Abstract
The incorporation of trees on traditional agricultural land has the potential for providing beneficial conditions for understory crops by altering the microclimate. Under these assumptions, we conducted a study on maize productivity intercropped in a 14-year-old walnut orchard by measuring growth and yield parameters, and water and nutrient uptake. Overall, we found that walnut trees decreased maximum air temperature and increased air humidity, especially during hot summer months characterized by precipitation deficit. A 30% reduction in maize yield per total area was a result of significantly reduced plant density, which could be a walnut-specific effect due to juglone excretion. Productivity per plant increased as shown by a significantly higher harvest index and 1000 kernel weight. No meaningful differences were found in terms of maize grain nutrient productivity, nutrient recovery, or nutrient use efficiency. On the systems level, we observed an advantage of the walnut-maize system compared to its respective monoculture systems—land and water equivalent ratios showed that for gaining the same yields as in intercropped system, walnut and maize grown separately would need 32% more land and 31% more water. Our study implies there are some beneficial outcomes to growing maize with trees, although further research should focus on investigating walnut as an option, due to its possible allelopathic effects.
1. Introduction
The trend of rising temperatures under climate change is well evidenced and many future climate projections indicate that the continuation of this trend is inevitable [1]. Generally, many regions will face warmer and drier conditions during summer, which insinuates a high risk for agricultural production. Maize (Zea mays L.), one of the most important crops, might be especially vulnerable [2], even though it favors relatively higher temperatures than other staple crops. Outside breeding efforts to produce more heat-stress resilient hybrids, maize adaptation will need to deal not only with increased temperature averages but also with the increasing frequency of extreme climate conditions [3]. Excellent potential for mitigating these conditions has been shown by introducing trees to traditional arable land and establishing agroforestry systems. Trees can modify the microclimate by influencing radiation flux, reducing temperature oscillations and wind strength, and increasing relative air humidity. These changes lead to a reduction of evapotranspiration and the improvement of water utilization for such systems, and ultimately, more stable conditions for crops grown underneath the trees [4]. Intercropping arable crops between trees provide many ecosystem services [5], and one of the most important potentials of these systems is the reduction of reliance on chemical inputs, via better utilization of soil nutrients [6]. Trees in agroforestry systems may promote advantages in nutrient acquisition of understory crops by reduction of losses via safety nets, uplift of deep soil nutrients, changes in chemical processes at the rhizosphere by root activity, and the addition of nitrogen from the atmosphere in the case of N fixing trees [7]. However, many factors affect these processes and the balance between complementarity and competition for resources between species is crucial for the overall productivity of agroforestry systems. Probably the greatest concern for intercropping maize with trees is the reduction of incoming radiation under the shade of trees. Maize, as a C4 plant, is sensitive to reduced radiation, and the reduction of biomass and yield under shading is anticipated [8]. However, some studies have concluded that light competition is not a significant factor for the reduction of maize yields in agroforestry when tree lines follow a N–S orientation [9,10,11], and some studies in Africa have found even higher maize yields in agroforestry compared to monoculture maize [12,13]. Still, the extent of shade and its effect on understory crop productivity depends also on the tree species, age and morphology. In the case of high trees with wide or converging canopies, there will be little advantage of the N–S orientation. Another factor is the region, i.e., climate. The positive effects of trees which have been found in tropical regions may have the completely opposite effect in temperate regions. Common walnut (Juglans regia L.), also called English or Persian walnut, is cultivated around the world. It produces kernels with great nutritional value, but it also produces high-quality timber [14]. The suitability of walnut for agroforestry systems lies in its morphological and phenological features: its irregular, half-open crown allows more light to reach the understory, and its late leafing delays shading on intercrops, which is the basis of temporal complementarity in systems with winter crops. At the same time, this trait could be a competing factor in systems with summer crops. Furthermore, the walnut tree has a long and not very fibrous taproot, which has the potential to partially eliminate below-ground competition with crops [10,15]. However, these features can vary across different environmental conditions, soil types, pruning management processes, etc. Another important aspect to consider for intercropping with walnut trees is allelopathy. Namely, walnut trees produce a variety of organic substances that can have inhibitory effects on plants grown nearby, but the most notable one is juglone (5-hydroxy-1,4-naphthoquinone). Juglone is found in all tree organs but is especially abundant in leaves, fruit hulls, and roots [16].
Considering all of the above, our study aimed to quantify the potential microclimatic benefits of intercropped walnut orchards and evaluate whether they can aid maize growth under the hot and dry summers of temperate Europe, despite anticipated competition with walnuts. The novelty of our research lies in a system-level investigation, as well as a detailed study of maize growth, and water and nutrient-related productivity. It was conducted by measuring and analyzing (i) microclimate parameters; (ii) soil moisture; (iii) maize phenology, growth, yield, and nutrient uptake, and (iv) the land and water productivity of the system.
2. Materials and Methods
2.1. Field Experiments and Systems Design
Field experiments were conducted in 2021 in Eastern Croatia near the city of Ðakovo (45°18′24.09′′ N, 18°26′20.5′′). The site elevation is 111 m above sea level, with soil type luvisol pseudogley on loess, and an effective soil depth of 1500 mm. This area has a continental climate of warm summers and cold winters, with a mean annual rainfall of between 600 and 1000 mm, relatively evenly distributed throughout the year.
The experiment consisted of three plots, each approximately 1.3 ha: (a) a control plot of an arable field with only maize (further referred to as monoculture maize system); (b) a sole walnut orchard; (c) a walnut orchard intercropped with maize.
The orchard was 14 years old at the time of the experiment, with 8 m alleys between and a distance of 7 m within the rows of walnut trees (Juglans regia L.). Tree density was uniform for both the sole and intercropped orchards. Tree row orientation was N–S, and trees were pruned every year in March. In 2021, trees were on average 5.5 m high, with an average canopy width of 5 m. All the walnut trees received organic fertilization in 2015 and foliar topdressing yearly. Within the intercropped orchard, maize was sown in strips of 6 m in width, resulting in a crop area of 75%. Maize (hybrid PP9911) was sown on 26 April 2021 with a 75 × 19 cm interspace. There were eight maize rows between walnut trees. Maize was harvested on 14 October 2021. All systems were managed following organic agriculture principles. Soil management and fertilization were uniform for both maize plots and no irrigation was applied. Before maize sowing, the green manuring principle was applied (plowing of buckwheat in the flowering stage). During the 3–5 leaves stage, liquid N fertilizer acceptable in organic farming was applied to both the intercropped maize and arable field maize.
2.2. Meteorological Data
Temperature and humidity data were obtained from Tinytag (Gemini Data Loggers, West Sussex, UK) devices placed between maize rows at crop height. The height position of these devices was adjusted during vegetation to follow the growth of maize plants. Precipitation data were measured using the Vantage Pro2 meteorological station (Davis Instruments Corporation, Hayward, CA, USA).
To describe the broad effect of temperature and humidity, the hydrothermal coefficient (K) was calculated:
K = 10 ∗ sum of rainfall [mm]/number of days ∗ average daily air temperature [C].
Interpretation of the hydrothermal coefficient according to Selyaninov [17] was based on the following:
- K > 1.5: excessive humidity for most plants;
- 1 < K < 1.5: humidity sufficient for most plants;
- 0.5 < K < 1.0: insufficient humidity for most plants;
- K < 0.5: drought.
2.3. Soil Water Content
Soil volumetric water content was derived from soil water potential data. The matric potential of soil water was recorded using Watermark sensors (Environmental Measuring Systems s.r.o., Brno, Czech Republic) on each plot. Due to technical difficulties with the sensors, data was recorded during 10-day periods around each observed stage (see Section 2.4). Sensors were previously calibrated as described in Žalac et al. [18]. The soil water measurements were recorded for 10, 30, 60, and 90 cm of soil depth.
2.4. Sampling, Measurements, and Analysis
Ten maize plants were sampled at random across the width of the alley, four times during maize development: at the five leaves stage (V5), during the blister stage (R2), during the dough stage (R4), and at harvest (H). Plant samples were measured for height and dried in the oven at 105 °C for the first two hours and then at 70 °C until constant weight to determine aboveground dry matter biomass accumulation. The leaf area of a plant was determined by summing up the measurements of each leaf’s maximum width ∗ length ∗ 0.75 [19,20]. This value was then multiplied by the number of plants per 1 m2 to obtain the leaf area index (LAI). A SPAD-502 meter (Soil Plant Analysis Development, Minolta, Japan) was used to obtain so-called SPAD values of greenness, which indicate the relative amount of chlorophyll present in plant leaves (Minolta, 1989). Four soil samples were collected from both the middle of the alley (10 subsamples) and closer to the trees (10 subsamples) at depths of 0–30 and 30–60 cm. Soil chemical analysis included the determination of pH (HRN ISO 10390:2005), organic matter content (OM) (HRN ISO 14235:1998), and concentrations of available nitrogen (mineral N; NO3−-HRN EN ISO 13395:1998 and NH4+-HRN EN ISO 11732:2008), phosphorus (P2O5), and potassium (K2O) following the Egner et al. method (1960). The content of nitrogen, phosphorus, and potassium was determined from plant tissue and grain at harvest.
2.5. Yields Determination
Maize yields were determined by harvesting plants from a 10 m2 area, separating, drying, and weighing the grain. Total yields were expressed in kg ha−1. To account for the unsown area in intercropped orchards (tree row strip), the determined maize yield (per cropped area) was multiplied by 0.75 to obtain yield per total area. 1000 kernel weight was also reported on a dry matter basis. The harvest index was calculated as the ratio of dry matter grain yield to dry matter total biomass. Walnut fruit yields were determined by collecting nuts from each walnut system and weighing them.
