Variety and Sowing Date Affect Seed Yield and Chemical Composition of Linseed Grown under Organic Production System in a Semiarid Mediterranean Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Plant Material
2.2. Plant Sampling and Measurements
2.3. Oil and Protein Content and Fatty Acid Composition
2.4. Statistical Analysis
3. Results
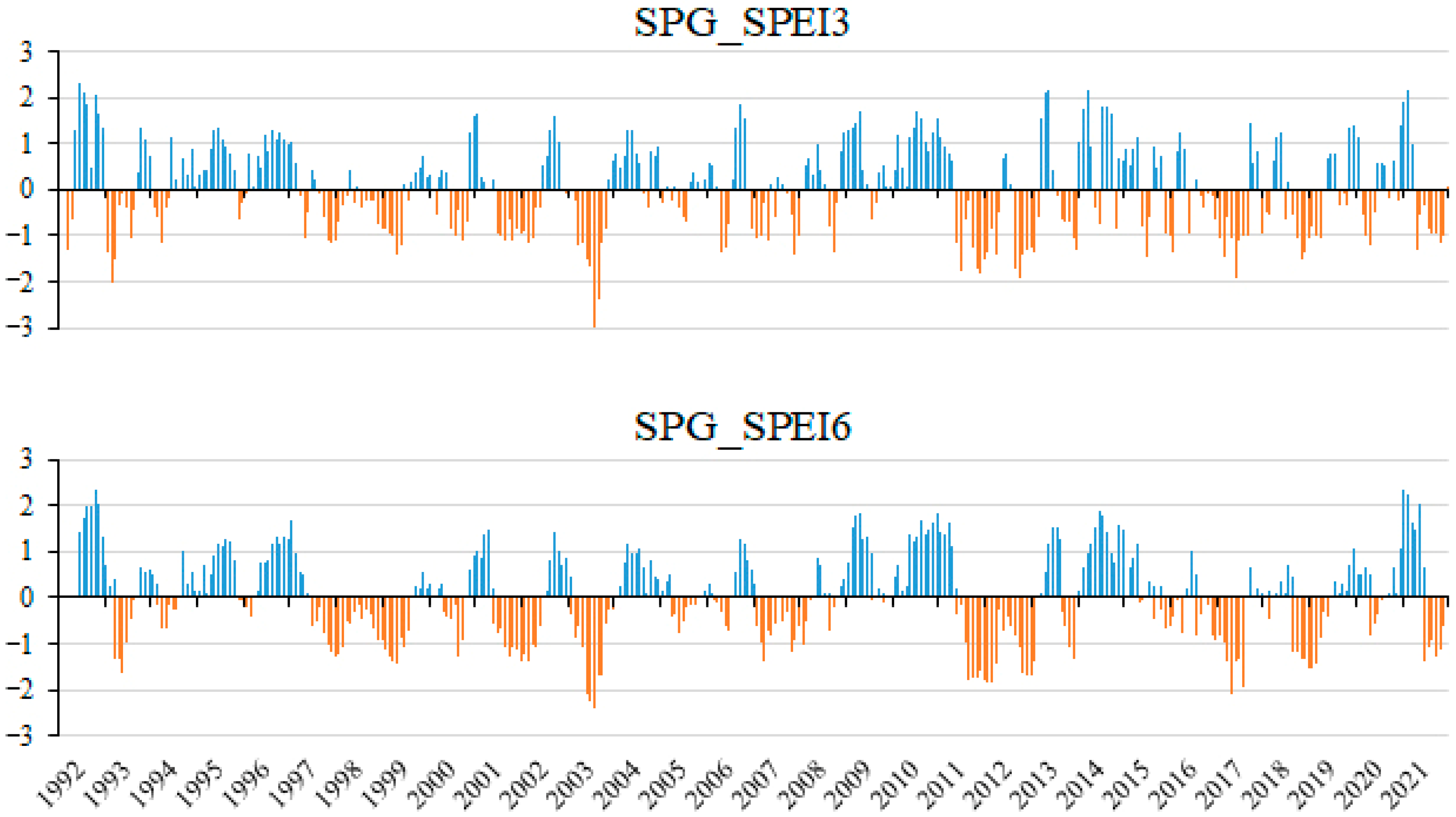
3.1. Weather Conditions and Crop Phenology
3.2. Yield and Yields Components
3.3. Additive Main Effects and Multiplicative Interaction Analysis: AMMI1 and AMMI2
3.4. Genotype Plus Genotype-vs.-Environment Interaction Analysis: GGE Biplot
3.5. Seed Oil Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health—A review. Global Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Lassaletta, L.; Aguilera, E.; del Prado, A.; Garnier, J.; Billen, G.; Iglesias, A.; Sánchez, B.; Guardia, G.; Abalos, D.; et al. Strategies for greenhouse gas emissions mitigation in Mediterranean agriculture: A review. Agric. Ecosyst. Environ. 2017, 238, 5–24. [Google Scholar] [CrossRef] [Green Version]
- Kehoe, L.; Romero-Muñoz, A.; Polaina, E.; Estes, L.; Kreft, H.; Kuemmerle, T. Biodiversity at risk under future cropland expansion and intensification. Nat. Ecol. Evol. 2017, 1, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, C.; Orsi, L.; Ferrazzi, G.; Corsi, S. The dimension of agricultural diversification: A spatial analysis of Italian municipalities. Rural Sociol. 2020, 85, 316–345. [Google Scholar] [CrossRef]
- Messéan, A.; Viguier, L.; Paresys, L.; Aubertot, J.-N.; Canali, S.; Iannetta, P.; Justes, E.; Karley, A.; Keillor, B.; Kemper, L.; et al. Enabling crop diversification to support transitions towards more sustainable European agrifood systems. Front. Agric. Sci. Eng. 2021, 8, 474–480. [Google Scholar] [CrossRef]
- Vanino, S.; Di Bene, C.; Piccini, C.; Fila, G.; Pennelli, B.; Zornoza, R.; Sanchez-Navarro, V.; Álvaro-Fuentes, J.; Hüppi, R.; Six, J.; et al. A comprehensive assessment of diversified cropping systems on agro-environmental sustainability in three Mediterranean long-term field experiments. Eur. J. Agron. 2022, 140, 126598. [Google Scholar] [CrossRef]
- Chehade, L.A.; Angelini, L.G.; Tavarini, S. Genotype and seasonal variation affect yield and oil quality of safflower (Carthamus tinctorius L.) under Mediterranean conditions. Agronomy 2022, 12, 122. [Google Scholar] [CrossRef]
