Two Better Than One? Potential Effects of Intraguild Predation on the Biological Control of Ceratitis capitata (Diptera: Tephritidae) by the Parasitoid Aganaspis daci (Hymenoptera: Figitidae) and the Predator Pseudoophonus rufipes (Coleoptera: Carabidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Center and Insect Rearing
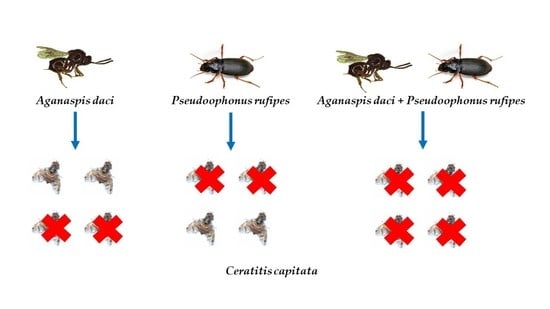
2.2. Experimental Design
2.2.1. Experiment 1: Functional Response of A. daci
2.2.2. Experiment 2: Functional Response of P. rufipes
2.2.3. Experiment 3: Functional Response of A. daci and P. rufipes When Acting Jointly on the Medfly
2.2.4. Experiment 4: Demographic Parameters of A. daci and P. rufipes
2.3. Statistical Analyses
3. Results
3.1. Experiment 1: Functional Response of A. daci
3.2. Experiment 2: Functional Response of P. rufipes
3.3. Experiment 3: Functional Response of A. daci and P. rufipes When Acting Jointly on the Medfly
3.4. Experiment 4: Demographic Parameters of A. daci and P. rufipes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denoth, M.; Frid, L.; Myers, J.H. Multiple agents in biological control: Improving the odds? Biol. Control. 2002, 24, 20–30. [Google Scholar] [CrossRef]
- Vafaie, E.K.; Pemberton, H.B.; Gu, M.; Kerns, D.; Eubanks, M.D.; Heinz, K.M. A comparison of repetitive releases of single or multiple natural enemy species on the suppression of Bemisia tabaci infesting poinsettias. Biol. Control. 2020, 151, 104407. [Google Scholar] [CrossRef]
- Martin, E.A.; Reineking, B.; Seo, B.; Steffan-Dewenter, I. Natural enemy interactions constrain pest control in complex agricultural landscapes. Proc. Natl. Acad. Sci. USA 2013, 110, 5534–5539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borer, E.T.; Briggs, C.J.; Murdoch, W.W.; Swarbrick, S.L. Testing intraguild predation theory in a field system: Does numerical dominance shift along a gradient of productivity? Ecol. Lett. 2003, 6, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Bilu, E.; Coll, M. The importance of intraguild interactions to the combined effect of a parasitoid and a predator on aphid population suppression. Biol. Control. 2007, 52, 753–763. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Kaya, H.K.; Ehler, L.E.; Marois, J.J.; Jaffee, B.A. Intraguild predation among biological-control agents: Theory and evidence. Biol. Control. 1995, 5, 303–335. [Google Scholar] [CrossRef]
- Kindlmann, P.; Houdková, K. Intraguild predation: Fiction or reality? Popul. Ecol. 2006, 48, 317–322. [Google Scholar] [CrossRef]
- Hall, R.J. Intraguild predation in the presence of a shared natural enemy. Ecology 2011, 92, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Vance-Chalcraft, A.H.D.; Rosenheim, J.A.; Vonesh, J.R.; Craig, W.; Sih, A. The influence of intraguild predation on prey suppression and prey release: A meta-analysis. Ecology 2007, 88, 2689–2696. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, M.B.; Bruzzone, O.A.; Triapitsyn, S.V.; Diaz-Soltero, H.; Hight, S.D.; Logarzo, G.A. Influence of competition and intraguild predation between two candidate biocontrol parasitoids on their potential impact against Harrisia cactus mealybug, Hypogeococcus sp. (Hemiptera: Pseudococcidae). Sci. Rep. 2021, 11, 13377. [Google Scholar] [CrossRef]
- Frago, E. Interactions between parasitoids and higher order natural enemies: Intraguild predation and hyperparasitoids. Curr. Opin. Insect Sci. 2016, 14, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rosenheim, J.A.; Harmon, J.P. The influence of intraguild predation on the suppression of a shared prey population: An empirical reassessment. In Trophic and Guild Interactions in Biological Control; Brodeur, J., Boivin, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–20. [Google Scholar]
- White, I.; Elson-Harris, M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB Inc.: Oxford, UK, 1994. [Google Scholar]
- Aluja, M.; Mangan, R.L. Fruit fly (Diptera: Tephritidae) host status determination: Critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol. 2008, 53, 473–502. [Google Scholar] [CrossRef] [PubMed]
- García, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological control of tephritid fruit flies in the Americas and Hawaii: A review of the use of parasitoids and predators. Insects 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- CABI (Centre for Agricultural Bioscience International)—Invasive Species Compendium. Available online: https://www.cabi.org/isc/ (accessed on 20 January 2021).