2.6. Land Equivalent Ratio (LER)
LER (land equivalent ratio), which is used to determine the productivity advantage of intercropped systems, was calculated as follows [21]:
where pLERW and pLERC are so-called partial LERs of walnut and maize, i.e., relative yields of species in the intercropped system. Yint,W and Yint,M are yields of walnut and maize, respectively, in the intercropped system, and Ymono,W and Ymono,M are walnut and maize yields, respectively, in the monoculture plot. When LER < 1, there is no agronomic advantage of intercropping over sole cropping, but when LER is >1, production in the intercropped system is higher than in the separate sole system, meaning that for production of the same yields as in monoculture systems, intercropping would require less land area.
LER = pLERW + pLERM = Yint,W/Ymono,W + Yint,M/Ymono,M
2.7. Water Use (WU), Water Productivity (WP), and Water Equivalent Ratio (WER)
For calculating growing season evapotranspiration, soil water content and precipitation data were inputs of the water balance equation [22], which represents actual water use (WU, mm):
where P is the amount of rainfall (mm) during the maize growing season, S1 is the water content (mm) within 0–90 cm soil depth at crop sowing, and S2 is the water content for the same depth at maize harvest. Water runoff and capillary rise have been ignored because the experimental fields were flat, and the water table was low (below 10 m). Due to the presence of a poorly permeable Btg subsoil horizon with higher clay content, downward drainage was negligible and is therefore excluded from the equation. Water use was not partitioned for each plant species, because maize and walnut roots overlap in intercropped systems; instead, it was representative of the system as a whole. Water use was determined by averaging WU measurements from the middle of an intercropped alley and within tree rows.
WU = P + S1 − S2
Water productivity (WP, kg ha−1 mm−1) was calculated as the ratio of the yield and the water use of the system [23,24]:
where Y is maize or walnut fruit yield (kg ha−1) and WU is the actual water use (mm).
WP = Y/WU
Water equivalent ratio (WER) was used to determine if water was used more efficiently in intercropping than in monoculture systems [25], and it was defined analogously to LER. WER was calculated as the ratio of intercropped walnut WP to the walnut monoculture WP plus the ratio of maize WP in the intercropped system to the maize monoculture WP:
WER = pWERW + pWERM = WPint,W/WPmono,W + WPint,M/WPmonoM
WER values quantify the amount of water needed in monoculture plots for walnut and maize to achieve the same yield as produced with one unit of water in the intercropped system. WER > 1 indicates a water use advantage for the intercropped system, meaning that yields in the intercropped system are produced with less water than needed for the same yields in monoculture plots. If both LER > 1 and WER > 1, then the intercropped system requires less land and less water than monoculture cultivation.
2.8. Nutrient Indices
For the estimation of nutrient efficiency in observed maize systems, different nutrient indices were calculated. First, nutrient accumulation (nA) was calculated by multiplying total aboveground dry matter by nutrient content to obtain the accumulated nutrient in kg ha−1. GnA is the amount of nutrient accumulated in the dry matter of grain.
Nutrient use efficiency (nUE) was used as an expression of maize grain productivity per amount of nutrient acquired [26]:
where Y is dry grain yield and nA is nutrient accumulation, with n denoting different nutrients—either nitrogen, phosphorus, or potassium.
nUE = Y/nA (kg ha−1 [kg total accumulated nutrient ha−1]−1),
Nutrient productivity (nP) was calculated as the ratio of grain yield and available soil nutrient content. It is derived from a concept of nutrient-response efficiency [24,26,27]:
where Y is dry grain yield and Sn is available soil nutrient content, with n being N, P, or K.
nP = Y/Sn (kg ha−1 [kg soil nutrient ha−1]−1),
Nutrient recovery index (GnRI) was used to determine how efficient maize was in relocating available soil nutrient into grain [24,28]:
where GnA is grain nutrient accumulation and Sn is available soil nutrient content, with n being N, P, or K.
nRI = GnA/Sn (kg grain accumulated nutrient ha−1 [kg soil nutrient ha−1]−1),
These indices were calculated for the three macronutrients analyzed; nitrogen (NUE, NP, NRI), phosphorus (PUE, PP, PRI), and potassium (KUE, KP, KRI).
2.9. Statistical Analysis
Statistical analysis of the obtained data was conducted in R software [29] using analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) post hoc test. Non-parametric alternative tests were applied in the case of non-normal data distribution and/or variance heterogeneity.
3. Results and Discussion
3.1. Microclimate Conditions
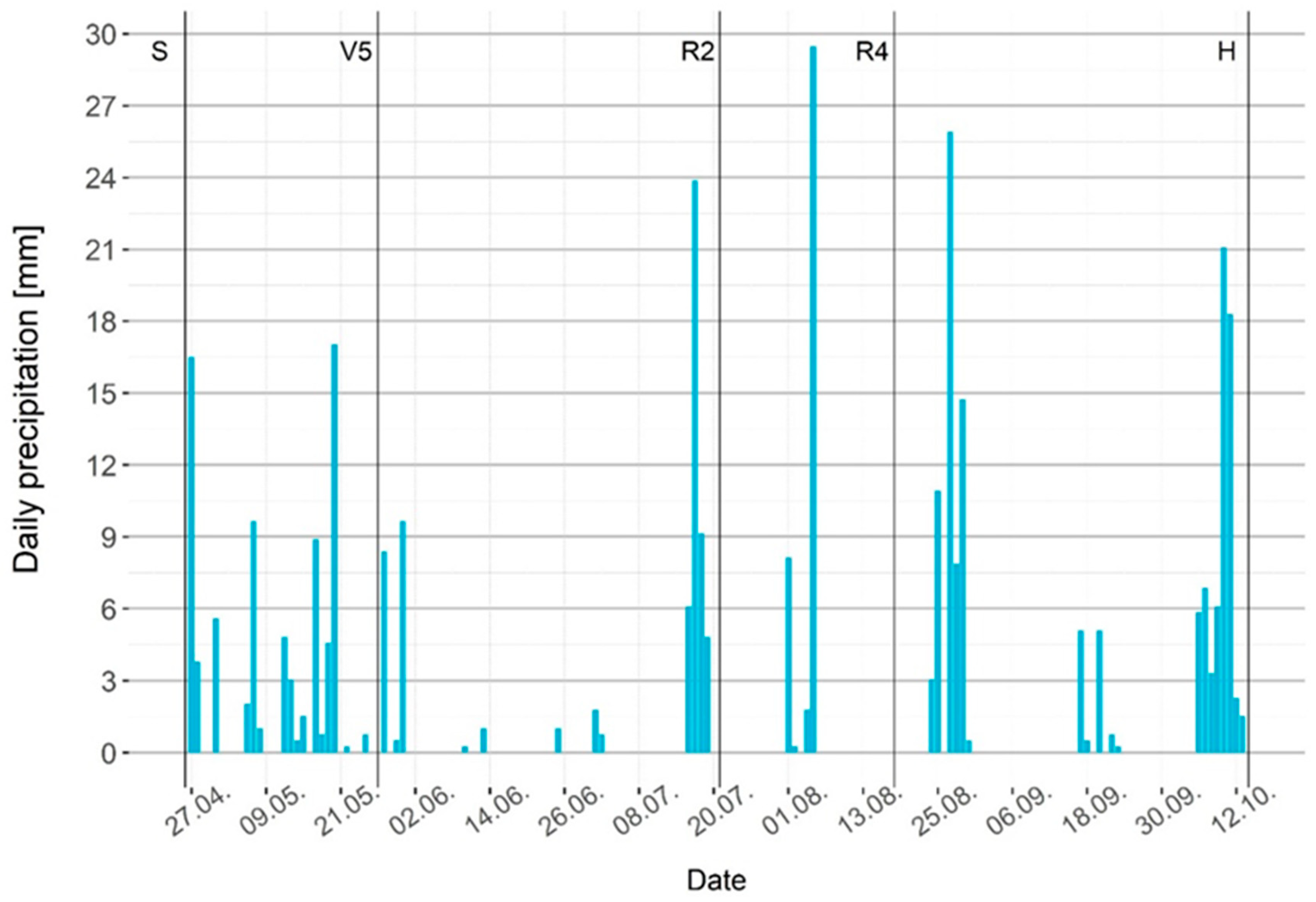
Different simulation models for a future climate in Europe predict warmer and drier conditions with increased risk of heat stress and drought during summer, especially for the Pannonian region [30,31]. In comparison with average measurements for our region in the period from 1981–2010, April and May of 2021 were much colder than normal [32], and the summer months of 2021 were relatively hot and dry. During maize vegetation, there was only 327.5 mm of rain, which was not distributed uniformly. The greatest precipitation deficit occurred during June 2021 (Figure 1).

Figure 1.
Daily precipitation for 2021 during maize vegetation. S—maize sowing date; V5—maize five leaves stage; R2—maize blister stage; R4—maize dough stage; H—maize harvest.
During the hot summer months of June, July, and most of August (from maize stage V5 to stage R4), there was not sufficient humidity for plants, according to the hydrothermal coefficient (Table 1).

Table 1.
Hydrothermal coefficient for 2021 during maize vegetation. S—maize sowing date; V5—maize five leaves stage; R2—maize blister stage; R4—maize dough stage; H—maize harvest.