- Hussain, M.I.; Lyra, D.-A.; Farooq, M.; Nikoloudakis, N.; Khalid, N. Salt and drought stresses in safflower: A review. Agron. Sustain. Dev. 2016, 36, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal agricultural land low-input systems for biomass production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef] [Green Version]
- Karg, S. New research on the cultural history of the useful plant Linum usitatissimum L. (Flax), a resource for food and textiles for 8000 Years. Veg. Hist. Archaeobot. 2012, 21, 79. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tavarini, S.; Antichi, D.; Foschi, L.; Mazzoncini, M. On-farm evaluation of seed yield and oil quality of linseed (Linum usitatissimum L.) in inland areas of Tuscany, central Italy. Ital. J. Agron. 2016, 11, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.R.; Severino, P.; Ferreira, C.S.; Zielinska, A.; Santini, A.; Souto, S.B.; Souto, E.B. Linseed essential oil—Source of lipids as active ingredients for pharmaceuticals and nutraceuticals. Curr. Med. Chem. 2019, 26, 4537–4558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzon, E.; Guigues, P.; Habas, J. Biobased structural epoxy foams derived from plant-oil: Formulation, manufacturing and characterization. Ind. Crops Prod. 2020, 144, 111994. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy agents. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yan, Z.; Niu, Z.; Coulter, J.A.; Niu, J.; Zhang, J.; Wang, B.; Yan, B.; Zhao, W.; Wang, L. Yield, Oil content, and fatty acid profile of flax (Linum usitatissimum L.) as affected by phosphorus rate and seeding rate. Ind. Crops Prod. 2020, 145, 112087. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [Green Version]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Darabighane, B.; Miccoli, F.E.; Gómez-Cortés, P.; Gonzalez-Ronquillo, M.; Mele, M. Effect of dietary vegetable sources rich in unsaturated fatty acids on milk production, composition, and cheese fatty acid profile in sheep: A meta-analysis. Front. Vet. Sci. 2021, 8, 641364. [Google Scholar] [CrossRef]
- Migliore, G.; Rizzo, G.; Bonanno, A.; Dudinskaya, E.C.; Tóth, J.; Schifani, G. Functional food characteristics in organic food products—the perspectives of Italian consumers on organic eggs enriched with Omega-3 polyunsaturated fatty acids. Org. Agric. 2022, 12, 149–161. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 September 2022).
