Multiple Resistance to Three Modes of Action of Herbicides in a Single Italian Ryegrass (Lolium multiflorum L.) Population in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Herbicides and Chemicals
2.3. Whole-Plant Dose Response
2.4. DNA Sequencing of Target Genes from Three Herbicide MoAs
| Primers | Sequence (5′-3′) | Product Size (bp) | Tm (°C) | Usage | Sequence Source |
|---|---|---|---|---|---|
| ALS-F | CCGCAAGGGCGCCGACATCCTCGT | 1719 | 62 | Sequencing | AF310684.2 |
| ALS-R | CGAAATCCTGCCATCACCTTCCAT | ||||
| ACCase-F | AATGGGTCGTGGGGCACTCCTATAATTCC | 1600 | 61 | Sequencing | Reference [13] |
| ACCase-R | CTCCCTGGAGTTGTGCTTTC | ||||
| psbA-F | ATGACTGCAATTTTAGAGAGACGC | 1023 | 60 | Sequencing | EU360732.1 |
| psbA-R | TAGAGGGAAGTTGTGAGCAT | ||||
| RGTP a-F | GATGTGACTGACCAAGAGAGCTTCA | 117 | 60 | qRT-PCR | Reference [38] |
| RGTP-R | CTCAGCTAAGTCGCATTTGTTCCCC | ||||
| psbA-F | ATTCCAGGCAGAGCACAACAT | 156 | 60 | qRT-PCR | EU360732.1 |
| psbA-R | GTAACCCTCATTAGCAGATTCATTT |
2.5. psbA Gene Expression Analysis
