Assessment of Drought Stress Tolerance of Mangifera indica L. Autotetraploids
Abstract
:1. Introduction
2. Materials and Methods
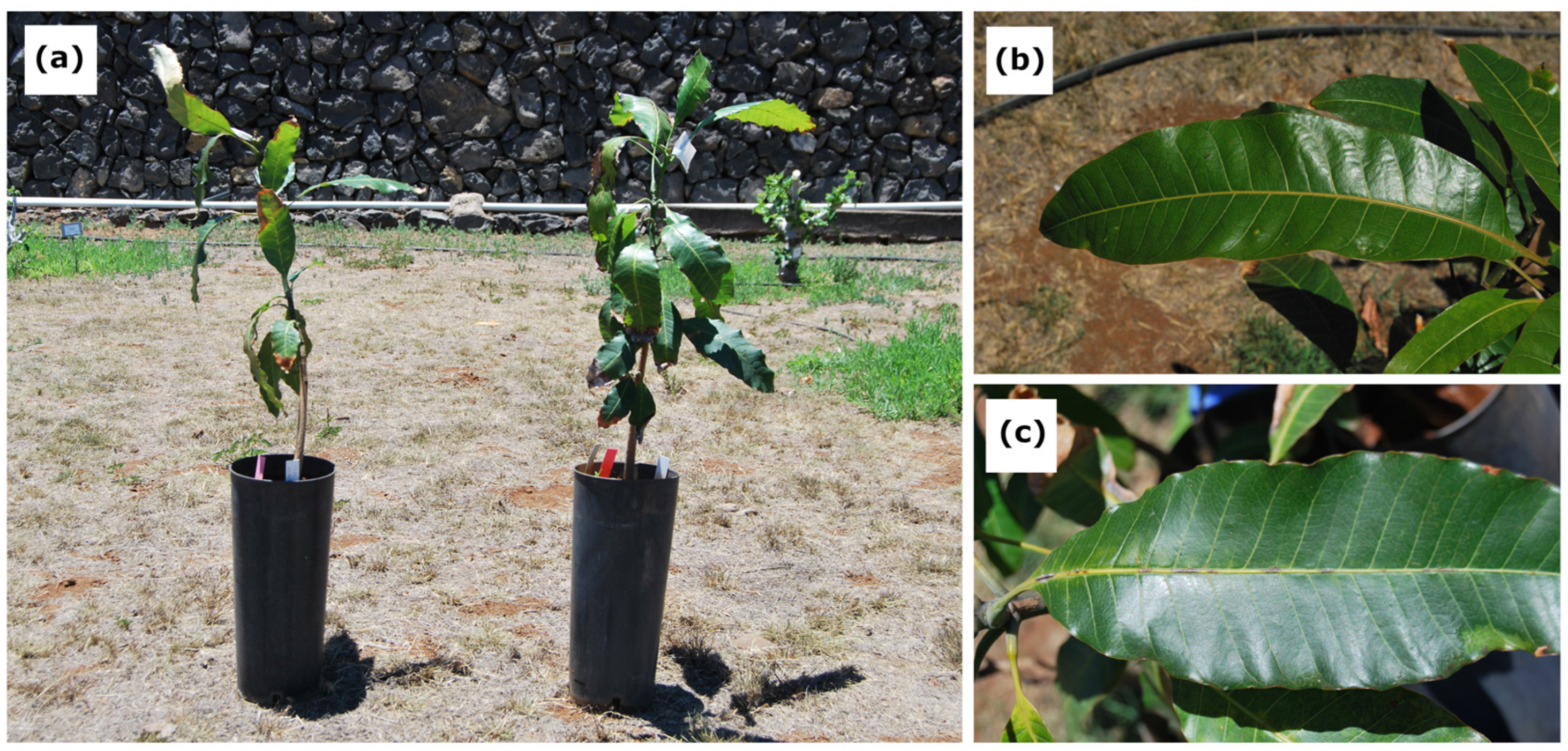
2.1. Plant Material and Study Site
2.2. Drought Treatment
2.3. Water Status and Leaf Morphology
2.4. Chlorophyll Fluorescence and Gas Exchange
2.5. Free Proline Determination
2.6. Statistical Analysis
3. Results
3.1. Ploidy Effect on Leaf Morphology and Physiology
3.2. Drought Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOstat. Food and Agriculture Organization Corporate Statistical Database. Available online: https://www.fao.org/faostat/en/#data (accessed on 24 October 2022).