- Sela, S.; Nestel, D.; Pinto, R.; Nemny-Lavy, E.; Bar-Joseph, M. Mediterranean fruit fly as a potential vector of bacterial pathogens. Appl. Environ. Microbiol. 2005, 71, 4052–4056. [Google Scholar] [CrossRef] [Green Version]
- Monzó, C.; Sabater-Muñoz, B.; Urbaneja, A.; Castañera, P. The ground beetle Pseudophonus rufipes revealed as predator of Ceratitis capitata in citrus orchards. Biol. Control. 2011, 56, 17–21. [Google Scholar] [CrossRef]
- Monzó, C.; Vanaclocha, P.; Outerelo, R.; Ruiz-Tapiador, I.; Tortosa, D.; Pina, T.; Castañera, P.; Urbaneja, A. Catalogación de especies de las familias Carabidae, Cicindelidae y Staphylinidae en el suelo de los cítricos de la provincia de Valencia, España. Boletín Sanid. Veg. Plagas 2005, 31, 492. [Google Scholar]
- Liquido, N.J.; Shinoda, L.A.; Cunningham, R.T. Host plants of the Mediterranean Fruit fly (Diptera, Tephritidae). An Annotated World List; Miscellaneous Publication: Lanham, MD, USA, 1991. [Google Scholar]
- Papadopoulos, N.T.; Papachristos, D.P.; Ioannou, C. Citrus fruits and the Mediterranean fruit fly. Acta Hortic. 2015, 1065, 1009–1018. [Google Scholar] [CrossRef]
- Farinós, G.P.; de la Poza, M.; Hernández-Crespo, P.; Ortego, F.; Castañera, P. Diversity and seasonal phenology of aboveground arthropods in conventional and transgenic maize crops in Central Spain. Biol. Control. 2008, 44, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Miñarro, M.; Espadaler, X.; Melero, V.X.; Suárez-Álvarez, V. Organic versus conventional management in an apple orchard: Effects of fertilization and tree-row management on ground-dwelling predaceous arthropods. Agric. For. Entomol. 2009, 11, 133–142. [Google Scholar] [CrossRef]
- Weld, L.H. A New Species of Trybliographa (Hymenoptera: Cynipidae). Proc. Hawaiian Entomol. Soc. 1951, 14, 331–332. [Google Scholar]
- Clausen, C.P. Tephritidae (Trypetidae, Trupaneidae). In Introduced Parasites and Predators of Arthropod Pests and Weeds: A World Review; Clausen, C.P., Ed.; USDA-ARS: Washington, DC, USA, 1978; pp. 320–335. [Google Scholar]
- Wharton, R.A.; Gilstrap, F.E.; Rhode, R.H.; Fischel-m, M.; Hart, W.G. Hymenopterous egg-pupal and larval-pupal parasitoids of Ceratitis capitata and Anastrepha spp. (Dip.: Tephritidae) in Costa Rica. Entomophaga 1981, 26, 285–290. [Google Scholar] [CrossRef]
- El-Heneidy, A.; Hosny, M.; Ramadan, M. Potential of the parasitoid species, Aganaspis daci (Weld) (Hymenoptera: Eucoilidae) against the peach fruit fly Bactrocera zonata (Saund.) (Diptera: Tephritidae). In Proceedings of the Ninth International Symposium on Fruit Flies of Economic Importance (ISFFEI), Bangkok, Thailand, 12–16 May 2014; Sabater-Muñoz, B., Vera, T., Pereira, R., Orankanok, W., Eds.; pp. 395–400. [Google Scholar]
- Papadopoulos, N.T.; Katsoyannos, B.I. Field parasitism of Ceratitis capitata larvae by Aganaspis daci in Chios, Greece. Biol. Control. 2003, 48, 191–195. [Google Scholar]