2.6. Effects of CYP450 and/or GST Inhibitors on Isoproturon and Pyroxsulam Resistance
2.7. Data Analysis
3. Results
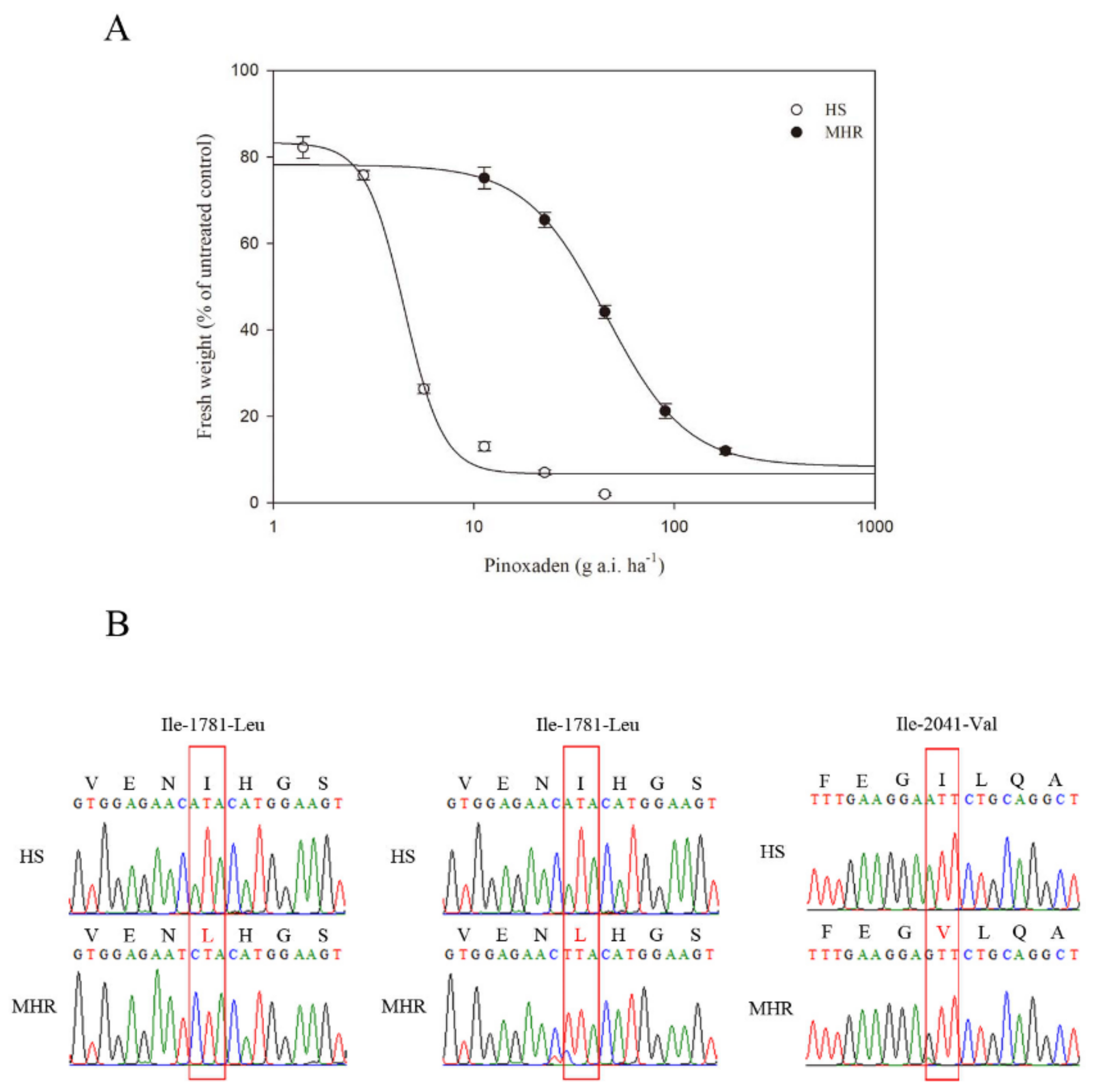
3.1. Resistance to ACCase-Inhibiting Pinoxaden
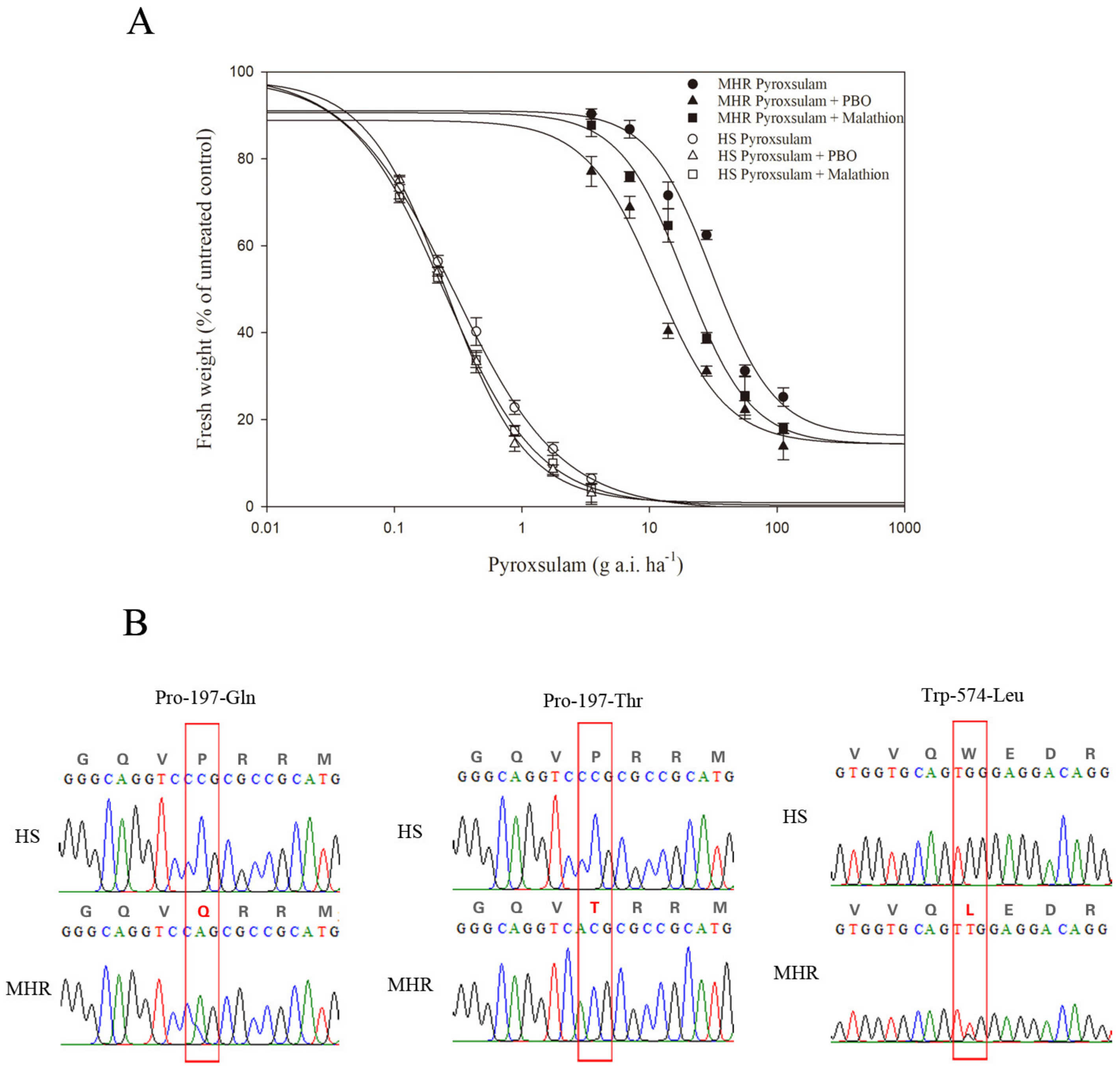
3.2. Resistance to ALS-Inhibiting Pyroxsulam
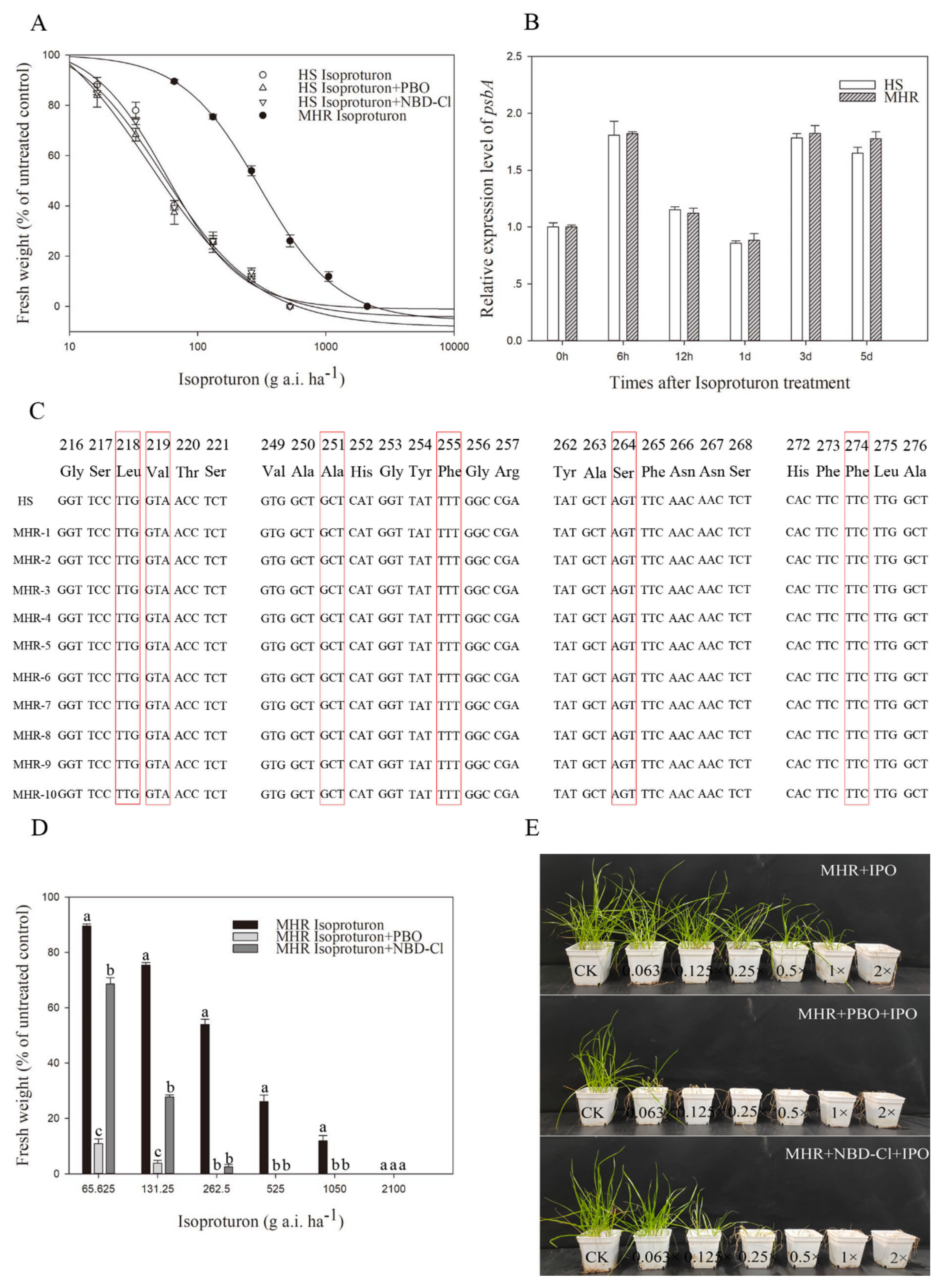
3.3. Resistance to PSII-Inhibiting Isoproturon
3.4. Sensitivity of L. multiflorum to Other Herbicides
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.org (accessed on 20 October 2022).