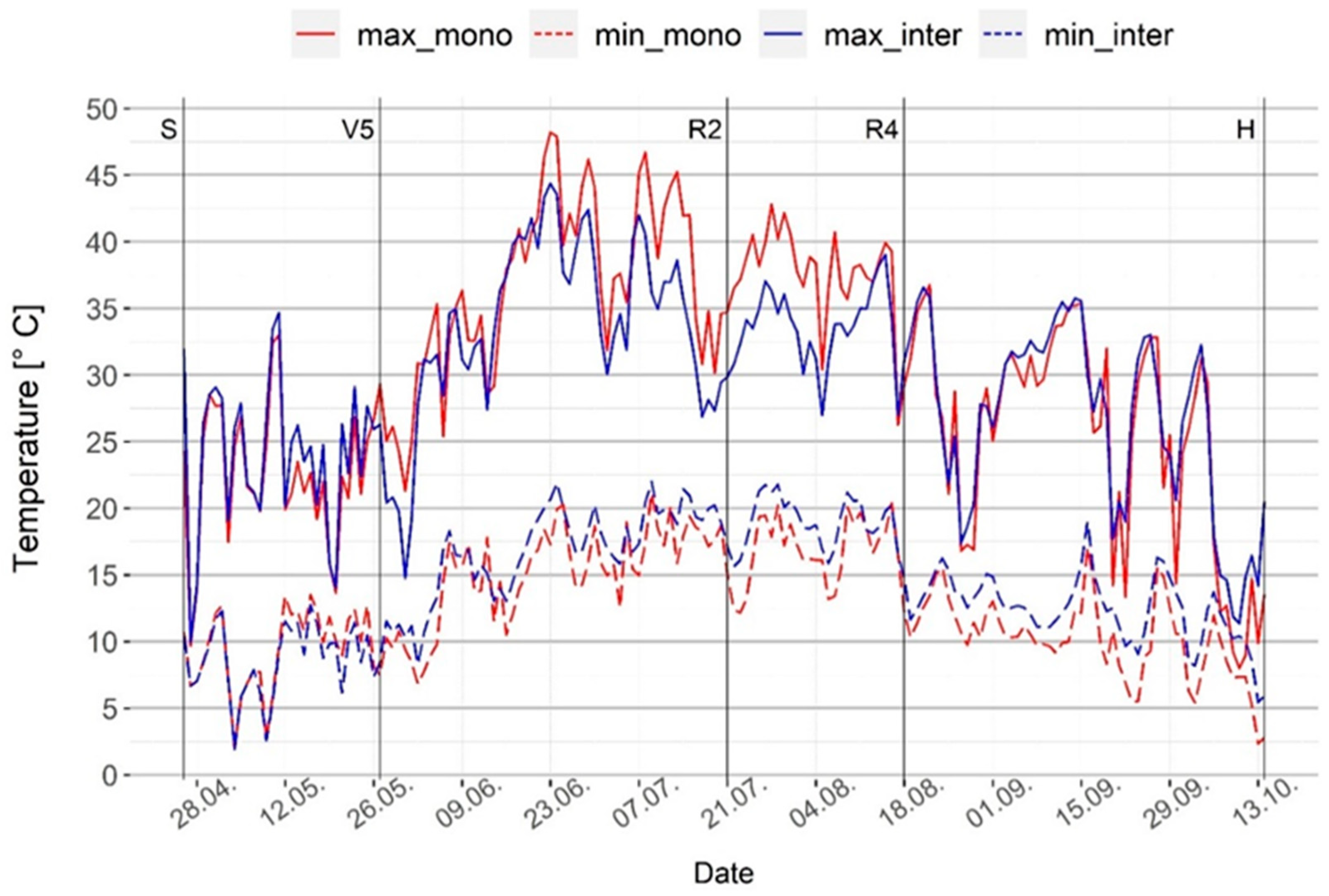
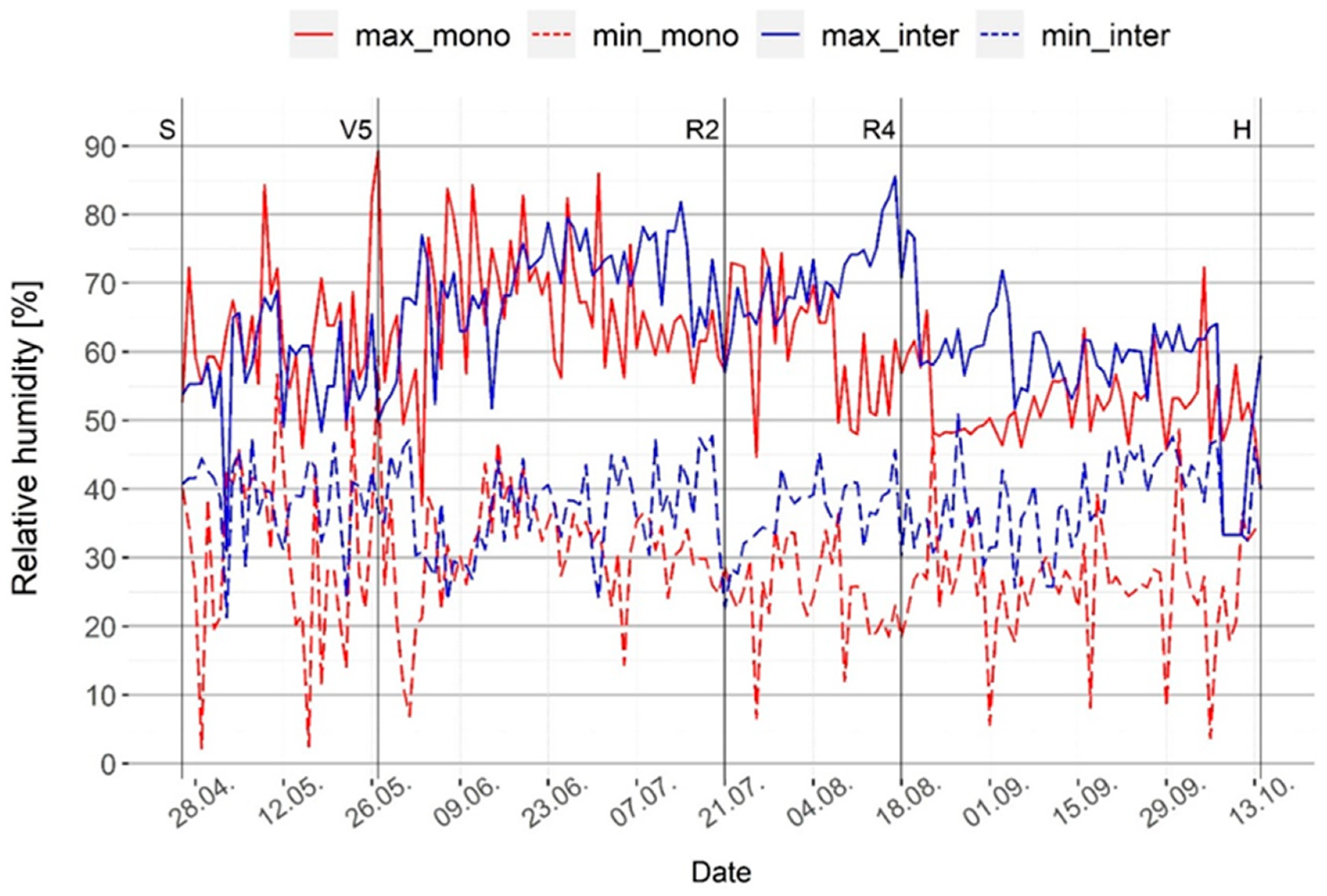
However, the intercropped system did seem to mediate these conditions in terms of microclimate changes. Generally, it had lower maximum and higher minimum temperature and humidity than the maize monoculture system (Figure 2 and Figure 3), meaning there was less oscillation on daily basis, which can help plants adapt and resist stress conditions. There was no significant difference in daily temperature extremes between the monoculture and intercropped systems during the first month of maize growth, as this is when walnut only begins to develop foliage and thereby does not yet cause significant shading of the understory. However, significant differences were found in terms of both maximum and minimum daily temperatures during the period from the maize five leaves stage (V5) to the dough stage (R4), as well as minimum temperature from the dough stage (R4) until harvest (H). This was when the intercropped system had lower maximum temperatures and higher minimum temperatures, providing more stable conditions (Tukey’s HSD, p < 0.05) (Figure 2). Regarding the daily relative humidity, the analysis of variance showed a statistically significant difference between the two systems during all maize growth stages, i.e., the whole vegetation. The intercropped system had higher maximum and minimum relative humidity, except during the first month of maize growth when the monoculture system had higher maximum humidity (Tukey’s HSD, p < 0.05) (Figure 3). These positive changes in microclimate in the intercropped system with trees were mostly due to shading and wind reduction, which can buffer the temperature, increase air humidity, and result in reduced evapotranspiration [33]. Similar results have been obtained in other studies on tree-based systems across different regions [4,34,35,36].

Figure 2.
Daily temperature range (minimum and maximum) inside maize rows in the monoculture and intercropped systems. S—maize sowing date; V5—maize five leaves stage; R2—maize blister stage; R4—maize dough stage; H—maize harvest.

Figure 3.