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Magnifera indica L.), “The king of fruits”—An overview. Food Rev. Int. 2007, 22, 95–123. [Google Scholar] [CrossRef]
- Warschefsky, E.; von Wettberg, E.J.B. Population genomic analysis of mango (Mangifera indica) suggests a complex history of domestication. New Phytol. 2019, 222, 2023–2037. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, J.A. Mangifera indica L. Mango. Anacardiaceae. Cashew Family; USDA Forest Service, International Institute of Tropical Forestry: Washington, DC, USA, 1993; Volume 63, p. 6. [Google Scholar]
- Grajal-Martín, M.J.; Rosell, P.; Galán-Saúco, V.; Fernández, D. Filipino, un nuevo tetraploide de mango. In XI Congreso de la Sociedad Española de Ciencias Hortícolas (SECH); Bioberica: Albacete, Spain, 2007. [Google Scholar]
- Galán-Saúco, V. El Cultivo del Mango; Ediciones Mundi-Prensa: Madrid, España, 2009. [Google Scholar]
- Yadav, D.; Singh, S.P. Mango: History origin and distribution. J. Pharmacog. Phytochem. 2017, 6, 1257–1262. [Google Scholar]
- Durán Zuazo, V.H.; García-Tejero, I.F.; Rodríguez, B.C.; Tarifa, D.F.; Ruiz, B.G.; Sacristán, P.C. Deficit irrigation strategies for subtropical mango farming. A review. Agron. Sustain. 2014, 1, 13. [Google Scholar]
- Pavel, E.W.; Vanassche, F.M.G.; Grossman, Y.L. Optimization of Irrigation Management in Mango Trees by Determination of Water and Carbon Demands to Improve Water Use Efficiency and Fruit Quality; Final Report to the Water Research Commission, WRC Report No.: 1136/1/03; Water Research Commission: Pretoria, South Africa, 2003; p. 106. [Google Scholar]
- Habiba, U.; Shaw, R.; Takeuchi, Y. Farmers’ adaptive practices for drought risk reduction in the northwest region of Bangladesh. Nat. Hazards 2014, 72, 337–359. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Bertaki, M. Sustainable water management in agriculture under climate change. Agric. Agric. Sci. Procedia 2015, 4, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Elsheery, N.I.; Cao, K.F. Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant. 2008, 30, 769–777. [Google Scholar] [CrossRef]
- Luvaha, E.; Netondo, G.W.; Ouma, G. Effect of water deficit on the physiological and morphological characteristics of mango (Mangifera indica) rootstock seedlings. Am. J. Plant Physiol. 2010, 5, 7–21. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio./Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Van Laere, K.; França, S.C.; Vansteenkiste, H.; Van Huylenbroeck, J.; Steppe, K.; Van Labeke, M.C. Influence of ploidy level on morphology, growth and drought susceptibility in Spathiphyllum wallisii. Acta Physiol. Plant. 2011, 33, 1149–1156. [Google Scholar] [CrossRef]
- González-Rodríguez, A.M.; Peters, J.; del Olmo, A.; Grajal-Martín, M.J. Selección de patrones de mango frente al estrés hídrico. Actas Hortic. 2008, 51, 155–156. [Google Scholar]
- Grajal-Martín, M.J.; Peters, J.; del Olmo Aínsua, A.I.; González-Rodríguez, A.M. Respuesta Fisiológica de Varias Especies del Género Mangifera al Estrés Hídrico; VI Congreso Ibérico de Ciencias Hortícolas (SECH 6°): Logroño, Spain, 2009. [Google Scholar]
- Trojak-Goluch, A.; Kawka-Lipińska, M.; Wielgusz, K.; Praczyk, M. Polyploidy in Industrial Crops: Applications and Perspectives in Plant Breeding. Agronomy 2021, 11, 2574. [Google Scholar] [CrossRef]
- Barker, M.S.; Arrigo, N.; Baniaga, A.E.; Li, Z.; Levin, D.A. On the relative abundance of autopolyploids and allopolyploids. New Phytol. 2015, 210, 391–398. [Google Scholar] [CrossRef]
- Soltis, D.E.; Visger, C.J.; Marchant, D.B.; Soltis, P.S. Polyploidy: Pitfalls and paths to a paradigm. Am. J. Bot. 2016, 103, 1146–1166. [Google Scholar] [CrossRef] [Green Version]