- Verdú, M.J.; Falcó, J.V.; Beitia, F.; Sabater-Muñoz, B. Identificación de un nuevo agente de control biológico de Ceratitis capitata en España, el himenóptero eucoilino Aganaspis daci. In Proceedings of the XXVIII Jornadas de la Asociación Española de Entomología (AeE), Book of Abstracts, Ponferrada, Spain, 7–8 July 2011; p. 25. [Google Scholar]
- Sabater-Muñoz, B.; Falcó, J.V.; de Pedro, L.; Tormos, J.; Asís, J.D.; Papadopoulos, N.; Verdú, M.J.; Beitia, F. First record, surveillance and biological parameters of Aganaspis daci (Hymenoptera: Figitidae), as parasitoid of Ceratitis capitata (Diptera: Tephritidae) in Spain. In Proceedings of the Second TEAM (Tephritid Workers of Europe Africa and the Middle East) Meeting; Biological Invasions of Tephritidae: Ecological and Economic Impacts, Book of Abstracts, Kolymbari, Crete, Greece, 6 July 2012; p. 117. [Google Scholar]
- Ali, A.Y.; Ahmad, A.M.; Amar, J.A. Hymenopteran parasitoids (Figitidae and Pteromalidae) of Ceratitis capitata (Diptera: Tephritidae) on loquat and guava in Tartous, Syria. Biocontrol Sci. Technol. 2015, 25, 223–228. [Google Scholar] [CrossRef]
- Ali, A.Y.; Ahmad, A.M.; Amar, J.A.; Darwish, R.Y.; Izzo, A.M.; Al-Ahmad, S.A. Field parasitism levels of Ceratitis capitata larvae (Diptera: Tephritidae) by Aganaspis daci on different host fruit species in the coastal region of Tartous, Syria. Biocontrol Sci. Technol. 2016, 26, 1617–1625. [Google Scholar] [CrossRef]
- De Pedro, L.; Beitia, F.; Sabater-Muñoz, B.; Asís, J.D.; Tormos, J. Effect of temperature on the developmental time, survival of immatures and adult longevity of Aganaspis daci (Hymenoptera: Figitidae), a natural enemy of Ceratitis capitata (Diptera: Tephritidae). Crop Prot. 2016, 85, 17–22. [Google Scholar] [CrossRef]
- De Pedro, L.; Beitia, F.; Sabater-Muñoz, B.; Harbi, A.; Ferrara, F.; Polidori, C.; Asís, J.D.; Tormos, J. Parasitism of Aganaspis daci against Ceratitis capitata under Mediterranean climate conditions. Entomol. Exp. Appl. 2017, 163, 287–295. [Google Scholar] [CrossRef]
- De Pedro, L.; Tormos, J.; Asís, J.D.; Sabater-Muñoz, B.; Beitia, F. Biology of Aganaspis daci (Hymenoptera: Figitidae), parasitoid of Ceratitis capitata (Diptera: Tephritidae): Mode of reproduction, biological parameters and superparasitism. Crop Prot. 2018, 108, 54–61. [Google Scholar] [CrossRef]
- Rendon, P.; Sivinski, J.; Holler, T.; Bloem, K.; Lopez, M.; Martinez, A.; Aluja, M. The effects of sterile males and two braconid parasitoids, Fopius arisanus (Sonan) and Diachasmimorpha krausii (Fullaway) (Hymenoptera), on caged populations of Mediterranean fruit flies, Ceratitis capitata (Wied.) (Diptera: Tephritidae) at various sites. Biol. Control. 2006, 36, 224–231. [Google Scholar] [CrossRef]