- Owen, M.J.; Martinez, N.J.; Powles, S.B. Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Res. 2014, 54, 314–324. [Google Scholar] [CrossRef]
- Lan, Y.; Li, W.; Wei, S.; Huang, H.; Liu, Z.; Huang, Z. Multiple resistance to ACCase- and ALS-inhibiting herbicides in black-grass (Alopecurus myosuroides Huds.) in China. Pestic. Biochem. Physiol. 2022, 184, 105127. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, S.; Kamidate, Y.; Yamaguchi, T.; Ishizaka, M.; Endo, M.; Suda, H.; Nagai, K.; Sunohara, Y.; Toki, S.; Uchino, A.; et al. CYP81A P450s are involved in concomitant cross-resistance to acetolactate synthase and acetyl-CoA carboxylase herbicides in Echinochloa phyllopogon. New Phytol. 2019, 221, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Guo, Q.; Wang, J.; Shi, L.; Yang, X.; Zhou, Y.; Yu, Q.; Bai, L. CYP81A68 confers metabolic resistance to ALS and ACCase-inhibiting herbicides and its epigenetic regulation in Echinochloa crusgalli. J. Hazard. Mater. 2022, 428, 128225. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, Y.; Liu, Z.; Wei, S.; Huang, H.; Lan, Y.; Sun, Y.; Huang, Z. Investigation of resistance mechanisms to bentazone in multiple resistant Amaranthus retroflexus populations. Pestic. Biochem. Physiol. 2022, 186, 105164. [Google Scholar] [CrossRef]
- Shergill, L.S.; Barlow, B.R.; Bish, M.D.; Bradley, K.W. Investigations of 2,4-D and Multiple Herbicide Resistance in a Missouri Waterhemp (Amaranthus tuberculatus) Population. Weed Sci. 2018, 66, 386–394. [Google Scholar] [CrossRef]
- Mahmood, K.; Mathiassen, S.K.; Kristensen, M.; Kudsk, P. Multiple Herbicide Resistance in Lolium multiflorum and Identification of Conserved Regulatory Elements of Herbicide Resistance Genes. Front. Plant Sci. 2016, 7, 1160. [Google Scholar] [CrossRef] [Green Version]
- Beckie, H.J.; Jasieniuk, M. Chapter 12—Lolium rigidum and Lolium multiflorum. In Biology and Management of Problematic Crop Weed Species; Chauhan, B.S., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 261–283. [Google Scholar]
- Zhan, X.; Li, Y.; Gou, W.; Zhang, R. Research development of Italian ryegrass. Pratacultural Sci. 2009, 26, 55–60. [Google Scholar]
- Wang, H.; Song, Y.; Li, J.; Yang, G.; He, W.; Luo, Z. Precaution against malaria weed ryegrass in wheat field. Plant Prot. 2008, 34, 149–151. [Google Scholar]
- Hao, P.; Zhang, L. Highly alert to the spread of harmful ryegrass in weeds. Shanxi J. Agric. Sci. 2015, 61, 50–51. [Google Scholar]
- Zhang, P.; Wu, H.; Xu, H.; Gao, Y.; Zhang, W.; Dong, L. Mechanism of Fenoxaprop-P-ethyl Resistance in Italian Ryegrass (Lolium perenne ssp. multiflorum) from China. Weed Sci. 2017, 65, 710–717. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef]
- Chipman, D.M.; Duggleby, R.G.; Tittmann, K. Mechanisms of acetohydroxyacid synthases. Curr. Opin. Chem. Biol. 2005, 9, 475–481. [Google Scholar] [CrossRef]
- Duggleby, R.G.; McCourt, J.A.; Guddat, L.W. Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol. Biochem. 2008, 46, 309–324. [Google Scholar] [CrossRef]
- Takano, H.K.; Ovejero, R.F.L.; Belchior, G.G.; Maymone, G.P.L.; Dayan, F.E. ACCase-inhibiting herbicides: Mechanism of action, resistance evolution and stewardship. Sci. Agricola 2020, 78, e20190102. [Google Scholar] [CrossRef]
- Sørensen, S.R.; Bending, G.D.; Jacobsen, C.S.; Walker, A.; Aamand, J. Microbial degradation of isoproturon and related phenylurea herbicides in and below agricultural fields. FEMS Microbiol. Ecol. 2003, 45, 1–11. [Google Scholar] [CrossRef]