- Bomblies, K. When everything changes at once: Finding a new normal after genome duplication. Proc. R Soc. London B 2020, 287, 20202154. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Hegarty, M.; Hiscock, S. Polyploidy: Doubling up for evolutionary success. Current. Biol. 2007, 17, 927–929. [Google Scholar] [CrossRef] [Green Version]
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubešová, M.; Pyšek, P. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012, 109, 19–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, R.F.; Queiroga, D.; Vilela, E.; Moraes, A.P. Polyploidy and high environmental tolerance increase the invasive success of plants. J. Plant Res. 2021, 134, 105–114. [Google Scholar] [CrossRef]
- Nassar, N.M. Chromosome doubling induces apomixis in a cassava × Manihot anomala hybrid. Hereditas 2006, 143, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H.; Walden, A.E.; Husband, B.C. Genome duplication and the evolution of physiological responses to water stress. New Phytol. 2009, 184, 721–731. [Google Scholar] [CrossRef]
- Manzaneda, A.J.; Rey, P.J.; Bastida, J.M.; Weiss-Lehman, C.; Raskin, E.; Mitchell-Olds, T. Environmental aridity is associated with cytotype segregation and polyploidy occurrence in Brachypodium distachyon (Poaceae). New Phytol. 2012, 193, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Du, W.; Liu, C.; Sun, W.; Tian, S.; Dong, H. Antioxidant response to drought, cold and nutrient stress in two ploidy levels of tobacco plants: Low resource requirement confers polytolerance in polyploids? Plant Growth Regul. 2012, 66, 37–47. [Google Scholar] [CrossRef]
- Allario, T.; Brumos, J.; Colmenero-Flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L.; Talon, M.; Ollitrault, P.; Morillon, R. Tetraploid Rangpur lime rootstock increases drought tolerance via enhanced constitutive root abscisic acid production. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penner, S.; Dror, B.; Aviezer, I.; Bar-Lev, Y.; Salman-Minkov, A.; Mandakova, T.; Šmarda, P.; Mayrose, I.; Sapir, Y. Phenology and polyploidy in annual Brachypodium species (Poaceae) along the aridity gradient in Israel. J. Syst. Evol. 2020, 58, 189–199. [Google Scholar] [CrossRef]
- Buggs, R.J.; Pannell, J.R. Ecological differentiation and diploid superiority across a moving ploidy contact zone. Evolution 2007, 61, 125–140. [Google Scholar] [CrossRef]
- Chen, S.; Tang, P. Studies on colchicine-induced autotetraploid barley. III. Physiological studies. Am. J. Bot. 1945, 32, 177–179. [Google Scholar] [CrossRef]
- Yang, P.M.; Huang, Q.C.; Qin, G.Y.; Zhao, S.P.; Zhou, J.G. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 2014, 52, 193–202. [Google Scholar] [CrossRef]
- Chen, P.; Chen, J.; Sun, M.; Yan, H.; Feng, G.; Wu, B.; Zhang, X.; Wang, X.; Huang, L. Comparative transcriptome study of switchgrass (Panicum virgatum L.) homologous autopolyploid and its parental amphidiploid responding to consistent drought stress. Biotechnol. Biofuels 2020, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Berlyn, G.P.; Ashton, P.M.S. Polyploids and their structural and physiological characteristics relative to water deficit in Betula papyrifera (Betulaceae). Am. J. Bot. 1996, 83, 15–20. [Google Scholar] [CrossRef]
- Pustovoitova, T.N.; Eremin, G.V.; Rassvetaeva, E.G.; Zhdanova, N.E.; Zholkevich, V.N. Drought resistance, recovery capacity, and phytohormone content in polyploid plum meaves. Russ J. Plant Physiol. 1996, 43, 232–235. [Google Scholar]
- Zhang, F.; Xue, H.; Lu, X.; Zhang, B.; Wang, F.; Ma, Y.; Zhang, Z. Autotetraploidization enhances drought stress tolerance in two apple cultivars. Trees 2015, 29, 1773–1780. [Google Scholar] [CrossRef]