- García-Medel, D.; Sivinski, J.; Díaz-Fleischer, F.; Ramirez-Romero, R.; Aluja, M. Foraging behavior by six fruit fly parasitoids (Hymenoptera: Braconidae) released as single- or multiple-species cohorts in field cages: Influence of fruit location and host density. Biol. Control. 2007, 43, 12–22. [Google Scholar] [CrossRef]
- Miranda, M.; Sivinski, J.; Rull, J.; Cicero, L.; Aluja, M. Niche breadth and interspecific competition between Doryctobracon crawfordi and Diachasmimorpha longicaudata (Hymenoptera: Braconidae), native and introduced parasitoids of Anastrepha spp. fruit flies (Diptera: Tephritidae). Biol. Control. 2015, 82, 86–95. [Google Scholar] [CrossRef]
- Tormos, J.; Beitia, F.; Asís, J.D.; Pedro, L. de Intraguild interactions between two biological control agents in citrus fruit: Implications for biological control of medfly. Ann. Appl. Biol. 2018, 172, 321–331. [Google Scholar] [CrossRef]
- Pérez-Hinarejos, M.; Beitia, F. Parasitism of Spalangia cameroni (Hymenoptera, Pteromalidae), an idiobiont parasitoid on pupae of Ceratitis capitata (Diptera, Tephritidae). IOBC-WPRS Bull. 2008, 38, 130–133. [Google Scholar]
- De Pedro, L. Bases Para Implementar la Lucha Biológica Contra Ceratitis capitata (Diptera: Tephritidae) Mediante el Empleo de Aganaspis daci (Hymenoptera: Figitidae). Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2017. [Google Scholar]
- Solomon, M.E. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Juliano, S.A. Nonlinear curve fitting. Predation and functional response curves. In Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 178–196. [Google Scholar]
- Fernández-Arhex, V.; Corley, J.C. The functional response of parasitoids and its implications for biological control. Biocontrol Sci. Technol. 2003, 13, 403–413. [Google Scholar] [CrossRef]
- Vanaclocha, P.; Papacek, D.; Monzó, C.; Verdú, M.J.; Urbaneja, A. Intra-guild interactions between the parasitoid Aphytis lingnanensis and the predator Chilocorus circumdatus: Implications for the biological control of armoured scales. Biol. Control. 2013, 65, 169–175. [Google Scholar] [CrossRef]
- Hassell, M.P. Functional responses. In The Dynamics of Arthropod Predator-Prey Systems; Hassell, M.P., Ed.; Princeton University Press: Princeton, NJ, USA, 1978; pp. 28–49. [Google Scholar]
- Monzó, C. Artrópodos Depredadores Potenciales de Ceratitis capitata (Wiedemann) Presentes en el Suelo de Cítricos. Ph.D. Thesis, Universidad Politécnica de Valencia, Valencia, Spain, 2010. [Google Scholar]
- Colfer, R.G.; Rosenheim, J.A. Predation on immature parasitoids and its impact on aphid suppression. Oecologia 2001, 126, 292–304. [Google Scholar] [CrossRef]
- Brodeur, J.; Boivin, G. Functional ecology of immature parasitoids. Annu. Rev. Entomol. 2004, 49, 27–49. [Google Scholar] [CrossRef]
- Lucas, È.; Coderre, D.; Brodeur, J. Intraguild predation among aphid predators: Characterization and influence of extraguild prey density. Ecology 1998, 79, 1084–1092. [Google Scholar] [CrossRef]