- Qin, X.; Yang, C.; Hu, M.; Duan, Y.; Zhang, N.; Wang, J.; Wang, L.; Liu, W. Molecular Basis of Resistance to Mesosulfuron-Methyl in a Black-Grass (Alopecurus myosuroides Huds.) Population from China. Agronomy 2022, 12, 2203. [Google Scholar] [CrossRef]
- Zhao, N.; Bi, Y.; Wu, C.; Wang, D.; You, L.; Liu, W.; Wang, J. Cross-resistance to acetolactate synthase (ALS) inhibitors associated with different mutations in Japanese foxtail (Alopecurus japonicus). Weed Sci. 2019, 67, 389–396. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, F.; Li, Z.; Wang, H.; Wang, Q.; Wang, J.; Liu, W.; Bai, L. Target-site and non-target-site-based resistance to tribenuron-methyl in multiply-resistant Myosoton aquaticum L. Pestic. Biochem. Physiol. 2019, 155, 8–14. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Chi, Y.; Guo, W.; Luo, X.; Wang, J. Molecular Mechanism of Mesosulfuron-Methyl Resistance in Multiply-Resistant American Sloughgrass (Beckmannia syzigachne). Weed Sci. 2015, 63, 781–787. [Google Scholar] [CrossRef]
- Guo, W.; Lv, L.; Zhang, L.; Li, Q.; Wu, C.; Lu, X.; Liu, W.; Wang, J. Herbicides cross resistance of a multiple resistant short-awn foxtail (Alopecurus aequalis Sobol.) population in wheat field. Chil. J. Agric. Res. 2016, 76, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, J.; Fu, R.; Li, J.; Pan, L.; Dong, L. Effect of an adjuvant, Jijian, on isoproturon to control fenoxaprop-P-ethyl-resistant Beckmannia syzigachne. Int. J. Pest Manag. 2021. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Permingeat, H.R. Herbicide resistant weeds: A call to integrate conventional agricultural practices, molecular biology knowledge and new technologies. Plant Sci. 2020, 290, 110255. [Google Scholar] [CrossRef]
- Fang, J.; Yang, D.; Zhao, Z.; Chen, J.; Dong, L. A novel Phe-206-Leu mutation in acetolactate synthase confers resistance to penoxsulam in barnyardgrass (Echinochloa crusgalli (L.) P. Beauv). Pest Manag. Sci. 2022, 78, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Tranel, P.J.; Wright, T.R.; Heap, I. ALS Mutations from Herbicide-Resistant Weeds to Inhibition of Acetolactate Synthase. Available online: http://www.weedscience.com (accessed on 20 October 2022).
- Kaundun, S.S. Resistance to acetyl-CoA carboxylase-inhibiting herbicides. Pest Manag. Sci. 2014, 70, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, Q.; Han, H.; Owen, M.J.; Powles, S.B. A novel psbA mutation (Phe274–Val) confers resistance to PSII herbicides in wild radish (Raphanus raphanistrum). Pest Manag. Sci. 2019, 75, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Christophe, D. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2013, 2, 176–187. [Google Scholar]
- Suzukawa, A.; Bobadilla, L.K.; Mallory-Smith, C.; Brunharo, C. Non-target-Site Resistance in Lolium spp. Globally: A Review. Front. Plant Sci. 2021, 11, 609209. [Google Scholar] [CrossRef]
- Preston, C. Herbicide resistance in weeds endowed by enhanced detoxification: Complications for management. Weed Sci. 2004, 52, 448–453. [Google Scholar] [CrossRef]
- Li, W.; Wu, C.; Wang, M.; Jiang, M.; Zhang, J.; Liao, M.; Cao, H.; Zhao, N. Herbicide Resistance Status of Italian Ryegrass (Lolium multiflorum Lam.) and Alternative Herbicide Options for Its Effective Control in the Huang-Huai-Hai Plain of China. Agronomy 2022, 12, 2394. [Google Scholar] [CrossRef]