- Barceló-Anguiano, M.; Holbrook, N.M.; Hormaza, J.I.; Losada, J.M. Changes in ploidy affect vascular allometry and hydraulic function in Mangifera indica trees. Plant J. 2021, 108, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Sarr, M.S.; Seiler, J.R.; Sullivan, J.; Diallo, A.M.; Strahm, B.D. Drought resistance and gum yield performances in a Senegalia senegal (L.) Britton progeny trial in Senegal. New For. 2021, 52, 943–957. [Google Scholar] [CrossRef]
- Wójcik, D.; Marat, M.; Marasek-Ciołakowska, A.; Klamkowski, K.; Buler, Z.; Podwyszyńska, M.; Tomczyk, P.P.; Wójcik, K.; Treder, W.; Filipczak, J. Apple autotetraploids—Phenotypic characterisation and response to drought stress. Agronomy 2022, 12, 161. [Google Scholar] [CrossRef]
- Nassar, N.M.; Graciano-Ribeiro, D.; Fernandes, S.D.C.; Araujo, P.C. Anatomical alterations due to polyploidy in cassava, Manihot esculenta Crantz. Genet. Mol. Res. 2008, 7, 276–283. [Google Scholar] [CrossRef]
- Ntuli, N.R.; Zobolo, A.M. Effect of water stress on growth of colchicine induced polyploid Coccinia palmata and Lagenaria sphaerica plants. Afr. J. Biotechnol. 2008, 7, 3548–3652. [Google Scholar]
- Wulansari, A.; Purwito, A.; Sukma, D.; Ermayanti, T.M. Growth response of diploid and tetraploid taro (Colocasia esculenta (L.) Schott) shoot culture to drought stress using polyethylene glycol. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012016. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Chen, Y.; Guan, Z.; Yin, D.; Chen, F. In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci. Hortic. 2011, 127, 411–419. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hortic. Res. 2020, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, P.; Bisht, M.S.; Miyazawa, S.I.; Yano, S.; Noguchi, K.; Terashima, I.; Nayama-Noguchi, S. Effects of polyploidy on photosynthetic properties and anatomy in leaves of Phlox drummondii. Funct. Plant Biol. 2007, 34, 673–682. [Google Scholar] [CrossRef]
- Li, W.D.; Biswas, D.K.; Xu, H.; Xu, C.Q.; Wang, X.Z.; Liu, J.K.; Jiang, G.M. Photosynthetic responses to chromosome doubling in relation to leaf anatomy in Lonicera japonica subjected to water stress. Funct. Plant Biol. 2009, 36, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.C.; Ramirez-Parra, E. Deciphering the molecular bases for drought tolerance in Arabidopsis autotetraploids. Plant Cell Environ. 2014, 37, 2722–2737. [Google Scholar] [CrossRef]
- Xu, J.; Jin, J.; Zhao, H.; Li, K. Drought stress tolerance analysis of Populus ussuriensis clones with different ploidies. J. For. Res. 2019, 30, 1267–1275. [Google Scholar] [CrossRef]
- Hao, G.Y.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef]
- Saúco, V.G.; Martín, M.J.G.; Galván, D.F.; Torres, A.C.; Juárez, J.; Navarro, L. Occurrence of spontaneous tetraploid nucellar mango plants. HortScience 2001, 36, 755–757. [Google Scholar] [CrossRef]
- Mukherjee, S.K. Mango: Its allopolyploid nature. Nature 1950, 4213, 196–197. [Google Scholar] [CrossRef]
- García-García, A.L.; Grajal-Martín, M.J.; González-Rodríguez, Á.M. Polyploidization enhances photoprotection in the first stages of Mangifera indica. Sci. Hortic. 2020, 264, 109198. [Google Scholar] [CrossRef]
- AgroCabildo. Available online: https://www.agrocabildo.org/agrometeorologia_estaciones_detalle.asp?id=44 (accessed on 30 November 2022).
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 1 August 2022).
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Software 2011, 40, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar DR Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 2019, 3, 1–143. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 2019, 1, 2–3. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 1 August 2022).