- Muratori, F.B.; Borlee, S.; Messing, R.H. Induced niche shift as an anti-predator response for an endoparasitoid. Proc. R. Soc. B Biol. Sci. 2010, 277, 1475–1480. [Google Scholar] [CrossRef] [Green Version]
- Meyhöfer, R.; Klug, T. Intraguild predation on the aphid parasitoid Lysiphlebus fabarum (Marshall) (Hymenoptera: Aphidiidae): Mortality risks and behavioral decisions made under the threats of predation. Biol. Control. 2002, 25, 239–248. [Google Scholar] [CrossRef]
- Müller, C.B.; Brodeur, J. Intraguild predation in biological control and conservation biology. Biol. Control. 2002, 25, 216–223. [Google Scholar] [CrossRef]
- Taylor, A.J.; Müller, C.B.; Godfray, H.C.J. Effect of aphid predators on oviposition behavior of aphid parasitoids. J. Insect Behav. 1998, 11, 297–302. [Google Scholar] [CrossRef]
- Martinou, A.F.; Raymond, B.; Milonas, P.G.; Wright, D.J. Impact of intraguild predation on parasitoid foraging behaviour. Ecol. Entomol. 2010, 35, 183–189. [Google Scholar] [CrossRef]
- Snyder, W.E.; Ives, A.R. Generalist predators disrupt biological control by a specialist parasitoid. Ecology 2001, 82, 705–716. [Google Scholar] [CrossRef]
- Snyder, W.E.; Ives, A.R. Interactions between specialist and generalist natural enemies: Parasitoids, predators, and pea aphid biocontrol. Ecology 2003, 84, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Traugott, M.; Bell, J.R.; Raso, L.; Sint, D.; Symondson, W.O.C. Generalist predators disrupt parasitoid aphid control by direct and coincidental intraguild predation. Bull. Entomol. Res. 2012, 102, 239–247. [Google Scholar] [CrossRef]
- Durán, J.; Trotta, V.; Di Nardo, E.; Forlano, P.; Fanti, P.; Battaglia, D. Intraguild predation between Macrolophus pygmaeus and Aphidius ervi. Bull. Insectol. 2018, 71, 113–120. [Google Scholar]
- Fernández-Arhex, V.; Corley, J.C. La respuesta funcional: Una revisión y guía experimental. Ecol. Austral 2004, 14, 83–93. [Google Scholar]
- Hassell, M.P.; Lawton, J.H.; Beddington, J.R. Sigmoid Functional Responses by Invertebrate Predators and Parasitoids. J. Anim. Ecol. 1977, 46, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Aluja, M. Functional response and superparasitism by Diachasmimorpha longicaudata (Hymenoptera: Braconidadae), a parasitoid of fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2000, 93, 47–54. [Google Scholar] [CrossRef]
- Collins, M.D.; Ward, S.A.; Dixon, A.F.G. Handling time and the functional response of Aphelinus thomsoni, a predator and parasite of the aphid Drepanosiphum platanoidis. J. Anim. Ecol. 1981, 50, 479–487. [Google Scholar] [CrossRef]
- De Pedro, L.; Beitia, F.; Ferrara, F.; Sabater-Muñoz, B.; Asís, J.D.; Tormos, J. Effect of host density and location on the percentage parasitism, fertility and induced mortality of Aganaspis daci (Hymenoptera: Figitidae), a parasitoid of Ceratitis capitata (Diptera: Tephritidae). Crop Prot. 2017, 92, 160–167. [Google Scholar] [CrossRef]