- Wu, C.; Song, M.; Zhang, T.; Zhou, C.; Liu, W.; Jin, T.; Zhao, N. Target-site mutation and cytochrome P450s confer resistance to multiple herbicides in Italian ryegrass (Lolium multiflorum Lam.) from China. Crop Prot. 2022, 161, 106068. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Chen, X.; Dong, L. Cross resistance patterns to acetyl-CoA carboxylase inhibiting herbicides associated with different mutations in Italian ryegrass from China. Crop Prot. 2021, 143, 105479. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, Y.; Zhang, Y.; Dong, L.; Li, J. Mechanisms of Resistance to Pyroxsulam and ACCase Inhibitors in Japanese Foxtail (Alopecurus japonicus). Weed Sci. 2016, 64, 695–704. [Google Scholar] [CrossRef]
- Gaines, T.A.; Lorentz, L.; Figge, A.; Herrmann, J.; Maiwald, F.; Ott, M.; Han, H.; Busi, R.; Yu, Q.; Powles, S.B.; et al. RNA-Seq transcriptome analysis to identify genes involved in metabolism-based diclofop resistance in Lolium rigidum. Plant J. 2014, 78, 865–876. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Cocker, K.M.; Northcroft, D.S.; Coleman, J.O.D.; Moss, S.R. Resistance to ACCase-inhibiting herbicides and isoproturon in UK populations of Lolium multiflorum: Mechanisms of resistance and implications for control. Pest Manag. Sci. 2001, 57, 587–597. [Google Scholar] [CrossRef]
- Murphy, B.; Tranel, P. Target-Site Mutations Conferring Herbicide Resistance. Plants 2019, 8, 382. [Google Scholar] [CrossRef] [Green Version]
- Jhala, A.J.; Beckie, H.J.; Mallory-Smith, C.; Jasieniuk, M.; Busi, R.; Norsworthy, J.K.; Bagavathiannan, M.V.; Tidemann, B.D.; Geddes, C.M. Transfer of resistance alleles from herbicide-resistant to susceptible grass weeds via pollen-mediated gene flow. Weed Technol. 2021, 35, 869–885. [Google Scholar] [CrossRef]
- Scarabel, L.; Panozzo, S.; Varotto, S.; Sattin, M. Allelic variation of the ACCase gene and response to ACCase-inhibiting herbicides in pinoxaden-resistant Lolium spp. Pest Manag. Sci. 2011, 67, 932–941. [Google Scholar] [CrossRef]
- Délye, C.; Zhang, X.-Q.; Chalopin, C.; Michel, S.; Powles, S. An Isoleucine Residue within the Carboxyl-Transferase Domain of Multidomain Acetyl-Coenzyme A Carboxylase Is a Major Determinant of Sensitivity to Aryloxyphenoxypropionate but Not to Cyclohexanedione Inhibitors. Plant Physiol. 2003, 132, 1716–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Z.; Peng, Y.J.; Chen, W.; Yu, Q.; Bai, L.Y.; Pan, L. The Ile-2041-Val mutation in the ACCase gene confers resistance to clodinafop-propargyl in American sloughgrass (Beckmannia syzigachne Steud). Pest Manag. Sci. 2021, 77, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Hongchun, W.; Jun, L.; Bo, L.; Yuanlai, L.; Liyao, D. The role of cytochrome P450 monooxygenase in the different responses to fenoxaprop-P-ethyl in annual bluegrass (Poa annua L.) and short awned foxtail (Alopecurus aequalis Sobol.). Pestic. Biochem. Physiol. 2013, 107, 334–342. [Google Scholar] [CrossRef]
- Kumar, N. Herbicide resistance mechanism of P. minor in Uttarakhand. J. Crop Weed 2016, 12, 129–133. [Google Scholar]
- Burnet, M.; Loveys, B.; Holtum, J.; Powles, S. Increased Detoxification Is a Mechanism of Simazine Resistance in Lolium rigidum. Pestic. Biochem. Physiol. 1993, 46, 207–218. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Lei, Q.; Lu, B.; Jin, C.; Liu, X.; Wang, Y.; Bai, L. Multiple herbicide resistance in Eleusine indica from sugarcane fields in China. Pestic. Biochem. Physiol. 2022, 182, 105040. [Google Scholar] [CrossRef]