Daily humidity range (minimum and maximum) inside maize rows in the monoculture and intercropped systems. S—maize sowing date; V5—maize five leaves stage; R2—maize blister stage; R4—maize dough stage; H—maize harvest.
3.2. Soil Water Content
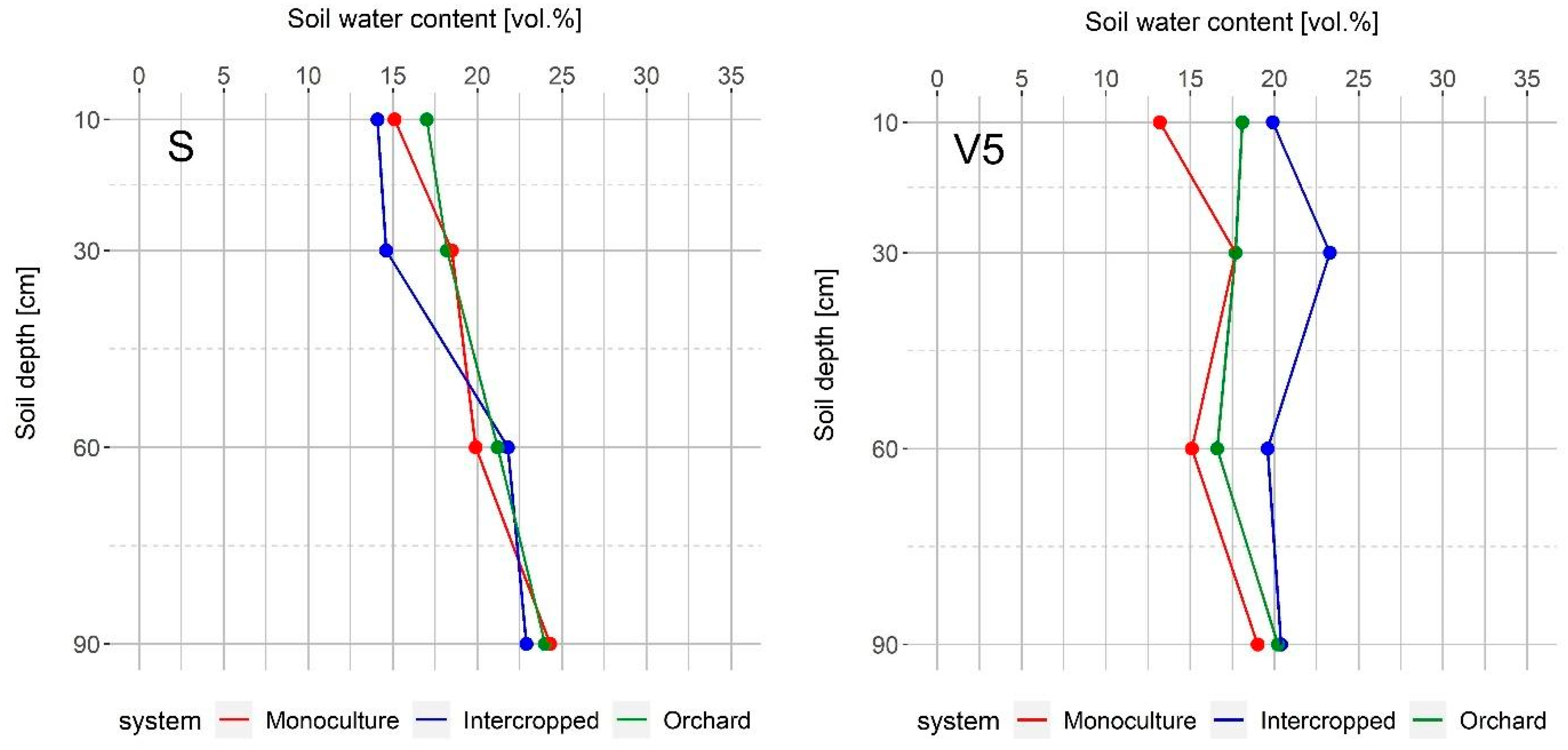
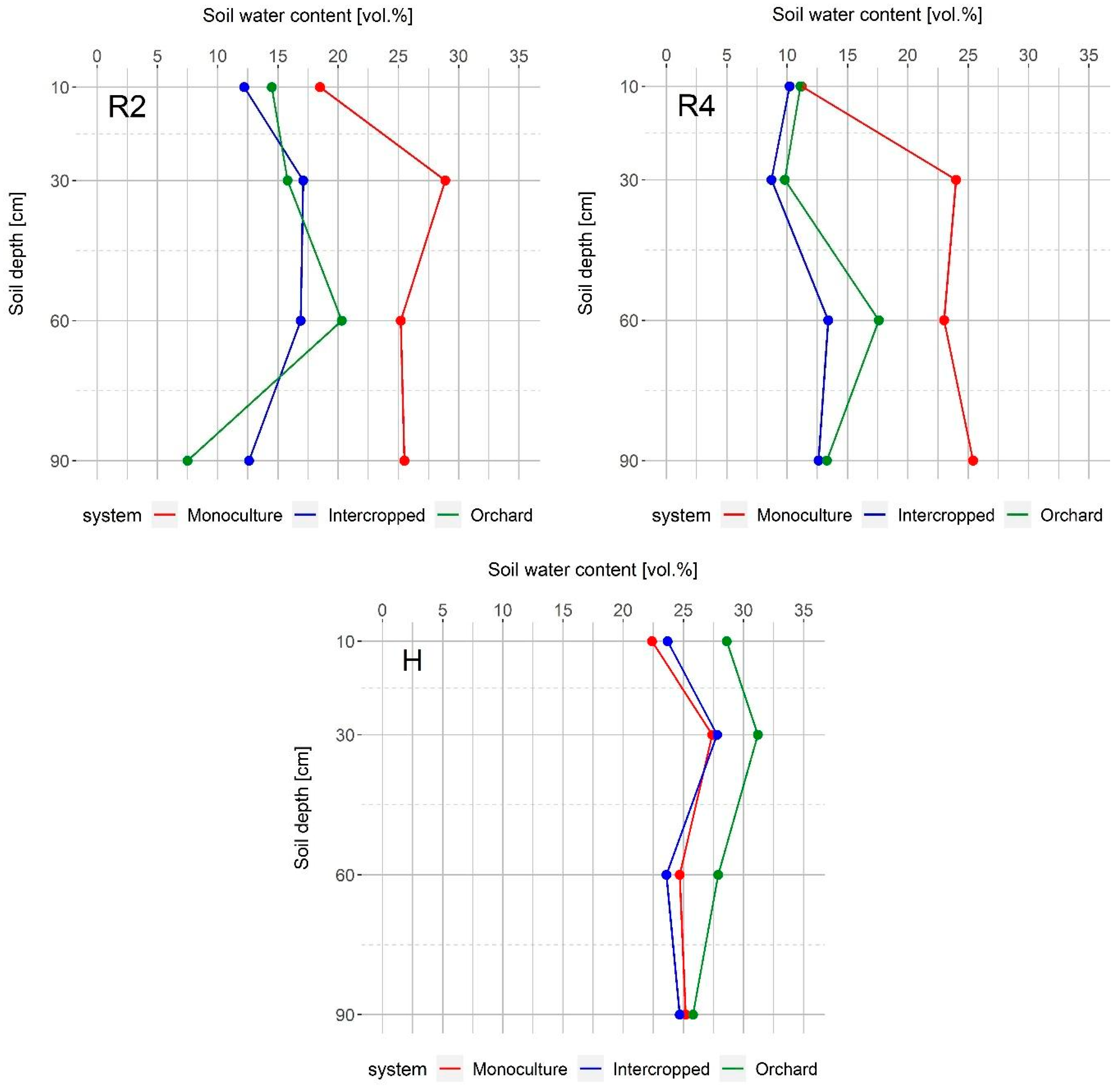
Initially, soil water content was similar in the three observed systems, as well as at the end of maize vegetation. In April, walnut trees have not yet produced foliage, and in October, when maize is harvested, walnut trees have already lost the majority of their leaves. Accordingly, walnut water demand during these periods is not high; therefore, soil water content is not affected by water uptake. The intercropped system conserved more water during the V5 stage than both the monoculture maize and the orchard, but later in vegetation, it had much less than the monoculture maize, especially in the deeper soil layers, which could be an indicator of the walnut water uptake pattern (Figure 4). Positive effects of trees on the microclimate in terms of decreased day temperatures, increased air humidity, and the lower transpiration rate of plants in shade could lead to greater conservation of soil water in intercropped systems. This outcome was found by Panozzo et al. [34] in their study conducted in Southern France—greater water availability was observed in the olive-wheat system than in the wheat monoculture system, and these differences were most pronounced during the last period of wheat vegetation (May and June). Even though we observed similar microclimate changes in our study (Figure 2 and Figure 3) and there was more water in the intercropped system during late May (maize V5 stage) (Figure 4), the competition for water between the walnut trees and the maize increased significantly during dry summer months, which led to lower soil water content than in the monoculture maize system. These observations were not unexpected considering the precipitation deficit and high temperatures that occurred during this period, but also, in this period walnut consumed most of the water due to fruit expansion [37] and the peak of fine root production [38,39]. Simpson [9] also found that below-ground competition between trees and maize led to lower soil water content than in monoculture maize, even in shallow soil layers.