- Anastasiu, A.E.; Chira, N.A.; Banu, I.; Ionescu, N.; Stan, R.; Rosca, S.I. Oil productivity of seven Romanian linseed varieties as affected by weather conditions. Ind. Crops Prod. 2016, 86, 219–230. [Google Scholar] [CrossRef]
- Green, A.G. Effect of temperature during seed maturation on the oil composition of low-linolenic genotypes of flax. Crop Sci. 1986, 26, 961–965. [Google Scholar] [CrossRef]
- Cross, R.H.; McKay, S.A.B.; McHughen, A.G.; Bonham-Smith, P.C. Heat-stress effects on reproduction and seed set in Linum usitatissimum L. (Flax). Plant Cell Environ. 2003, 26, 1013–1020. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D., Eds.; American Society of Agronomy, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Gardin, L.; Chiesi, M.; Fibbi, L.; Maselli, F. Mapping soil organic carbon in Tuscany through the statistical combination of ground observations with ancillary and remote sensing data. Geoderma 2021, 404, 115386. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- Peña-Gallardo, M.; Martín Vicente-Serrano, S.; Domínguez-Castro, F.; Beguería, S. The impact of drought on the productivity of two rainfed crops in Spain. Nat. Hazards Earth Syst. Sci. 2019, 19, 1215–1234. [Google Scholar] [CrossRef] [Green Version]
- Vergni, L.; Vinci, A.; Todisco, F. Effectiveness of the new standardized deficit distance index and other meteorological indices in the assessment of agricultural drought impacts in Central Italy. J. Hydrol. 2021, 603, 126986. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements; United Nations Food and Agriculture Organization, Irrigation and Drain: Rome, Italy, 1998. [Google Scholar]
- Smith, J.M.; Froment, M.A. A growth stage key for winter linseed (Linum usitatissimum). Ann. Appl. Biol. 1998, 133, 297–306. [Google Scholar] [CrossRef]
- International Rules for Seed Testing; ISTA: Wallisellen, Switzerland, 2004; ISBN 3-906549-38-0.
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Čeh, B.; Štraus, S.; Hladnik, A.; Kušar, A. Impact of linseed variety, location and production year on seed yield, oil content and its composition. Agronomy 2020, 10, 1770. [Google Scholar] [CrossRef]
- Kurtenbach, M.E.; Johnson, E.N.; Gulden, R.H.; Duguid, S.; Dyck, M.F.; Willenborg, C.J. Integrating cultural practices with herbicides augments weed management in flax. Agron. J. 2019, 111, 1904–1912. [Google Scholar] [CrossRef]
- Dey, P.; Mahapatra, B.S.; Pramanick, B.; Pyne, S.; Pandit, P. Optimization of seed rate and nutrient management levels can reduce lodging damage and improve yield, quality and energetics of subtropical flax. Biomass Bioenergy 2022, 157, 106355. [Google Scholar] [CrossRef]
- Gajardo, H.A.; Quian, R.; Soto-Cerda, B. Agronomic and quality assessment of linseed advanced breeding lines varying in seed mucilage content and their use for food and feed. Crop Sci. 2017, 57, 2979–2990. [Google Scholar] [CrossRef]