- Nakka, S.; Godar, A.S.; Thompson, C.R.; Peterson, D.E.; Jugulam, M. Rapid detoxification via glutathioneS-transferase (GST) conjugation confers a high level of atrazine resistance in Palmer amaranth (Amaranthus palmeri). Pest Manag. Sci. 2017, 73, 2236–2243. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Cui, H.; Chen, J.; Li, X. PsbA gene over-expression and enhanced metabolism conferring resistance to atrazine in Commelina communis. Pestic. Biochem. Physiol. 2022, 188, 105260. [Google Scholar] [CrossRef]
- Preston, C.; Wakelin, A.; Dolman, F.; Bostamam, Y.; Boutsalis, P. A Decade of Glyphosate-Resistant Lolium around the World: Mechanisms, Genes, Fitness, and Agronomic Management. Weed Sci. 2009, 57, 435–441. [Google Scholar] [CrossRef]
- Wang, Y.; Han, H.; Chen, J.; Yu, Q.; Vila-Aiub, M.; Beckie, H.J.; Powles, S.B. A dinitroaniline herbicide resistance mutation can be nearly lethal to plants. Pest Manag. Sci. 2022, 78, 1547–1554. [Google Scholar] [CrossRef]
| Population | Suspected Biotype a | Geographical Location | ||
|---|---|---|---|---|
| Longitude | Latitude | Province | ||
| DH-JNXW-2020-2 | HS | 118.87° E | 32.03° N | Jiangsu Academy of Agricultural Sciences, Xuanwu District, Nanjing, Jiangsu Province |
| DH-HZYC-2020-1 | MHR | 113.95° E | 32.95° N | Dashu Au, Yicheng District, Zhumadian, Henan Province |
| Class a | Group a | Herbicide | Formulation | Manufacturer | Doses Applied g a.i. ha−1 b | |
|---|---|---|---|---|---|---|
| HS | MHR | |||||
| ALS | TP | Pyroxsulam | 7.5% WG | Dow AgroSciences, Beijing, China | 0,0.11,0.22,0.44,0.88,1.75,3.5 | 0,3.5,7,14,28,56,112 |
| Penoxsulam | 25g L−1 OD | Dow AgroSciences, Beijing, China | 0,0.47,0.94,1.88,3.75,7.5,15 | 0,15,30,60,120,240 | ||
| SU | Mesosulfuron-methyl | 30 g L−1 OF | Bayer, Hangzhou, China | 0,0.42,0.84,1.69,3.38,6.75,13.5 | 0,13.5,27,54,108,216 | |
| PTB | Bispyribac-sodium | 20% WP | Ruibang Agrochemical, Jiangsu, China | 0,0.94,1.88,3.75,7.5,15,30 | 0,30,60,120,240,480 | |
| IMI | Imazethapyr | 5% AS | Changqing Agrochemical, Jiangsu, China | 0,2.81,5.63,11.25,22.5,45,90 | 0,22.5,45,90,180,360 | |
| Imazamox | 4% AS | Flag Chemical, Jiangsu, China | 0,1.41,2.81,5.63,11.25,22.5,45 | 0,11.25,45,90,180 | ||
| SCT | Flucarbazone-sodium | 70% WG | Arysta Life Science, Shanghai, China | 0,1,2,4,8,16,32 | 0,32,64,128,216,512 | |
| ACCase | APP | Fenoxaprop-p-ethyl | 69 g L−1 EW | Bayer, Hangzhou, China | 0,60,120,240,480,960 | 0,240,480,960,1920, 3840 |
| Clodinafop-propargyl | 15% WP | Syngenta, Shanghai, China | 0,2.25,4.5,9,18,36 | 0,18,36,72,144,288 | ||
| CHD | Sethoxydim | 12.5% EC | Changqing Agrochemical, Jiangsu, China | 0,3.13,6.25,12.5,25,50,100 | 0,200,400,800,1600, 3200 | |
| Tralkoxydim | 40% WG | Jiangsu Agrochem Laboratory Co., Ltd., China | 0,0.39,1.56,6.25,25,100,400 | 0,50,100,200,400, 800,1600 | ||
| PPZ | Pinodexn | 5% EC | Syngenta, Shanghai, China | 0,1.41,2.81,5.63,11.25,22.5,45 | 0,11.25,22.5,45,90,180 | |
| PSII | Urea | Isoproturon | 50% WP | Jiangsu Futian Agrochemical Co., Ltd., China | 0,32.81,65.63,131.25,262.5,525,1050 | 0,65.63,131.25,262.5, 525,1050,2100 |
| Herbicides | Populations | Regression Parameters a | GR50(SE) b | RI c | |||
|---|---|---|---|---|---|---|---|
| c | d | b | R2 | ||||
| Pyroxsulam | HS | 0.26(2.03) | 98.47(9.24) | −1.18(0.15) | 0.9995 | 0.24(0.06) | 1.00 |
| MHR | 16.26(16.05) | 91.06(7.89) | −1.76(0.95) | 0.9896 | 29.22(1.51) | 121.75 | |
| Pyroxsulam+ PBO | HS | 0.88(2.58) | 98.15(11.72) | −1.38(0.24) | 0.9987 | 0.20(0.04) d | 0.83 |
| MHR | 14.33(7.75) | 88.86(19.76) | −1.57(0.87) | 0.9817 | 11.65(1.30) * | 58.25 | |
| Pyroxsulam+ Malathion | HS | −0.73(3.65) | 99.81(11.69) | −1.04(0.18) | 0.9992 | 0.21(0.03) d | 0.88 |
| MHR | 14.33(5.37) | 90.62(5.73) | −1.69(0.43) | 0.9953 | 20.48(0.92) * | 97.52 | |