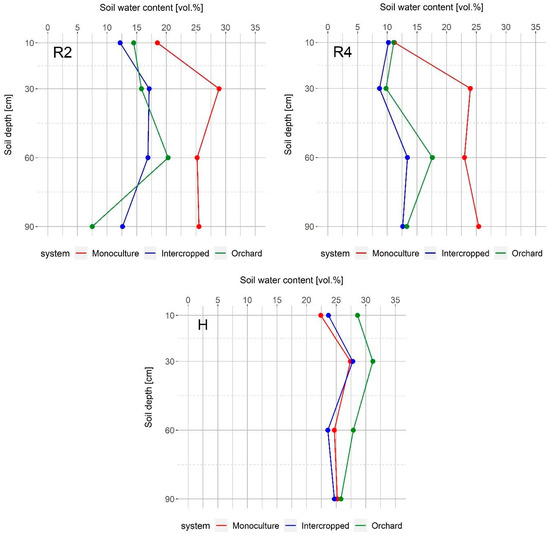
Figure 4.
Soil volumetric water content in the monoculture and intercropped systems, and the walnut orchard during: S—maize sowing date; V5—five leaves stage; R2—blister stage; R4—dough stage; H—harvest. Data show average values for the 10-day recording period.
3.3. Soil Chemical Properties
The soil chemical properties showed that soil in the intercropped system did not differ significantly from the soil in the sole walnut orchard, except for higher potassium content in the sole orchard. Differences between the soil in the intercropped system and monoculture maize showed mostly in the pH and organic matter content, which were significantly higher for the intercropped system (Table 2). Many studies have confirmed the significant contribution of tree-based systems to soil carbon sequestration [40,41,42,43], which in turn contributes to greater carbon pools in the soil and greater aboveground biomass than in agricultural monoculture systems [44,45]. Organic carbon is a major component of soil organic matter, and it is found in greater content in tree-based systems than in monoculture crop systems due to the increased input of litter and roots, and the reduction of soil temperature through shading [46,47]. The lower OM content in intercropped systems compared to sole orchards could be due to the disturbance of the soil by tillage practices [48], although the difference in our case was not significant.

Table 2.
Soil chemical properties during maize vegetation.
3.4. Maize Growth and Leaf Greenness during Vegetation
Regarding growth traits measured during vegetation, statistically significant differences were observed in favor of intercropped maize compared to monoculture maize, despite observed competition for water. During whole vegetation, maize in the intercropped system was higher, and had a greater leaf area index and higher SPAD values than maize in the monoculture system. Initially, it also had higher aboveground biomass (until R4 stage) (Table 3). All of these were a sign of maize adapting to shaded conditions—plants grown in shade tend to grow higher and produce greater leaf area in order to reach more light [49,50,51]. Even though incoming photosynthetically active radiation is a major determinant of plants’ dry matter production, photosynthetic efficiency is also driven by plant leaf area, leaf area density, and leaf area duration [52,53], which is why plants grown in mixed systems do not necessarily have reduced photosynthesis. Gillespie et al. [10] found no effect of tree shading on maize net photosynthesis, while Zhang et al. [54] found that net photosynthetic efficiencies on leaf level in mixed crops were even higher than in sole crops due to the increase in the proportion of diffuse radiation. Our earlier studies have shown that on the experimental site, solar radiation under the canopy during the summer months does not fall below 10,000 lux, which is sufficient for normal plant growth [55]. Higher aboveground biomass of intercropped maize correlates with increased height and leaf area; however, this comes at the cost of carbon invested in the stems [49]. It seems this could have been the case in our study—at the end of vegetation, when leaves senesced and began to fall apart, the monoculture maize had higher aboveground biomass due to more dry matter allocated to the stem (Table 3). Another case could be that the maize in the monoculture system prolonged carbon allocation in vegetative organs due to unfavorable microclimate conditions, instead of investing more carbon into grain filling.

Table 3.
Maize traits measured during: S—maize sowing date; V5—five leaves stage; R2—blister stage; R4—dough stage; H—harvest.
3.5. Yields, Yield Components, and LER
Tabolt et al. [56] argued that shading is crucial for crop yields in an intercropped system with trees. The shading effect on understory crops depends on many other factors. One of these is tree species, i.e., its crown structure. Reynolds et al. [8] reported that shading by poplar trees impacted maize performance to a greater extent than shading by silver maple trees, which was determined by the differences in height and canopy structure between these two tree species. Surprisingly, Rao et al. [57] found that maize yield was positively affected under the shade of Peltophorum, a slow-growing tree with a small canopy. The walnut trees’ round, irregular, half-open crown fits the criteria of a good woody species for intercropped systems with crops [15]. Tree row orientation also plays an important role. Unless canopies converge and overlap, N–S row orientation casts less shade on the middle of the alley than E–W orientation, especially in higher latitudes. Under N–S orientation, the shadows from trees at noon, when most plant photosynthesis occurs, lay mostly under tree rows and not on crop rows [8,58,59]. Even though we observed that intercropped maize development was driven by reduced light availability, considering all of the above, we believe that the shading was not detrimental to the reduced maize yield in our study. Instead, significantly lower maize plant density in the intercropped system was the main determinant of significantly lower grain yield per total area (Table 4). In our previous studies in the same walnut orchard, we found that crop plant density was always lowered in comparison to the monoculture systems; wheat by 16%, barley by 13%, and buckwheat by 29% (unpublished data). Lower maize density, i.e., reduced germination in intercropped systems with walnut could be associated with walnut allelopathic properties, more precisely juglone excretion to the soil [60]. Juglone (5-hydroxy-1,4-naphthoquinone) is an organic compound found in all plant parts of the Juglandaceae family and is known to cause inhibition of germination and growth [16]. However, more research is needed to confirm the potential and extent of juglone build-up in the soil under walnut orchards. To gain more insightful information about the effect of trees on maize yield, we also calculated the yield per cropped area, i.e., excluding the area occupied by trees, but still accounting for lowered maize plant density. In this scenario, maize yield per cropped area was 96.61% of that in the monoculture system, which shows that besides less area for growing maize and despite fewer plants emerging, maize yield was not significantly impacted by walnut trees (Table 4).

Table 4.
Maize yield (dry matter basis) and yield components.
Nevertheless, the reduction in the number of plants per area was probably compensated by increasing the productivity of individual plants [61,62], so intercropped maize achieved a higher harvest index and significantly greater 1000 kernel weight than monoculture maize (Table 4). Besides plant density, environmental factors should also be considered for observing these differences. Temperature thresholds for maize reproductive development are considerably lower than those for vegetative growth and are often exceeded for summer crops in our region. Biomass accumulation and transport capacity can be severely affected under such conditions, leading to a reduction in kernel number and weight [63]. Although the microclimate under tree rows can improve biomass remobilization towards grain [64], intercropped maize might also have preferentially began allocating assimilates to grains at the expense of total biomass due to limited water availability, i.e., competition with walnut trees. Similar observations have been previously reported for sorghum [65] and soybean [66]. This theory could explain our results for the maize plant aboveground biomass during vegetation, the 1000 kernel weight, as well as the improved harvest index. We found a 12% increase in the 1000 kernel weight of intercropped maize compared to monoculture maize. Similarly, Temani et al. [33] found that grain weights under olive orchards were increased by 17% for faba bean and 39% for wheat.
Walnut fruit yield amounted to a total of 1777 kg ha−1 in the intercropped system and 2997 kg ha−1 in the sole walnut orchard. This led to a walnut pLER of 0.59. The relatively low pLER of walnut in the intercropped system can not be ascribed to the competition with maize and is solely due to undefined differences between the two parts of the orchard. Namely, the orchard in this study had contrasting fruit yields between the first and last five rows of walnut trees since establishment, i.e., long before introducing intercrops. The first five tree rows always produced significantly less fruit yield than the last five, and the reason for intercropping crops between these first rows was to increase the total productivity of the area. Maize pLER was calculated on basis of total area (including the area occupied by trees), and amounted to 0.72. Together, these pLERs gave the intercropped system a high LER of 1.32, meaning it was 32% more productive per unit of land than cultivating these species separately. Some other studies, under different climates and designs, have also shown that tree-based intercropping systems with maize can achieve LER > 1 [67,68,69,70]. Our previous work [71] on simulating the productivity of intercropped systems using the Yield-SAFE model calibrated for our walnut orchard showed that, although intercropped maize could achieve surprisingly high yield while trees are young, by the time they reach year 13, maize pLER drops drastically and ranges from 0.18 to 0.55 depending on tree density scenario. However, by this time, walnut is in full fruit production maturity and its pLER could leverage a total LER towards LER > 1 (1.20 in the worst-case scenario with the highest tree density). Nevertheless, it would be expected that due to temporal complementarity in resource use between trees and crops, intercropping winter crops results in greater LER than intercropping summer crops. Under our experiments so far, we found that the best intercrops with walnut trees in terms of LER were as follows: winter barley—1.53 [18], perennial ryegrass—1.44 (unpublished data), maize—1.32, winter wheat—1.18 [72], buckwheat—1.05 [18].
3.6. Water Use, Water Productivity, and Water Equivalent Ratio
Complementarity in water use in intercropped systems with deciduous trees could be maintained either spacially [37,73]—by choosing deep-rooting trees with a long taproot and little or no lateral spreading [15], or temporally—by intercropping winter crops that can satisfy most of its water needs before the trees begin leafing and consume more water [74,75]. Such design and species selections could lead to maximum efficiency in water use for the intercropped system and maximize productivity.