- De Pedro, L.; Tormos, J.; Harbi, A.; Ferrara, F.; Sabater-Muñoz, B.; Asís, J.D.; Beitia, F. Combined use of the larvo-pupal parasitoids Diachasmimorpha longicaudata and Aganaspis daci for biological control of the medfly. Ann. Appl. Biol. 2018, 174, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Ovruski, S.; Van Nieuwenhove, G.; Bezdjian, L.; Albornoz-Medina, P.; Schliserman, P. Evaluation of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) as a mortality factor of Ceratitis capitata (Diptera: Tephritidae) infesting Citrus species under laboratory and field-cage conditions. Biocontrol Sci. Technol. 2012, 22, 187–202. [Google Scholar] [CrossRef]
- Sánchez, G.; Murúa, F.; Suárez, L.; Van Nieuwenhove, G.; Taret, G.; Pantano, V.; Bilbao, M.; Schliserman, P.; Ovruski, S.M. Augmentative releases of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) for Ceratitis capitata (Diptera: Tephritidae) control in a fruit-growing region of Argentina. Biol. Control. 2016, 103, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Tormos, J.; Asís, J.; Sabater-Muñoz, B.; Baños, L.; Gayubo, S.F.; Beitia, F. Superparasitism in laboratory rearing of Spalangia cameroni (Hymenoptera: Pteromalidae), a parasitoid of medfly (Diptera: Tephritidae). Bull. Entomol. Res. 2012, 102, 51–61. [Google Scholar] [CrossRef]
- King, B.H. Offspring sex ratios in parasitoid wasps. Q. Rev. Biol. 1987, 62, 367–396. [Google Scholar] [CrossRef]
- He, L.F.; Feng, D.D.; Li, P.; Zhou, Z.S.; Xu, Z.F. Reproductive modes and daily fecundity of Aenasius bambawalei (Hymenoptera: Encyrtidae), a parasitoid of Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Florida Entomol. 2015, 98, 358–360. [Google Scholar] [CrossRef]
- Brower, J.H.; Press, J.W. Interactions between the egg parasite Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) and a predator, Xylocoris flavipes (Hemiptera: Anthocoridae) of the almond moth, Cadra cautella (Lepidoptera: Pyralidae). J. Entomol. Sci. 1988, 23, 342–349. [Google Scholar] [CrossRef]
- Van Driesche, R.; Hoddle, M.; Center, T. Control of Pests and Weeds by Natural Enemies: An Introduction to Biological Control; Blackwell: Hoboken, NJ, USA, 2008; ISBN 978-1-405-14571-8. [Google Scholar]
- Cabello, T.; Bonfil, F.; Gallego, J.R.; Fernandez, F.J.; Gamez, M.; Garay, J. Can interactions between an omnivorous hemipteran and an egg parasitoid limit the level of biological control for the tomato pinworm? Environ. Entomol. 2015, 44, 12–26. [Google Scholar] [CrossRef]
- Ferguson, K.I.; Stiling, P. Non-additive effects of multiple natural enemies on aphid populations. Oecologia 1996, 108, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ferrer, M.T.; Campos-Rivela, J.M.; Fibla, J.M. Detection of Aganaspis daci (Weld) (Hymenoptera: Eucoilidae) parasitizing Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) in different hosts in northeastern Spain. IOBC/WPRS Bull. 2018, 132, 150–153. [Google Scholar]




