossRef]
- Carleton, A.E.; Austin, R.D.; Stroh, J.R.; Wiesner, L.E.; Scheetz, J.G. Cicer milkvetch (Astragalus cicer L.): Seed germination, scarification and field emergence studies. Mont. Agric. Exp. Stn. Bull. 1971, 655, 21. [Google Scholar]
- Kaye, T.N. From flowering to dispersal: Reproductive ecology of an endemic plant, Astragalus australis var. olympicus (Fabaceae). Am. J. Bot. 1999, 86, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jeon, Y.S. Critical seed moisture content for germination in crop species. Korean J. Intl. Agri. 2009, 21, 159–164. [Google Scholar]
- Joyce, G.; Melissa, L. Comparative studies of seed priming and pelleting on percentage and meantime to germination of seeds of tomato (Lycopersicon esculentum Mill.). Afr. J. Agric. Res. 2008, 3, 725–731. [Google Scholar]
- Vaughn, K.J.; Young, T.P. Short-term priority over exotic annuals increases the initial density and longer-term cover of native perennial grasses. Ecol. Appl. 2015, 25, 791–799. [Google Scholar] [CrossRef]
- Madsen, M.D.; Davies, K.W.; Boyd, C.S.; Kerby, J.D.; Svejcar, T.J. Emerging seed enhancement technologies for overcoming barriers to restoration. Restor. Ecol. 2016, 24, S77–S84. [Google Scholar] [CrossRef]
- Madsen, M.D.; Hulet, A.; Phillips, K.; Staley, J.L.; Davies, K.W.; Svejcar, T.J. Extruded seed pellets: A novel approach for enhancing sagebrush seedling emergence. Native Plants J. 2016, 17, 230–243. [Google Scholar] [CrossRef]
- Arshadi, J.; Asgharipour, M.R. The effects of seed size on germination and early seedling growth of pelleted seeds of sugar beet. J. Appl. Sci. Res. 2011, 7, 1257–1260. [Google Scholar]
- Tuğrul, K.M.; Kaya, R. The effect of seed coating thickness on sugar beet (Beta vulgaris L.) yield and quality under different irrigation conditions. Appl. Ecol. Env. Res. 2020, 18, 6969–6979. [Google Scholar] [CrossRef]
- Podlaski, S.Z.; Wzorek, H.; Chomontowski, C.M. Effects of the physicochemical properties of pellets on the germination of pelleted sugar beet seeds. Int. Agrophys. 2019, 33, 175–183. [Google Scholar] [CrossRef]
- Blunk, S.; Hoffer, J.; Brosda, S.; de Heer, M.I.; Sturrock, C.J.; Mooney, S.J. Impact of fruit orientation and pelleting material on water uptake and germination performance in artificial substrate for sugar beet. PLoS ONE 2020, 15, e0232875. [Google Scholar] [CrossRef]
- Nawrot-Chorabik, K.; Osmenda, M.; Słowiński, K.; Latowski, D.; Tabor, S.; Woodward, S. Stratification, scarification and application of phytohormones promote dormancy breaking and germination of pelleted scots pine (Pinus sylvestris L.) seeds. Forests 2021, 12, 621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).