The monoculture maize system in our experiment used the most water, i.e., it had the highest evapotranspiration of the three observed systems (Table 5). Considering the higher temperatures and lower humidity in this system, this is probably a result of a greater share of soil evaporation than in systems with trees [76,77,78]. The sole walnut orchard used the least water, which is probably only due to the combination of reduced soil evaporation and the transpiration of trees. Due to its higher yield and absence of competition for water with trees, monoculture maize had higher water productivity (also called water use efficiency—WUE) than maize in the intercropped system, although this difference was not statistically significant (Tukey’s HSD, p > 0.05) (Table 5). Sole walnuts used less water than the intercropped system, and considering its much higher yield, it was also more productive per unit of water used. However, the intercropped system reached a WER of 1.31, which means that maize and walnuts grown together were 31% more efficient in using water than the monoculture system. The WER value followed closely the LER value, as has been seen in some other studies [25,79].

Table 5.
Water use (WU [mm]), water productivity (WP [kg/ha/mm]), and water equivalent ratio (WER).
3.7. Nutrient Indices
Trees provide environmental services by reducing nutrient losses via a safety net, the uplift of deep soil nutrients, fixation of N2, and changing morphological and chemical processes at the rhizosphere [7], which can then indirectly benefit the nutrient uptake of crops in agroforestry systems. Studies have found that crops in an intercropped system with trees gained higher nutrient content in biomass and/or grain in comparison with monoculture systems [12,59,74,80,81]. In our previous study, we also found that barley in the intercropped system had significantly higher N, P, and K grain content [82]. However, we observed the contrary with intercropped maize (Table 6).

Table 6.
Grain nutrient contents and nutrient indices; NP, PP, KP—grain productivity [kg ha−1 [kg soil nutrient ha−1]−1], NUE, PUE, KUE—nutrient use efficiencies [kg ha−1 [kg total accumulated nutrient ha−1]−1], NRI, PRI, KRI—nutrient recovery indices [kg grain accumulated nutrient ha−1 [kg soil nutrient ha−1]−1].
Gillespie [83] stated that greater competition between trees and crops in agroforestry systems is most likely to occur for nitrogen as nitrate and potassium. We found that monoculture maize had higher grain nitrogen and potassium content (N% and K%) than intercropped maize (Table 6). Even though intercropped maize had higher nitrogen, phosphorus, and potassium use efficiencies (NUE, PUE, KUE), meaning that it produced more dry grain mass per kg of those nutrients accumulated (Table 6), the difference was significant only for potassium. Our results are comparable with those observed by Ciampitti and Vyn [84] and Ciampitti et al. [85]—nitrogen and phosphorus use efficiency (denoted as NIE and PIE) increased exponentially as the grain concentration of those nutrients declined. Higher nutrient use efficiencies in the intercropped system in this study could be related to decreased plant density, as there is less intra-species competition (Table 3). Furthermore, intercropped maize also produced slightly higher grain yield per available nitrogen and phosphorus (NP and PP), which could be related to lower nitrogen and phosphorus content in the soil [24]. Schmidt et al. [27] examined grain nutrient productivity (denoted as NRE—nutrient response efficiency) in crops in intercropped systems across three different sites, i.e., different soil types. They reported that nutrient productivities were comparable between crops in monoculture and intercropped systems, due to similar yields and nutrient availability in the soil. Similarly to our results, they did not observe any KP advantage during their study but found a higher NP of intercrops on gleyic cambisol. The nutrient recovery indices (NRI, PRI, KRI) showed no significant difference for maize between the monoculture and intercropped systems, indicating that the ability of maize to partition nutrients in grain was not affected by its possible competition with walnut trees.
4. Conclusions
Maintaining agricultural production at a high level while ensuring sustainability to face climate change challenges is a high priority around the globe. The role of trees in modifying microclimate conditions on agricultural land can be significant in mitigating heat stress. Our study showed that combining walnut orchards with maize in temperate regions has a lot of potential for sustaining the high land and water productivity of the system while having the benefit of improved microclimate conditions for crops. Land and water equivalent ratios of 1.32 and 1.31, respectively, showed an advantage for the walnut-maize intercropped system, meaning that for achieving equivalent yields in monoculture systems, these species would need both more land and more water. Despite the anticipated negative effects of shade on C4 crops such as maize, we found this was not a limiting factor for maize productivity in the system intercropped with walnut. Moreover, the maize seemed to proficiently adapt its growth to these conditions by increasing its aboveground growth. It is important to note here the importance of tree row orientation, as E–W orientation can limit light availability for intercrops to a greater extent than N–S orientation. Belowground, we did observe possible water competition with trees during the walnut fruit expansion stage, but the main limiting factor for decreased maize yield per unit of the total area was reduced germination. Many studies have reported the negative effects of walnut juglone excretion on plants grown nearby, and this may have been the case in our 14-year-old orchard. However, more research is needed to evaluate the extent of this allelopathic potential regarding tree age, soil type, etc. Nevertheless, the reduction in the number of plants per area was compensated by the increased productivity of individual plants, so intercropped maize achieved a higher harvest index and significantly greater 1000 kernel weight than monoculture maize. Furthermore, we did not find an increase in grain nutrient content as observed in previous studies and seen in the literature. On the contrary, monoculture maize achieved higher nitrogen and potassium content. No significant differences were found in terms of maize grain productivity per unit of soil nutrients or grain nutrient recovery. However, intercropped maize did produce more grain mass per unit of accumulated nitrogen and potassium. Further research should focus on providing insights into the trade-off between radiation decrease and microclimate improvements in intercropped systems with summer crops. Also, potential allelopathic associations in mature walnut orchards should be assessed.
Author Contributions
Conceptualization, H.Ž. and V.I.; methodology, H.Ž., G.H., L.E., J.J., V.Z. and V.I.; software, H.Ž. and V.Z.; validation, J.J., V.Z. and V.I.; formal analysis and investigation, H.Ž., resources and data curation, H.Ž., L.E., G.H., A.B., J.J., V.Z. and V.I.; writing—original draft preparation, H.Ž.; writing—review and editing, H.Ž. and V.I.; visualization, H.Ž.; supervision, V.Z. and V.I.; project administration, V.I.; funding acquisition, V.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Croatian Science Foundation project “Intercropping of wood species and agricultural crops as an innovative approach in agroecosystems (7103)”.
Data Availability Statement
The data are contained within the article.
Acknowledgments
The authors would like to thank the Croatian Science Foundation for financing the project, as well as the Faculty of Agrobiotechnical Sciences Osijek, who made this research possible. Thanks also go to our associate farmer Ivan Paponja for his cooperation in conducting the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raftery, A.E.; Zimmer, A.; Frierson, D.M.W.; Startz, R.; Liu, P. Less than 2 °C warming by 2100 unlikely. Nat. Clim. Chang. 2017, 7, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Kamali, B.; Abbaspour, K.C.; Wehrli, B.; Yang, H. Drought vulnerability assessment of maize in Sub-Saharan Africa: Insights from physical and social perspectives. Glob. Planet. Chang. 2018, 162, 266–274. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.K., Allen, S.K., et al., Eds.; A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; pp. 1–19. [Google Scholar]
- Gosme, M.; Inurreta-Aguirre, H.D.; Dupraz, C. Microclimatic effect of agroforestry on diurnal temperature cycle. In Proceedings of the 3rd European Agroforestry Conference, European Agroforestry Federation, Montpellier, France, 23–25 May 2016; pp. 183–186. [Google Scholar]
- van Noordwijk, M. Agroforestry-based ecosystem services. Land 2021, 10, 770. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, W.; Chen, J.; Bruijnzeel, L.A.; Mao, Z.; Yang, X.; Cardinael, R.; Meng, F.-R.; Sidle, R.C.; Seitz, S.; et al. Reductions in water, soil and nutrient losses and pesticide pollution in agroforestry practices: A review of evidence and processes. Plant Soil 2019, 453, 45–86. [Google Scholar] [CrossRef]
- Isaac, M.E.; Borden, K.A. Nutrient acquisition strategies in agroforestry systems. Plant Soil 2019, 444, 1–19. [Google Scholar] [CrossRef]
- Reynolds, P.E.; Simpson, J.A.; Thevathasan, N.V.; Gordon, A.M. Effects of tree competition on corn and soybean photosynthesis, growth, and yield in a temperate tree-based agroforestry intercropping system in southern Ontario, Canada. Ecol. Eng. 2007, 29, 362–371. [Google Scholar] [CrossRef]
- Simpson, J.A. Effects of Shade on Maize and Soybean Productivity in a Tree Based Intercrop System. Master’s Thesis, The University of Guelph, Guelph, ON, Canada, 1999. [Google Scholar]