- Hassan, F.U.; Leitch, M.H. Influence of seeding density on contents and uptake of N, P and K in linseed (Linum usitatissimum L.). J. Agron. Crop Sci. 2000, 185, 193–199. [Google Scholar] [CrossRef]
- Benaragama, D.I.; Johnson, E.N.; Gulden, R.H.; Willenborg, C.J. Integrated agronomy for high yield and stable flax production in Canada. Agron. J. 2022, 114, 2230–2242. [Google Scholar] [CrossRef]
- Casa, R.; Russell, G.; Lo Cascio, B.; Rossini, F. Environmental effects on linseed (Linum usitatissimum L.) yield and growth of flax at different stand densities. Eur. J. Agron. 1999, 11, 267–278. [Google Scholar] [CrossRef]
- Kalinina, E.Y.; Lyakh, V.A. Combining ability according to the traits of stem branching and plant height in linseed lines. Cytol. Genet. 2011, 45, 293–297. [Google Scholar] [CrossRef]
- Korres, N.E.; Froud-Williams, R.J. Effects of winter wheat cultivars and seed rate on the biological characteristics of naturally occurring weed flora. Weed Res. 2002, 42, 417–428. [Google Scholar] [CrossRef]
- Sulas, L.; Re, G.A.; Sanna, F.; Bullitta, S.; Piluzza, G. Fatty acid composition and antioxidant capacity in linseed grown as forage in Mediterranean environment. Ital. J. Agron. 2019, 14, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Ercoli, L.; Arduini, I.; Mariotti, M.; Masoni, A. Post-anthesis dry matter and nitrogen dynamics in durum wheat as affected by nitrogen and temperature during grain filling. Cereal Res. Commun. 2010, 38, 294–303. [Google Scholar] [CrossRef]
- Hocking, P.J.; Pinkerton, A. Response of growth and yield components of linseed to the onset or relief of nitrogen stress at several stages of crop development. Field Crops Res. 1991, 27, 83–102. [Google Scholar] [CrossRef]
- Dordas, C.A. Variation of physiological determinants of yield in linseed in response to nitrogen fertilization. Ind. Crops Prod. 2010, 31, 455–465. [Google Scholar] [CrossRef]
- Luhs, W.; Friedt, W. Major oil crops. In Designer Oil Crops: Breeding, Processing and Biotechnology; Murphy, D.J., Ed.; VCH Press: Weinheim, Germany, 1994; pp. 5–71. [Google Scholar]
- Tavarini, S.; Angelini, L.G.; Casadei, N.; Spugnoli, P.; Lazzeri, L. Agronomical evaluation and chemical characterization of Linum usitatissimum L. as oilseed crop for bio-based products in two environments of Central and Nothern Italy. Ital. J. Agron. 2016, 11, 122–132. [Google Scholar] [CrossRef] [Green Version]
- D’Antuono, L.F.; Rossini, F. Yield potential and ecophysiological traits of the Altamurano linseed (Linum usitatissimum L.), a landrace of southern Italy. Genet. Resour. Crop Evol. 2006, 53, 65–75. [Google Scholar] [CrossRef]
- Diepenbrock, W.A.; Leon, J.; Clasen, K. Yielding ability and yield stability of linseed in Central Europe. Agron. J. 1995, 87, 84–88. [Google Scholar] [CrossRef]
- Dordas, C.A. Nitrogen nutrition index and its relationship to N use efficiency in linseed. Eur. J. Agron. 2011, 34, 124–132. [Google Scholar] [CrossRef]
