Nutritional, Functional Properties and Applications of Mee (Madhuca longifolia) Seed Fat
Abstract
:1. Introduction
2. Value-Added Products from Mee Fruits and Seeds
2.1. Mee Seed Fat
2.2. Defatted Mee Seed Cake
3. Physical, Biological, and Chemical Properties of Mee Seed Fat
3.1. Physico-Chemical Properties of Mee Seed Fat
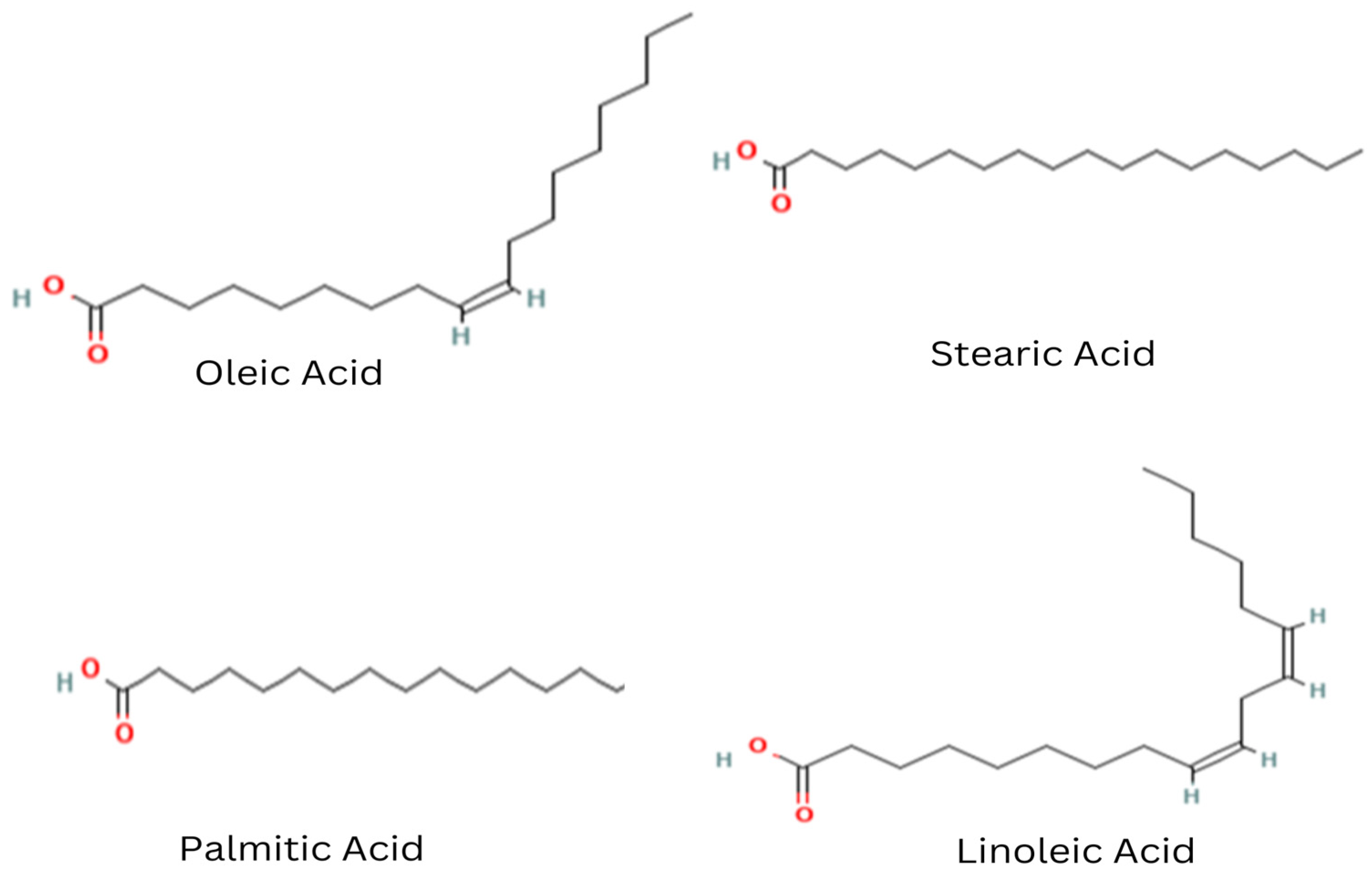
3.2. Fat Content and Fatty Acid Profile of Mee Seed Fat
3.3. Unsaponifiable Matters Composition of Mee Seed Fat
3.4. Antioxidant Potential of Mee Seed Fat
3.5. Mee Seed Fat Content and Quality upon Storage
4. Processing of Mee Seed Fat
4.1. Mechanical Press Fat Extraction
4.2. Solvent Oil Extraction (Chemical Extraction)
4.3. Ultrasonic-Assisted Bio-Oil Extraction
5. Potential Industrial Applications of Mee Seed Fat
5.1. Stir Frying Application
5.2. An Alternative Ingredient for Halal Fats
5.3. A cocoa Butter Substitute
5.4. Development of Food Packaging Material
5.5. Pharmaceutical Product Manufacturing
5.6. Formulation of Coating Binders
5.7. Biodiesel Production
6. Limitations and Gaps in Processing and Applications of Mee Fat
7. Therapeutic Potential and Nutritional Properties of Mee Seed Fat
8. Future Perspectives of Edible Mee (Madhuca longifolia) Seed Fat
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramadan, M.F.; Mörsel, J.T. Madhuca longifolia Butter. In Fruit Oils: Chemistry and Functionality; Springer: Cham, Switzerland, 2019; pp. 291–300. [Google Scholar] [CrossRef]
- Thangamani, D.; Rajan, S.P.; Karunamoorthi, J.; Lalitha, S. Spiritually significant natural resource of Madhuca longifolia (J. Koenig ex L.) J.F. Macbr. Conservation and its value added products management. Pharma Innov. J. 2022, 11, 792–796. [Google Scholar]
- Ramadan, M.F.; Abdel-Hamed, E.M.W. Health-promoting Potential and Nutritional Value of Madhuca longifolia Seeds. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Khare, P.; Kishore, K.; Sharma, D.K. Medicinal uses, Phytochemistry and Pharmacological profile of Madhuca longifolia. Asian J. Pharm. Pharmacol. 2018, 4, 570–581. [Google Scholar] [CrossRef]
- Kendre, N.; Wakte, P. A review on Phytochemicals and biological attributes of Madhuca longifolia. Asian J. Pharm. Pharmacol. 2021, 7, 74–84. [Google Scholar] [CrossRef]
- Saif, M.; Varma, R.; Kant, R.; Gupta, R. Madhuca longifolia (Mahua): A comprehensive ethno pharmacological review. Int. J. Chem. Stud. 2020, 8, 172–175. [Google Scholar] [CrossRef]