- Gillespie, A.R.; Jose, S.; Mengel, D.B.; Hoover, W.L.; Pope, P.E.; Seifert, J.R. Defining competition vectors in a temperate alley cropping system in the midwestern USA: 1. Production physiology. Agrofor. Syst. 2000, 48, 25–40. [Google Scholar] [CrossRef]
- Jose, S.; Gillespie, A.R.; Seifert, J.R.; Biehle, D.J. Defining competition vectors in a temperate alley cropping system in the midwestern USA: 2. Competition for water. Agrofor. Syst. 2000, 48, 41–59. [Google Scholar] [CrossRef]
- Harawa, R.; Lehmann, J.; Akinnifesi, F.; Fernandes, E.; Kanyama-Phiri, G. Nitrogen dynamics in maize-based agroforestry systems as affected by landscape position in Southern Malawi. Nutr. Cycl. Agroecosyst. 2006, 75, 271–284. [Google Scholar] [CrossRef]
- Amadi, D.C.; Idiege, D.A.; Sobola, O.O. Agroforestry technique and its influence on maize crop yield in Gombi local government, Adamawa state, Nigeria. IOSR J. Agric. Vet. Sci. 2013, 4, 52–55. [Google Scholar] [CrossRef]
- Taha, N.A.; Al-wadaan, M.A. Utility and importance of walnut, Juglans regia Linn: A review. Afr. J. Microbiol. Res. 2011, 5, 5796–5805. [Google Scholar] [CrossRef]
- Tengnas, B. Agroforestry Extension Manual for Kenya. Nairobi; International Centre for Research in Agroforestry: Nairobi, Kenya, 1994. [Google Scholar]
- Kocacë Aliskan, I.; Terzi, I. Allelopathic effects of walnut leaf extracts and juglone on seed germination and seedling growth. J. Hortic. Sci. Biotechnol. 2001, 76, 436–440. [Google Scholar] [CrossRef]
- Selyaninov, G.T. About climate agricultural estimation. Proc. Agric. Meteorol. 1928, 20, 165–177. [Google Scholar]
- Žalac, H.; Zebec, V.; Ivezić, V.; Herman, G. Land and water productivity in intercropped systems of walnut—buckwheat and walnut–barley: A case study. Sustainability 2022, 14, 6096. [Google Scholar] [CrossRef]
- McKee, G.W. A coefficient for computing leaf area in hybrid corn. Agron. J. 1964, 56, 240–241. [Google Scholar] [CrossRef]
- Yi, L.; Shenjiao, Y.; Shiqing, L.; Xinping, C.; Fang, C. Growth and development of maize (Zea mays L.) in response to different field water management practices: Resource capture and use efficiency. Agric. For. Meteorol. 2010, 150, 606–613. [Google Scholar] [CrossRef]
- Mead, R.; Willey, R.W. The concept of a ‘land equivalent ratio’ and advantages in yields from intercropping. Exp. Agric. 1980, 16, 217–228. [Google Scholar] [CrossRef]
- Hillel, D. Introduction to Environmental Soil Physics, 1st ed.; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Machado, S.; Petrie, S.; Rhinhart, K.; Ramig, R.E. Tillage effects on water use and grain yield of winter wheat and green pea in rotation. Agron. J. 2008, 100, 154–162. [Google Scholar] [CrossRef]
- Sainju, U.M.; Lenssen, A.W.; Allen, B.L.; Jabro, J.D.; Stevens, W.B. Crop water and nitrogen productivity in response to long-term diversified crop rotations and management systems. Agric. Water Manag. 2021, 257, 107149. [Google Scholar] [CrossRef]
- Mao, L.; Zhang, L.; Li, W.; van der Werf, W.; Sun, J.; Spiertz, H.; Li, L. Yield advantage and water saving in maize/pea intercrop. Field Crop. Res. 2012, 138, 11–20. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Pastor, J.; McClaugherty, C.A.; Richardson, C.J. Nutrient-use efficiency: A litterfall index, a model, and a test along a nutrient-availability gradient in North Carolina peatlands. Am. Nat. 1995, 145, 1–21. [Google Scholar] [CrossRef]
- Schmidt, M.; Corre, M.D.; Kim, B.; Morley, J.; Göbel, L.; Sharma AS, I.; Setriuc, S.; Veldkamp, E. Nutrient saturation of crop monocultures and agroforestry indicated by nutrient response efficiency. Nutr. Cycl. Agroecosyst. 2020, 119, 69–82. [Google Scholar] [CrossRef]
- Allen, B.L.; Lenssen, A.W.; Sainju, U.M.; Jabro, J.D.; Stevens, W.B. Nitrogen use in barley hay influenced by crop diversification, tillage, and management. In Proceedings of the Great Plains Soil Fertility Conference, Denver, CO, USA, 1–2 March 2016; International Plant Nutrition Institute: Brookings, SD, USA; pp. 172–179. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 13 June 2022).
- Trnka, M.; Olesen, J.E.; Kersebaum, K.C.; Skjelvåg, A.O.; Eitzinger, J.; Seguin, B.; Peltonen-Sainio, P.; Rötter, R.; Iglesias, A.; Orlandini, S.; et al. Agroclimatic conditions in Europe under climate change. Glob. Chang. Biol. 2011, 17, 2298–2318. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Državni Hidrometeorološki Zavod, Ocjena Mjeseca, Sezone, Godine. 2022. Available online: https://meteo.hr/klima.php?section=klima_pracenje¶m=ocjena&el=msg_ocjena&MjesecSezona=4&Godina=2021. (accessed on 1 May 2022).
- Temani, F.; Bouaziz, A.; Daoui, K.; Wery, J.; Barkaoui, K. Olive agroforestry can improve land productivity even under low water availability in the south Mediterranean. Agric. Ecosyst. Amp Environ. 2021, 307, 107234. [Google Scholar] [CrossRef]
- Panozzo, A.; Huang, H.-Y.; Bernazeau, B.; Meunier, F.; Turc, O.; Duponnois, R.; Prin, Y.; Vamerali, T.; Desclaux, D. Impact of olive trees on the microclimatic and edaphic environment of the understorey durum wheat in an alley orchard of the Mediterranean area. Agronomy 2022, 12, 527. [Google Scholar] [CrossRef]
- Karki, U.; Goodman, M.S. Microclimatic differences between mature loblolly-pine silvopasture and open-pasture. Agrofor. Syst. 2014, 89, 319–325. [Google Scholar] [CrossRef]
- Kanzler, M.; Böhm, C.; Mirck, J.; Schmitt, D.; Veste, M. Microclimate effects on evaporation and winter wheat (Triticum aestivum L.) yield within a temperate agroforestry system. Agrofor. Syst. 2019, 93, 1821–1841. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhao, S.; Ma, H.; Qi, G.; Guo, S. The depth of water taken up by walnut trees during different phenological stages in an irrigated arid hilly area in the Taihang mountains. Forests 2019, 10, 121. [Google Scholar] [CrossRef]
- Germon, A.; Cardinael, R.; Prieto, I.; Mao, Z.; Kim, J.; Stokes, A.; Dupraz, C.; Laclau, J.-P.; Jourdan, C. Unexpected phenology and lifespan of shallow and deep fine roots of walnut trees grown in a silvoarable Mediterranean agroforestry system. Plant Soil 2015, 401, 409–426. [Google Scholar] [CrossRef]
- Mohamed, A.; Monnier, Y.; Mao, Z.; Jourdan, C.; Sabatier, S.; Dupraz, C.; Dufour, L.; Millan, M.; Stokes, A. Asynchrony in shoot and root phenological relationships in hybrid walnut. New For. 2019, 51, 41–60. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Cambou, A.; Béral, C.; Barthès, B.G.; Dupraz, C.; Durand, C.; Kouakoua, E.; Chenu, C. Increased soil organic carbon stocks under agroforestry: A survey of six different sites in France. Agric. Ecosyst. Environ. 2017, 236, 243–255. [Google Scholar] [CrossRef]
- Pandey, D.N. Carbon sequestration in agroforestry systems. Clim. Policy 2002, 2, 367–377. [Google Scholar] [CrossRef]
- Peichl, M.; Thevathasan, N.V.; Gordon, A.M.; Huss, J.; Abohassan, R.A. Carbon sequestration potentials in temperate tree-based intercropping systems, southern Ontario, Canada. Agrofor. Syst. 2006, 66, 243–257. [Google Scholar] [CrossRef]
- Weerasrekara, C.; Udawatta, R.P.; Jose, S.; Kremer, R.J.; WeerasRekara, C. Soil quality differences in a row-crop watershed with agroforestry and grass buffers. Agrofo. Syst. 2016, 90, 829–838. [Google Scholar] [CrossRef]
- Muchane, M.N.; Sileshi, G.W.; Gripenberg, S.; Jonsson, M.; Pumariño, L.; Barrios, E. Agroforestry boosts soil health in the humid and sub-humid tropics: A meta-analysis. Agric. Ecosyst. Environ. 2020, 295, 106899. [Google Scholar] [CrossRef]
- Oelbermann, M.; Voroney, R.P.; Gordon, A. Carbon sequestration in tropical and temperate agroforestry systems: A review with examples from Costa Rica and southern Canada. Agric. Ecosyst. Environ. 2004, 104, 359–377. [Google Scholar] [CrossRef]
- Chander, K.; Goyal, S.; Nandal, D.P.; Kapoor, K.K. Soil organic matter, microbial biomass and enzyme activities in a tropical agroforestry system. Biol. Fertil. Soils 1998, 27, 168–172. [Google Scholar] [CrossRef]
- Pinho, R.C.; Miller, R.P.; Alfaia, S.S. Agroforestry and the improvement of soil fertility: A view from Amazonia. Appl. Environ. Soil Sci. 2012, 2012, 616383. [Google Scholar] [CrossRef]
- Sainepo, B.M.; Gachene, C.K.; Karuma, A. Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag. 2018, 13, 4. [Google Scholar] [CrossRef]
- Irving, L. Carbon Assimilation, Biomass Partitioning and Productivity in Grasses. Agriculture 2015, 5, 1116–1134. [Google Scholar] [CrossRef]
- Lee, D.W.; Baskaran, K.; Mansor, M.; Mohamad, H.; Yap, S.K. Irradiance and spectral quality affect Asian tropical rain forest tree seedling development. Ecology 1995, 77, 568–580. [Google Scholar] [CrossRef]
- Weselek, A.; Bauerle, A.; Hartung, J.; Zikeli, S.; Lewandowski, I.; Högy, P. Agrivoltaic system impacts on microclimate and yield of different crops within an organic crop rotation in a temperate climate. Agron. Sustain. Dev. 2021, 41, 59. [Google Scholar] [CrossRef]
- Monteith, J.L. Solar radiation and productivity in tropical ecosystems. J. Appl. Ecol. 1972, 9, 747. [Google Scholar] [CrossRef]
- Lawlor, D.W. Photosynthesis, productivity and environment. J. Exp. Bot. 1995, 46, 1449–1461. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Sun, Z.; Bai, W.; Wang, Q.; Feng, L.; Zheng, J.; Zhang, Z.; Liu, Y.; Yang, S.; et al. Agroforestry enables high efficiency of light capture, photosynthesis and dry matter production in a semi-arid climate. Eur. J. Agron. 2018, 94, 1–11. [Google Scholar] [CrossRef]
- Ivezić, V.; Žalac, H.; Jović, J.; Stošić, M.; Iljkić, D.; Zebec, V. Shading effect on crop yields in intercropped systems of walnut and agricultural crops. In Book of Abstracts, Proceedings of the 5th European Agroforestry Conference: Agroforestry for the Transition towards Sustainability and Bioeconomy, Nuoro, Italy, 17–19 May 2021; European Agroforestry Federation: Nuoro, Italy; pp. 111–112.