- Klein, J.; Zikeli, S.; Claupen, W.; Gruber, S. Linseed (Linum usitatissimum) as an oil crop in organic farming: Abiotic impacts on seed ingredients and yield. Org. Agric. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Berti, M.; Fischer, S.; Wilckens, R.; Hevia, F.; Johnson, B. Adaptation and genotype × environment interaction of flaxseed (Linum usitatissimum L.) genotypes in South Central Chile. Chil. J. Agric. Res. 2010, 70, 345–356. [Google Scholar]
- Bhatty, R.S.; Cherdkiatgumchai, P. Compositional analysis of laboratory- prepared and commercial samples of linseed meal and of hull isolated from flax. J. Am. Oil Chem. Soc. 1990, 67, 79–84. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Pihlava, J.M.; Eurola, M.; Klemola, A.; Jauhiainen, L.; Hietaniemi, V. Yield, SDG lignan, cadmium, lead, oil and protein contents of linseed (Linum usitatissimum L.) cultivated in trials and at different farm conditions in the southwestern part of Finland. Agric. Food Sci. 2013, 22, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Fofana, B.; Cloutier, S.; Duguid, S.; Ching, J.; Rampitsch, C.G. Gene expression of stearoyl-ACP desaturase and Δ12 fatty acid desaturase 2 is modulated during seed development of flax (Linum usitatissimum). Lipids 2006, 41, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Ataii, E.; Mirlohi, A.; Sabzalian, M.R.; Goli, S.A.H.; Sadri, N.; Sharif-Moghaddam, N.; Gheysari, M. Genetic variability of seed yield and oil nutritional attributes in linseed dominated by biennial variation. Crop Pasture Sci. 2021, 72, 443–457. [Google Scholar] [CrossRef]





| SPG—Spring | SPG–Autumn | ALB—Spring | ALB–Autumn | |
|---|---|---|---|---|
| Sand (%) | 55.4 | 50.9 | 82.5 | 48.1 |
| Silt (%) | 32.2 | 38.0 | 2.4 | 35.3 |
| Clay (%) | 12.4 | 11.1 | 15.2 | 16.6 |
| pH | 8.0 | 8.2 | 8.0 | 7.9 |
| Total Nitrogen (‰) | 1.1 | 1.2 | 1.0 | 0.8 |
| Organic matter (%) | 1.9 | 2.9 | 1.7 | 1.1 |
| Available phosphorus (mg kg−1) | 8.9 | 5.8 | 9.3 | 28.4 |
| Electrical conductivity (meq 100−1g) | 6.5 | 10.0 | 39.3 | 13.7 |
| SPG | ALB | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (Days) | GDD (°C d) | Rainfall (mm) | ET0 (mm) | Duration (Days) | GDD (°C d) | Rainfall (mm) | ET0 (mm) | |||||||||
| V | R | V | R | V | R | V | R | V | R | V | R | V | R | V | R | |
| Spring sowing | ||||||||||||||||
| Galaad | 49 | 49 | 549 | 782 | 98 | 96 | 157 | 203 | 83 | 52 | 471 | 774 | 97 | 31 | 211 | 270 |
| Libra | 57 | 40 | 659 | 659 | 102 | 91 | 193 | 164 | 83 | 52 | 471 | 774 | 97 | 31 | 211 | 270 |
| Sideral | 56 | 42 | 643 | 688 | 103 | 91 | 188 | 172 | 87 | 48 | 515 | 730 | 97 | 31 | 229 | 252 |
| Szafir | 56 | 49 | 643 | 810 | 103 | 91 | 188 | 204 | 87 | 48 | 515 | 730 | 97 | 31 | 229 | 252 |
| Kaolin | 56 | 47 | 643 | 810 | 103 | 91 | 188 | 195 | 87 | 48 | 515 | 730 | 97 | 31 | 229 | 252 |
| Mean ± SD | 55 ± 3 | 45 ± 4 | 627 ± 44 | 750 ± 71 | 102 ± 2 | 92 ± 2 | 183 ± 15 | 188 ± 18 | 85 ± 2 | 50 ± 2 | 497 ± 24 | 748 ± 24 | 97 ± 0 | 31 ± 0 | 222 ± 10 | 259 ± 10 |