| Penoxsulam | HS | 15.79(12.13) | 90.25(1.14) | −1.33(0.41) | 0.9957 | 4.66(1.14) | 1.00 |
| MHR | −8.97(4.43) | 94.34(6.60) | −1.12(0.53) | 0.9983 | 161.29(27.73) | 34.61 | |
| Mesosulfuron-methyl | HS | 6.53(4.67) | 94.44(11.84) | −1.70(0.50) | 0.9941 | 1.34(0.26) | 1.00 |
| MHR | 10.11(18.90) | 91.59(17.26) | −1.38(0.87) | 0.9938 | 56.57(16.41) | 42.22 | |
| Bispyribac-sodium | HS | −13.89(4.92) | 97.54(3.31) | −0.99(0.29) | 0.9985 | 29.33(10.42) | 1.00 |
| MHR | −1.83(7.64) | 94.02(3.88) | −1.10(0.30) | 0.9995 | 351.96(56.95) | 12.00 | |
| Imazethapyr | HS | 12.02(1.90) | 87.32(3.75) | −3.45(0.60) | 0.9969 | 8.66(0.57) | 1.00 |
| MHR | 18.66(4.97) | 81.49(3.70) | −2.38(0.63) | 0.9971 | 105.34(11.12) | 12.16 | |
| Imazamox | HS | 8.85(1.01) | 88.61(3.51) | −2.22(0.22) | 0.9994 | 3.86(0.24) | 1.00 |
| MHR | 6.66(6.70) | 95.56(5.39) | −2.04(0.49) | 0.9980 | 50.24(5.10) | 13.02 | |
| Flucarbazone-sodium | HS | 2.83(5.74) | 97.79(39.16) | −1.15(0.51) | 0.9946 | 1.77(0.25) | 1.00 |
| MHR | 4.27(29.31) | 82.74(3.75) | −1.37(0.62) | 0.9980 | 407.46(31.24) | 230.20 | |
| Fenoxaprop-P-ethyl | HS | 3.42(2.90) | 87.84(12.74) | −1.68(0.42) | 0.9964 | 66.01(14.71) | 1.00 |
| MHR | −26.44(5.27) | 102.50(1.17) | −1.14(0.06) | 0.9996 | 2030.86 (139.61) | 30.77 | |
| Clodinafop-propargyl | HS | 5.12(2.29) | 83.62(9.71) | −1.96(0.44) | 0.9965 | 5.27(0.83) | 1.00 |
| MHR | −16.23(5.52) | 93.61(7.83) | −0.95(0.43) | 0.9991 | 232.49(21.57) | 44.12 | |
| Sethoxydim | HS | 4.04(8.68) | 83.95(20.41) | −1.72(1.01) | 0.9867 | 8.00(4.81) | 1.00 |
| MHR | 6.04(7.00) | 88.87(4.07) | −3.06(0.81) | 0.9960 | 1220.73 (122.43) | 152.67 | |
| Tralkoxydim | HS | 1.07(6.02) | 100.48(10.87) | −0.91(0.27) | 0.9932 | 5.71(1.89) | 1.00 |
| MHR | 8.72(1.71) | 90.07(0.83) | −1.46(0.07) | 0.9999 | 578.35(13.54) | 101.34 | |
| Pinoxaden | HS | 6.70(3.04) | 83.29(5.53) | −4.47(1.42) | 0.9921 | 3.55(0.44) | 1.00 |
| MHR | 8.41(1.16) | 78.21(1.03) | −2.16(0.12) | 0.9999 | 33.28(1.01) | 9.39 | |
| Isoproturon | HS | 0.04(1.13) | 111.32(14.28) | −1.16(0.12) | 0.9997 | 42.15(9.05) | 1.00 |
| MHR | 7.17(1.13) | 97.38(3.19) | −2.21(0.17) | 0.9998 | 307.21(12.31) | 7.29 | |
| Isoproturon+ PBO | HS | −1.04(11.61) | 104.53(25.73) | −1.48(0.79) | 0.9845 | 38.32(7.48) d | 0.91 |
| Isoproturon+ NBD-Cl | HS | −4.53(2.34) | 99.68(8.78) | −1.12(0.15) | 0.9995 | 41.09(4.26) d | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Wang, H.; Gao, H.; Liu, Y.; Li, J.; Feng, Z.; Dong, L. Multiple Resistance to Three Modes of Action of Herbicides in a Single Italian Ryegrass (Lolium multiflorum L.) Population in China. Agronomy 2023, 13, 216. https://doi.org/10.3390/agronomy13010216
Zhu G, Wang H, Gao H, Liu Y, Li J, Feng Z, Dong L. Multiple Resistance to Three Modes of Action of Herbicides in a Single Italian Ryegrass (Lolium multiflorum L.) Population in China. Agronomy. 2023; 13(1):216. https://doi.org/10.3390/agronomy13010216
Chicago/Turabian StyleZhu, Guangtao, Hao Wang, Haitao Gao, Ying Liu, Jun Li, Zhike Feng, and Liyao Dong. 2023. "Multiple Resistance to Three Modes of Action of Herbicides in a Single Italian Ryegrass (Lolium multiflorum L.) Population in China" Agronomy 13, no. 1: 216. https://doi.org/10.3390/agronomy13010216
APA StyleZhu, G., Wang, H., Gao, H., Liu, Y., Li, J., Feng, Z., & Dong, L. (2023). Multiple Resistance to Three Modes of Action of Herbicides in a Single Italian Ryegrass (Lolium multiflorum L.) Population in China. Agronomy, 13(1), 216. https://doi.org/10.3390/agronomy13010216