- Talbot, G.; Roux, S.; Graves, A.; Dupraz, C.; Marrou, H.; Wery, J. Relative yield decomposition: A method for understanding the behaviour of complex crop models. Environ. Model. Amp Softw. 2014, 51, 136–148. [Google Scholar] [CrossRef]
- Rao, M.R.; Nair, P.K.R.; Ong, C.K. Biophysical interactions in tropical agroforestry systems. Dir. Trop. Agrofor. Res. 1998, 38, 3–50. [Google Scholar] [CrossRef]
- Dufour, L.; Metay, A.; Talbot, G.; Dupraz, C. Assessing light competition for cereal production in temperate agroforestry systems using experimentation and crop modelling. J. Agron. Crop. Sci. 2012, 199, 217–227. [Google Scholar] [CrossRef]
- Artru, S.; Garré, S.; Dupraz, C.; Hiel, M.-P.; Blitz-Frayret, C.; Lassois, L. Impact of spatio-temporal shade dynamics on wheat growth and yield, perspectives for temperate agroforestry. Eur. J. Agron. 2017, 82, 60–70. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Widhalm, J.R. Agricultural uses of juglone: Opportunities and challenges. Agronomy 2020, 10, 1500. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Schubert, S. Harvest index of maize (Zea mays L.): Are there possibilities for improvement? Adv. Agron. 2017, 146, 37–82. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crop. Res. 2011, 121, 2–18. [Google Scholar] [CrossRef]
- Shao, R.-X.; Yu, K.-K.; Li, H.-W.; Jia, S.-J.; Yang, Q.-H.; Zhao, X.; Zhao, Y.-L.; Liu, T.-X. The effect of elevating temperature on the growth and development of reproductive organs and yield of summer maize. J. Integr. Agric. 2021, 20, 1783–1795. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Effects of shading on morphology, physiology and grain yield of winter wheat. Eur. J. Agron. 2021, 33, 267–275. [Google Scholar] [CrossRef]
- Wenzel, W.; Ayisi, K.K.; Donaldson, G. Importance of harvest index in drought resistance of sorghum. J. Appl. Bot. 2000, 74, 203–205. [Google Scholar]
- Bunce, J.A. Abscisic acid mimics effects of dehydration on area expansion and photosynthetic partitioning in young soybean leaves. Plant Cell Environ. 1990, 13, 295–298. [Google Scholar] [CrossRef]
- Jama, B.A.; Nair, P.K.R.; Rao, M.R. Productivity of hedgerow shrubs and maize under alleycropping and block planting systems in semiarid Kenya. Agrofor. Syst. 1995, 31, 257–274. [Google Scholar] [CrossRef]
- Karimuna, L.; Halim; Ansi, A.; Marfi, W.E.; Wijayanto, T.; Hasanuddin, L. Growth and yields of two varieties of maize (Zea mays L.) intercropped with peanut (Arachys hypogaea L.) applied by bokashi plus fertilizer between the rows of teak trees based agroforestry system. IOP Conf. Ser. Earth Environ. Sci. 2022, 951, 012041. [Google Scholar] [CrossRef]
- Selim, M.A.F.; Shams, A.S. Maximizing efficiency of land and water utilization and profitability of interplanting maize with mandarin trees using irrigation with fish waste water under sandy soil and drip irrigation conditions. Middle East J. Agric. Res. 2019, 8, 1240–1252. [Google Scholar] [CrossRef]
- Bellow, J.G.; Nair, P.K.R.; Martin, T.A. Tree-Crop interactions in fruit tree-based agroforestry systems in the western Highlands of Guatemala: Component yields and system performance. Adv. Agrofor. 2008, 4, 111–131. [Google Scholar] [CrossRef]
- Žalac, H.; Burgess, P.; Graves, A.; Giannitsopoulos, M.; Paponja, I.; Popović, B.; Ivezić, V. Modelling the yield and profitability of intercropped walnut systems in Croatia. Agrofor. Syst. 2021, 14, 6096. [Google Scholar] [CrossRef]
- Ivezić, V.; Stošić, M.; Zebec, V.; Popović, B.; Puškarić, J.; Ilić, J.; Jović, J. Walnut and crop yields in walnut orchards intercropped with wheat. In Book of Abstracts of the 4th World Congress on Agroforestry, Montpellier, France, 20–25 May 2019; Springer: Berlin/Heidelberg, Germany, 2021; p. 318. [Google Scholar]
- Liu, Z.; Jia, G.; Yu, X. Water uptake and WUE of Apple tree-Corn Agroforestry in the Loess hilly region of China. Agric. Water Manag. 2020, 234, 106138. [Google Scholar] [CrossRef]
- Pardon, P.; Mertens, J.; Reubens, B.; Reheul, D.; Coussement, T.; Elsen, A.; Nelissen, V.; Verheyen, K. Juglans regia (walnut) in temperate arable agroforestry systems: Effects on soil characteristics, arthropod diversity and crop yield. Renew. Agric. Food Syst. 2019, 35, 533–549. [Google Scholar] [CrossRef]
- Broadhead, J.; Ong, C.; Black, C. Tree phenology and water availability in semi-arid agroforestry systems. For. Ecol. Manag. 2003, 180, 61–73. [Google Scholar] [CrossRef]
- Jackson, N.; Wallace, J. Soil evaporation measurements in an agroforestry system in Kenya. Agric. For. Meteorol. 1999, 94, 203–215. [Google Scholar] [CrossRef]
- Lin, B.B. The role of agroforestry in reducing water loss through soil evaporation and crop transpiration in coffee agroecosystems. Agric. For. Meteorol. 2010, 150, 510–518. [Google Scholar] [CrossRef]
- Siriri, D.; Wilson, J.; Coe, R.; Tenywa, M.M.; Bekunda, M.A.; Ong, C.K.; Black, C.R. Trees improve water storage and reduce soil evaporation in agroforestry systems on bench terraces in SW Uganda. Agrofor. Syst. 2012, 87, 45–58. [Google Scholar] [CrossRef]
- Bai, W.; Sun, Z.; Zheng, J.; Du, G.; Feng, L.; Cai, Q.; Yang, N.; Feng, C.; Zhang, Z.; Evers, J.B.; et al. Mixing trees and crops increases land and water use efficiencies in a semi-arid area. Agric. Water Manag. 2016, 178, 281–290. [Google Scholar] [CrossRef]
- Haggar, J.; Tanner, E.; Beer, J.; Kass, D. Nitrogen dynamics of tropical agroforestry and annual cropping systems. Soil Biol. Biochem. 1993, 25, 1363–1378. [Google Scholar] [CrossRef]
- Isaac, M.E.; Timmer, V.R.; Quashie-Sam, S.J. Shade tree effects in an 8-year-old cocoa agroforestry system: Biomass and nutrient diagnosis of Theobroma cacao by vector analysis. Nutr. Cycl. Agroecosyst. 2007, 78, 155–165. [Google Scholar] [CrossRef]
- Žalac, H.; Zebec, V.; Stošić, M.; Popović, B.; Bubalo, A.; Jović, J.; Herman, G.; Paponja, I.; Ivezić, V. Barley yield, yield components and nutrient content in intercropped system of walnut and barley. In Proceedings of the 56th Croatian and 16th International Symposium on Agriculture, Vodice, Croatia, 5–10 September 2021; pp. 460–464. [Google Scholar]
- Gillespie, A.R. Modelling nutrient flux and interspecies root competition in agroforestry interplantings. Agrofor. Syst. 1989, 8, 257–265. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Physiological perspectives of changes over time in maize yield dependency on nitrogen uptake and associated nitrogen efficiencies: A review. Field Crop. Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Camberato, J.J.; Murrell, S.T.; Vyn, T.J. Maize Nutrient Accumulation and Partitioning in Response to Plant Density and Nitrogen Rate: I. Macronutrients. Agron. J. 2013, 105, 783–795. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).