| Autumn sowing | ||||||||||||||||
| Galaad | 152 | 61 | 727 | 640 | 697 | 101 | 196 | 192 | 141 | 81 | 713 | 943 | 324 | 60 | 202 | 352 |
| Libra | 156 | 68 | 755 | 788 | 728 | 70 | 205 | 228 | 144 | 79 | 732 | 924 | 324 | 60 | 209 | 345 |
| Sideral | 166 | 54 | 821 | 668 | 729 | 69 | 235 | 184 | 168 | 55 | 894 | 762 | 354 | 30 | 285 | 269 |
| Szafir | 162 | 56 | 789 | 664 | 729 | 69 | 222 | 188 | 147 | 76 | 753 | 903 | 324 | 60 | 218 | 336 |
| Kaolin | 158 | 61 | 768 | 694 | 729 | 69 | 210 | 200 | 144 | 79 | 732 | 924 | 324 | 60 | 209 | 345 |
| Mean ± SD | 159 ± 5 | 60 ± 5 | 772 ± 35 | 691 ± 58 | 722 ± 14 | 76 ± 14 | 214 ± 15 | 198 ± 18 | 149 ± 11 | 74 ± 11 | 765 ± 74 | 891 ± 74 | 330 ± 13 | 54 ± 13 | 225 ± 34 | 329 ± 34 |
| Plant Height (cm) | Plant Density (n. m−2) | Stems (n. plant−1) | Capsule (n. stem−1) | Sterile Capsules (%) | Seeds (n. capsule−1) | |
|---|---|---|---|---|---|---|
| SPG | 59.2 ± 7.9 a | 341.3 ± 118.9 b | 1.3 ± 0.2 a | 18.4 ± 9.5 a | 9.7 ± 6.6 b | 4.0 ± 0.7 b |
| ALB | 58.7 ± 10.3 a | 432.0 ± 164.9 a | 1.1 ± 0.1 b | 9.3 ± 2.5 b | 14.5 ± 5.9 a | 4.4 ± 1.5 a |
| Spring | 52.6 ± 6.5 b | 369.6 ± 100.6 b | 1.3 ± 0.2 a | 10.0 ± 3.1 b | 12.4 ± 5.2 a | 3.6 ± 0.9 b |
| Autumn | 65.4 ± 6.4 a | 403.7 ± 186.6 a | 1.1 ± 0.1 b | 17.6 ± 10.0 a | 11.8 ± 8.0 a | 4.8 ± 1.1 a |
| Galaad | 54.3 ± 6.0 b | 324.8 ± 79.4 c | 1.2 ± 0.2 b | 12.8 ± 7.5 b | 10.2 ± 5.1 b | 4.4 ± 0.8 a |
| Libra | 62.5 ± 8.5 a | 276.0 ± 98.0 c | 1.1 ± 0.1 bc | 16.7 ± 11.4 a | 10.8 ± 7.3 b | 4.5 ± 1.5 a |
| Sideral | 54.8 ± 9.7 b | 542.5 ± 162.5 a | 1.3 ± 0.3 a | 10.1 ± 6.0 b | 12.6 ± 6.6 ab | 3.8 ± 1.2 b |
| Szafir | 63.6 ± 10.1 a | 326.0 ± 98.8 c | 1.2 ± 0.1 bc | 16.7 ± 8.6 a | 15.2 ± 7.3 a | 4.1 ± 1.2 ab |
| Kaolin | 59.8 ± 7.3 a | 463.9 ± 114.2 b | 1.1 ± 0.1 c | 12.9 ± 5.6 b | 11.7 ± 6.6 ab | 4.2 ± 1.1 ab |
| L | n.s. | *** | *** | *** | *** | * |
| SD | *** | * | *** | *** | n.s. | *** |
| G | *** | *** | *** | *** | *** | ** |
| L × SD | *** | *** | *** | *** | *** | *** |
| L × G | n.s. | n.s. | *** | *** | n.s. | *** |
| SD × G | ** | *** | ** | n.s. | * | n.s. |
| L × SD × G | * | *** | *** | n.s. | n.s. | n.s. |
| Seed Yield (Mg ha−1) | Crop Residues (Mg ha−1) | HI (%) | TSW (g) | Oil Content (g/100 g) | Crude Protein Content (g/100 g) | |
|---|---|---|---|---|---|---|
| SPG | 2.1 ± 0.6 a | 4.0 ± 1.4 a | 34.6 ± 5.0 a | 7.3 ± 1.0 a | 47.2 ± 1.0 a | 21.2 ± 1.0 b |
| ALB | 1.3 ± 0.7 b | 3.1 ± 2.2 b | 32.6 ± 6.7 b | 6.0 ± 0.8 b | 45.2 ± 1.4 b | 22.6 ± 1.5 a |
| Spring | 1.2 ± 0.6 b | 2.1 ± 1.2 b | 37.4 ± 4.2 a | 6.6 ± 0.7 b | 46.5 ± 1.7 a | 22.6 ± 1.4 a |
| Autumn | 2.1 ± 0.5 a | 5.0 ± 1.1 a | 29.8 ± 4.9 b | 7.5 ± 0.9 a | 46.6 ± 2.6 a | 21.3 ± 1.3 a |
| Galaad | 1.7 ± 0.8 a | 3.1 ± 1.6 b | 36.6 ± 7.2 a | 8.0 ± 0.7 a | 46.1 ± 1.2 b | 22.0 ± 1.2 abc |
| Libra | 1.3 ± 0.6 b | 3.2 ± 1.8 ab | 31.5 ± 4.6 b | 6.5 ± 0.6 d | 49.0 ± 0.9. a | 21.6 ± 1.1 bc |