| Parameter | Estimate | SE | χ2 | df | p | |
|---|---|---|---|---|---|---|
| A. daci | Linear | 0.035 | 0.0025 | 194.828 | 1 | <0.0001 |
| Quadratic | 0.00001 | 1.5552 × 10−5 | 159.518 | 1 | <0.0001 | |
| P. rufipes | Linear | −0.070 | 0.0037 | 361.345 | 1 | <0.0001 |
| Quadratic | 0.00001 | 2.1748 × 10−5 | 180.264 | 1 | <0.0001 | |
| A. daci (with P. rufipes) | Linear | −0.012 | 0.0025 | 24.203 | 1 | <0.0001 |
| Quadratic | 6.109 × 10−5 | 1.5428 × 10−5 | 15.582 | 1 | <0.0001 | |
| P. rufipes (with A. daci) | Linear | −0.016 | 0.0026 | 36.714 | 1 | <0.0001 |
| Quadratic | −8.926 × 10−5 | 1.618 × 10−5 | 30.429 | 1 | <0.0001 |
| FR | a | ±SE | 95% CI | Th | ±SE | 95% CI | R2 | |
|---|---|---|---|---|---|---|---|---|
| A. daci | III | b = 0.041 | 0.031 | −0.020–0.101 | 0.004 | 0.003 | −0.002–0.010 | 0.744 |
| c = 0.044 | 0.053 | −0.061–0.149 | ||||||
| P. rufipes | II | 1.549 | 0.233 | 1.090–2.008 | 0.024 | 0.002 | 0.021–0.027 | 0.584 |
| A. daci/P. rufipes | II | 0.572 | 0.055 | 0.464–0.681 | 0.002 | 0.002 | −0.002–0.005 | 0.659 |
| P. rufipes/A. daci | III | b = 0.050 | 0.042 | −0.032–0.132 | 0.003 | 0.003 | −0.003–0.010 | 0.791 |
| c = 0.104 | 0.111 | −0.115–0.322 |
| Parameter | 15 Larvae | 60 Larvae | 120 Larvae | Global (15–120 Larvae) | ||
|---|---|---|---|---|---|---|
| Percentage parasitoidism | F | 36.481 | 0.843 | 53.183 | 0.220 | |
| df | 1188 | 1188 | 1188 | 1572 | ||
| p | 0.003 * | 0.527 | 0.087 | 0.721 | ||
| σ2 (RV) | 7.445 | 27.555 | 12.333 | 27.120 | ||
| σ2 (BV) | 1 × 10−6 | 1 × 10−7 | 1 × 10−8 | 1 × 10−7 | ||
| Fertility | F | 146.689 | 0.875 | 53.127 | 0.007 | |
| df | 1188 | 1188 | 1188 | 1572 | ||
| p | 0.002 * | 0.548 | 0.089 | 0.948 | ||
| σ2 (RV) | 5.678 | 27.322 | 12.480 | 20.120 | ||
| A. daci | σ2 (BV) | 1 × 10−6 | 1 × 10−7 | 1 × 10−8 | 1 × 10−7 | |
| Induced mortality | F | 0.212 | 0.016 | 1.591 | 0.156 | |
| df | 1188 | 1188 | 1188 | 1572 | ||
| p | 0.725 | 0.921 | 0.427 | 0.761 | ||
| σ2 (RV) | 12.717 | 32.444 | 28.111 | 14.101 | ||
| σ2 (BV) | 1 × 10−6 | 1 × 10−7 | 1 × 10−7 | 1 × 10−7 | ||
| Population reduction | F | 220.061 | 2.250 | 2.317 | 0.414 | |
| df | 1188 | 1188 | 1188 | 1572 | ||
| p | 0.043 * | 0.374 | 0.370 | 0.636 | ||
| σ2 (RV) | 17.040 | 25.111 | 27.123 | 29.037 | ||
| σ2 (BV) | 1 × 10−6 | 1 × 10−7 | 1 × 10−6 | 1 × 10−7 | ||
| F | 1019.434 | 177.929 | 224,240.743 | 56.006 | ||
| df | 1188 | 1188 | 1188 | 1572 | ||
| P. rufipes | Predation | p | 0.020 * | 0.048 * | 0.001 * | 0.085 |
| σ2(RV) | 11.321 | 17.888 | 10.555 | 16.112 | ||
| σ2(BV) | 1 × 10−7 | 1 × 10−8 | 1 × 10−7 | 1 × 10−8 | ||
| Host Density | ||||
|---|---|---|---|---|
| Parameter | 15 Larvae | 60 Larvae | 120 Larvae | |
| A. daci (alone) | Parasitoidism (%) (Range; Mean ± SE) | 0–80; 35.90 ± 2.41 * | 0–83.3; 58.00 ± 1.92 | 0–88.3; 57.17 ± 2.00 |