- Xiong, D.; Nadal, M. Linking water relations and hydraulics with photosynthesis. Plant J. 2020, 101, 800–815. [Google Scholar] [CrossRef]
- Levitt, J. Responses of Plants to Environmental Stresses; Academic Press: New York, NY, USA; London, UK, 1972; p. 697. [Google Scholar]
- Hinckley, T.M.; Duhme, F.; Hinckley, A.R.; Richter, H. Drought relations of shrub species: Assessment of the mechanisms of drought resistance. Oecologia 1983, 59, 344–350. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agri. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Waseem, M.; Ali, A.; Tahir, M.; Nadeem, M.A.; Ayub, M.; Tanveer, A.; Ahmad, R.; Hussain, M. Mechanism of drought tolerance in plant and its management through different methods. Cont. J. Agri. Sci. 2011, 5, 10–25. [Google Scholar]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Molec. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- NeSmith, D.S.; Duval, J.R. The effect of container size. HortTechnology 1998, 8, 495–498. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A. Passive origins of stomatal control in vascular plants. Science 2011, 331, 582–585. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Buckley, T.N.; Egea, G.; de Cires, A.; Hernandez-Santana, V.; Martorell, S.; Diaz-Espejo, A. Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant Cell Environ. 2016, 39, 2014–2026. [Google Scholar] [CrossRef]
- Trueba, S.; Pan, R.; Scoffoni, C.; John, G.P.; Davis, S.D.; Sack, L. Thresholds for leaf damage due to dehydration: Declines of hydraulic function, stomatal conductance and cellular integrity precede those for photochemistry. New Phytol. 2019, 223, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef] [Green Version]
- Alhasnawi, A.N. Role of proline in plant stress tolerance: A mini review. Res. Crops 2019, 20, 223–229. [Google Scholar]
- Clifford, S.C.; Arndt, S.K.; Corlett, J.E.; Joshi, S.; Sankhla, N.; Popp, M.; Jones, H.G. The role of solute accumulation, osmotic adjustment and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk.). J. Exp. Bot. 1998, 49, 967–977. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Smith, R.H.; Newton, R.J. Physiological changes in cultured sorghum cells in response to induced water stress: I. Free proline. Plant Physiol. 1985, 79, 266–269. [Google Scholar] [CrossRef]
- Ibarra-Caballero, J.; Villanueva-Verduzco, C.; Molina-Galán, J.; Sánchez-de-Jiménez, E. Proline accumulation as a symptom of drought stress in maize: A tissue differentiation requirement. J. Exp. Bot. 1988, 39, 889–897. [Google Scholar] [CrossRef]
- Perez-Alfocea, F.; Santa-Cruz, A.; Guerrier, G.; Bolarin, M.C. NaCl stress-induced organic solute changes on leaves and calli of Lycopersicon esculentum, L. pennellii and their interspecific hybrid. J. Plant Physiol. 1994, 143, 106–111. [Google Scholar] [CrossRef]
- Anyia, A.O.; Herzog, H. Water-use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought. Eur. J. Agron. 2004, 20, 327–339. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Leaf size and leaf area index in Fagus sylvatica forests: Competing effects of precipitation, temperature, and nitrogen availability. Ecosystems 2008, 11, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Huang, B. Role of root morphological and physiological characteristics in drought resistance of plants. In Plant-Environment Interactions; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 39–64. [Google Scholar]
- Jensen, C.; Henson, I.; Turner, N. Leaf gas exchange and water relations of lupins and wheat. II. Root and shoot water relations of lupin during drought-induced stomatal closure. Funct. Plant Biol. 1989, 16, 415. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Brodribb, T.J. Declining root water transport drives stomatal closure in olive under moderate water stress. New Phytol. 2020, 225, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Blackman, C.J.; Brodribb, T.J.; Jordan, G.J. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 2010, 188, 1113–1123. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. Cell volume and the evolution of eukaryotic genome size. In The evolution of Genome Size; Cavalier-Smith, T., Ed.; John Wiley and Sons: Chichester, UK, 1985; pp. 105–184. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera-Castro, A.V.; Hernández, B.; Grajal-Martín, M.J.; González-Rodríguez, Á.M. Assessment of Drought Stress Tolerance of Mangifera indica L. Autotetraploids. Agronomy 2023, 13, 277. https://doi.org/10.3390/agronomy13010277
Perera-Castro AV, Hernández B, Grajal-Martín MJ, González-Rodríguez ÁM. Assessment of Drought Stress Tolerance of Mangifera indica L. Autotetraploids. Agronomy. 2023; 13(1):277. https://doi.org/10.3390/agronomy13010277
Chicago/Turabian StylePerera-Castro, Alicia V., Beatriz Hernández, Maria José Grajal-Martín, and Águeda M. González-Rodríguez. 2023. "Assessment of Drought Stress Tolerance of Mangifera indica L. Autotetraploids" Agronomy 13, no. 1: 277. https://doi.org/10.3390/agronomy13010277
APA StylePerera-Castro, A. V., Hernández, B., Grajal-Martín, M. J., & González-Rodríguez, Á. M. (2023). Assessment of Drought Stress Tolerance of Mangifera indica L. Autotetraploids. Agronomy, 13(1), 277. https://doi.org/10.3390/agronomy13010277