| Sideral | 1.6 ± 0.8 ab | 4.0 ± 2.3 a | 30.4 ± 4.9 b | 6.0 ± 0.4 e | 43.6 ± 1.2 c | 23.3 ± 1.1 a |
| Szafir | 1.7 ± 0.7 a | 3.5 ± 1.7 ab | 34.5 ± 5.4 a | 7.5 ± 0.6 b | 46.3 ± 1.0 b | 22.1 ± 1.1 ab |
| Kaolin | 1.9 ± 0.7 a | 3.9 ± 2.0 a | 35.1 ± 5.6 a | 7.4 ± 0.6 c | 47.9 ± 1.2 a | 20.6 ± 1.2 c |
| L | *** | *** | *** | *** | *** | *** |
| SD | *** | *** | *** | *** | n.s. | n.s. |
| G | *** | ** | *** | *** | *** | *** |
| L × SD | *** | *** | *** | *** | *** | n.s. |
| L × G | ** | n.s. | *** | *** | n.s. | n.s. |
| SD × G | n.s. | n.s. | n.s. | * | n.s. | n.s. |
| L × DS × G | n.s. | n.s. | ** | * | n.s. | n.s. |
| Location | Sowing Date | Plant Height (cm) | Plant Density (n. m−2) | Stems (n. plant−1) | Capsule (n. stem−1) | Sterile Capsules (%) | Seeds (n. Capsule−1) |
|---|---|---|---|---|---|---|---|
| SPG | Spring | 55.5 ± 7.7 c | 395.6 ± 100.6 b | 1.4 ± 0.3 a | 10.8 ± 3.7 b | 14.6 ± 5.4 b | 4.0 ± 0.8 b |
| Autumn | 62.9 ± 6.3 b | 286.9 ± 112.5 d | 1.1 ± 0.1 c | 26.0 ± 7.1 a | 4.7 ± 3.1 d | 4.1 ± 0.5 b | |
| ALB | Spring | 49.6 ± 3.2 d | 343.5 ± 96.0 c | 1.2 ± 0.1 b | 9.3 ± 2.4 b | 10.3 ± 4.1 c | 3.1 ± 0.7 c |
| Autumn | 67.9 ± 5.5 a | 520.5 ± 173.5 a | 1.0 ± 0.0 c | 9.3 ± 2.6 b | 18.8 ± 4.2 a | 5.6 ± 0.9 a |
| Plant Height | Plant Density | Stems per Plant | Capsules per Plant | Sterile Capsules | Seeds per Capsule | Seed Yield | Crop Residues | HI | TSW | Oil Content | Protein Content | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 1 | 0.07 | −0.58 | 0.37 | 0.25 | 0.69 | 0.55 | 0.77 | −0.71 | 0.34 | 0.18 | −0.50 |
| ns | ** | ns | ns | *** | * | *** | *** | ns | ns | * | ||
| Plant density | 1 | 0.04 | −0.57 | 0.59 | 0.21 | 0.10 | 0.33 | −0.43 | 0.35 | −0.63 | 0.16 | |
| ns | ** | ** | ns | ns | ns | ns | ns | ** | Ns | |||
| Stems per plant | 1 | −0.33 | −0.03 | −0.28 | −0.17 | −0.34 | 0.39 | −0.41 | −0.20 | 0.18 | ||
| ns | ns | ns | ns | ns | ns | ns | ns | ns | ||||
| Capsules per plant | 1 | −0.64 | −0.06 | 0.53 | 0.37 | −0.01 | 0.53 | 0.49 | −0.46 | |||
| ** | ns | * | ns | ns | * | * | * | |||||
| Sterile capsules | 1 | 0.32 | −0.11 | 0.12 | −0.40 | −0.24 | −0.44 | 0.20 | ||||
| ns | ns | ns | ns | ns | ns | ns | ||||||
| Seeds per capsule | 1 | 0.47 | 0.61 | −0.52 | 0.29 | 0.05 | −0.49 | |||||
| * | ** | * | ns | ns | * | |||||||
| Seed yield | 1 | 0.86 | −0.28 | 0.62 | 0.13 | −0.76 | ||||||
| *** | ns | ** | ns | *** | ||||||||
| Crop residues | 1 | −0.72 | 0.38 | −0.05 | −0.58 | |||||||
| *** | ns | ns | ** | |||||||||
| HI | 1 | 0.11 | 0.25 | 0.08 | ||||||||
| ns | ns | ns | ||||||||||
| TSW | 1 | 0.30 | −0.58 | |||||||||
| ns | ** | |||||||||||
| Oil content | 1 | −0.67 | ||||||||||
| ** | ||||||||||||
| Protein content | 1 |
| Location | Sowing Date | Seed Yield (Mg ha−1) | Crop Residues (Mg ha−1) | HI (%) | TSW (g) | Oil Content (g 100 g−1) | Crude Protein Content (g 100 g−1) |
|---|---|---|---|---|---|---|---|