| Fertility (Range; Mean ± SE) | 0–12; 5.38 ± 0.36 * | 0–50; 34.80 ± 1.15 | 0–106; 68.60 ± 2.40 | |
| Induced mortality (%) (Range; Mean ± SE) | 0–20; 9.44 ± 0.70 | 0–18.3; 8.07 ± 0.62 | 0–16.67; 6.63 ± 0.59 | |
| Population reduction (%) (Mean ± SE) | 45.34 ± 1.86 * | 66.07 ± 1.84 | 66.61 ± 2.01 | |
| Sex ratio (♂♂, ♀♀; ♀♀/♀♀ + ♂♂) | 196, 321; 0.62 * | 1352, 1654; 0.55 * | 2053, 4083; 0.62 * | |
| A. daci (with P. rufipes) | Parasitoidism (%) (Range; Mean ± SE) | 26.67–100; 62.43 ± 2.38 * | 23.33–93.33; 53.73 ± 2.08 | 20.83–90.83, 51.97 ± 2.03 |
| Fertility (Range; Mean ± SE) | 4–15; 9.37 ± 0.36 * | 14–56; 32.23 ± 1.25 | 25–109; 62.37 ± 2.44 | |
| Induced mortality (%) (Range; Mean ± SE) | 0–33.3; 8.61 ± 1.27 | 0–31.67; 7.86 ± 1.52 | 0–40; 9.98 ± 1.44 | |
| Population reduction (%) (Mean ± SE) | 71.11 ± 1.45 * | 61.59 ± 1.26 | 61.96 ± 1.10 | |
| Sex ratio (♂♂, ♀♀; ♀♀/♀♀ + ♂♂) | 243, 657; 0.73 * | 990, 2105; 0.68 * | 1715, 4004; 0.70 * | |
| P. rufipes (alone) | Predation (Range; Mean ± SE) [Percentage: Mean ± SE] | 9–15; 13.56 ± 0.23 * [90.41 ± 1.56] | 9–48; 31.18 ± 1.56 * [51.97 ± 2.61] | 9–51; 32.62 ± 1.59 * [27.18 ± 1.32] |
| P. rufipes (with A. daci) | Predation (Range; Mean ± SE) [Percentage: Mean ± SE] | 0–11; 4.31 ± 0.22 * [28.85 ± 1.44] | 4–46; 23.04 ± 0.76 * [38.40 ± 1.26] | 11–93; 45.64 ± 1.32 * [38.04 ± 1.11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pedro, L.; Beitia, F.; Tormos, J. Two Better Than One? Potential Effects of Intraguild Predation on the Biological Control of Ceratitis capitata (Diptera: Tephritidae) by the Parasitoid Aganaspis daci (Hymenoptera: Figitidae) and the Predator Pseudoophonus rufipes (Coleoptera: Carabidae). Agronomy 2023, 13, 87. https://doi.org/10.3390/agronomy13010087
de Pedro L, Beitia F, Tormos J. Two Better Than One? Potential Effects of Intraguild Predation on the Biological Control of Ceratitis capitata (Diptera: Tephritidae) by the Parasitoid Aganaspis daci (Hymenoptera: Figitidae) and the Predator Pseudoophonus rufipes (Coleoptera: Carabidae). Agronomy. 2023; 13(1):87. https://doi.org/10.3390/agronomy13010087
Chicago/Turabian Stylede Pedro, Luis, Francisco Beitia, and José Tormos. 2023. "Two Better Than One? Potential Effects of Intraguild Predation on the Biological Control of Ceratitis capitata (Diptera: Tephritidae) by the Parasitoid Aganaspis daci (Hymenoptera: Figitidae) and the Predator Pseudoophonus rufipes (Coleoptera: Carabidae)" Agronomy 13, no. 1: 87. https://doi.org/10.3390/agronomy13010087
APA Stylede Pedro, L., Beitia, F., & Tormos, J. (2023). Two Better Than One? Potential Effects of Intraguild Predation on the Biological Control of Ceratitis capitata (Diptera: Tephritidae) by the Parasitoid Aganaspis daci (Hymenoptera: Figitidae) and the Predator Pseudoophonus rufipes (Coleoptera: Carabidae). Agronomy, 13(1), 87. https://doi.org/10.3390/agronomy13010087