| SPG | Spring | 1.7 ± 0.4 b | 3.1 ± 0.7 b | 36.2 ± 4.5 b | 6.8 ± 0.8 c | 46.6 ± 1.7 b | 21.6 ± 0.9 a |
| Autumn | 2.4 ± 0.5 a | 5.0 ± 1.2 a | 32.9 ± 4.9 c | 7.9 ± 0.8 a | 47.8 ± 2.0 a | 20.8 ± 1.1 a | |
| ALB | Spring | 0.7 ± 0.3 c | 1.0 ± 0.3 c | 38.6 ± 3.5 a | 6.5 ± 0.6 d | 46.4 ± 1.9 b | 23.5 ± 1.1 a |
| Autumn | 1.9 ± 0.4 b | 5.1 ± 1.0 a | 26.7 ± 2.1 d | 7.2 ± 0.8 b | 45.4 ± 2.0 c | 21.8 ± 1.4 a |
| C16:0 | C16:1 | C17:0 | C18:1 | C18:2 | C18:3 | SFA | MUFA | PUFA | PUFA/SFA | n6/n3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | SPG | 6.35 b | 0.14 | 0.084 | 18.46 b | 13.85 b | 58.37 a | 6.50 b | 18.60 b | 72.31 a | 11.21 | 0.24 b |
| ALB | 6.80 a | 0.13 | 0.085 | 21.11 a | 16.80 a | 52.94 b | 6.95 a | 21.25 a | 69.74 b | 10.94 | 0.37 a | |
| Sowing date | Spring | 6.16 b | 0.13 | 0.067 b | 20.14 a | 13.88 b | 57.94 a | 6.33 b | 20.27 a | 71.82 a | 11.39 a | 0.24 b |
| Autumn | 6.95 a | 0.13 | 0.099 a | 19.45 b | 16.73 a | 53.59 b | 7.08 a | 19.61 b | 70.31 b | 10.79 b | 0.36 a | |
| Genotype | Galaad | 6.13 bc | 0.13 b | 0.076 b | 22.20 a | 17.75 a | 51.65 e | 6.24 c | 22.34 a | 69.40 c | 11.22 b | 0.40 a |
| Libra | 5.88 c | 0.09 c | 0.083 b | 17.05 d | 13.32 c | 61.17 a | 6.10 c | 17.15 d | 74.49 a | 12.31 a | 0.22 d | |
| Sideral | 6.25 bc | 0.11 bc | 0.079 b | 20.46 b | 13.19 c | 57.53 b | 6.46 b | 20.58 b | 70.72 b | 11.01 b | 0.23 c | |
| Szafir | 8.25 a | 0.21 a | 0.108 a | 18.76 c | 17.96 a | 52.42 d | 8.40 a | 18.99 c | 70.39 bc | 9.81 c | 0.40 a | |
| Kaolin | 6.38 b | 0.11 bc | 0.076 b | 20.41 b | 14.64 b | 55.50 c | 6.43 b | 20.54 b | 70.15 bc | 11.02 b | 0.26 b | |
| Variability factors | L | ** | n.s. | n.s. | ** | *** | *** | *** | *** | *** | n.s. | *** |
| SD | *** | n.s. | *** | * | *** | *** | *** | * | ** | *** | *** | |
| G | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| L × SD | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| L × G | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| SD × G | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| L × SD × G | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, A.; Clemente, C.; Tavarini, S.; Angelini, L.G. Variety and Sowing Date Affect Seed Yield and Chemical Composition of Linseed Grown under Organic Production System in a Semiarid Mediterranean Environment. Agronomy 2023, 13, 45. https://doi.org/10.3390/agronomy13010045
Rossi A, Clemente C, Tavarini S, Angelini LG. Variety and Sowing Date Affect Seed Yield and Chemical Composition of Linseed Grown under Organic Production System in a Semiarid Mediterranean Environment. Agronomy. 2023; 13(1):45. https://doi.org/10.3390/agronomy13010045
Chicago/Turabian StyleRossi, Alessandro, Clarissa Clemente, Silvia Tavarini, and Luciana G. Angelini. 2023. "Variety and Sowing Date Affect Seed Yield and Chemical Composition of Linseed Grown under Organic Production System in a Semiarid Mediterranean Environment" Agronomy 13, no. 1: 45. https://doi.org/10.3390/agronomy13010045