- Munasinghe, M.; Wansapala, J. Study on variation in seed morphology, oil content and fatty acid profile of Madhuca longifolia grown in different Agro-climatic zones in Sri Lanka. Sci. Res. 2015, 3, 105–109. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mohdaly, A.A.A.; Assiri, M.A.A.; Tadros, M.; Niemeyer, B. Functional characteristics, nutritional value and industrial applications of Madhuca longifolia seeds: An overview. J. Food Sci. Technol. 2016, 53, 2149–2157. [Google Scholar] [CrossRef]
- Saral, S.K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A.; Sharma, S.; Vijay, V.K. Comparative evaluation of raw and detoxified mahua seed cake for biogas production. Appl. Energy 2013, 102, 1514–1521. [Google Scholar] [CrossRef]
- Munasinghe, M.; Wansapala, J. Β-carotene content of M. longifolia seed oil in different Agro climatic zones in Sri Lanka, the effect of heat on its stability and the composition of seed cake. Potravinarstvo 2015, 9, 474–479. [Google Scholar] [CrossRef]
- Munasinghe, M.; Wansapala, J. Oil extraction from Madhuca longifolia (J. Konig) J.F. Macbr seeds and evaluation of physico- chemical properties, fatty acid profile, antioxidant potential and sensory characteristics. Trop. Agric. 2016, 9, 29–35. [Google Scholar]
- Seneviratne, K.; Jayathilaka, N. Coconut Oil: Chemistry and Nutrition; Lakva Publishers: Battaramulla, Sri Lanka, 2016; pp. 18–34. [Google Scholar]
- Bandara, D.M.S.P.; Dissanayake, C.A.K.; Rathnayaka, H.M.A.P.; Senanayake, D.P. Performance evaluation of the screw type oil expeller for extracting Mee (Madhuca longifolia) oil. J. Biosyst. Eng. 2016, 41, 177–183. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 445639, Oleic Acid. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Oleic-Acid (accessed on 10 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 985, Palmitic Acid. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Palmitic-Acid (accessed on 10 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281, Stearic Acid. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Stearic-Acid (accessed on 10 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280450, Linoleic Acid. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Linoleic-Acid (accessed on 12 February 2023).
- Punyawardena, B.V.R. Climate. In The Soils of Sri Lanka; Mapa, R.B., Ed.; World Soils Book Series; Springer: Cham, Switzerland, 2020; pp. 13–22. [Google Scholar]
- Nayak, S.; Sahoo, U.K.; Thangjam, U.; Garnayak, L.M. Provenance Variations of Morphometric Traits and oil contents of Madhuca latifolia Macbride in Odisha: Implication for tree improvement. J. Exp. Biol. Agric. Sci. 2020, 8, 224–232. [Google Scholar] [CrossRef]
- Suryawanshi, Y.C.; Mokat, D.N. GCMS and Elemental Analysis of Madhuca longifolia var. latifolia Seeds. IJPSR 2019, 10, 786–789. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Lee, F.S.; Tang, T.-K.; Chan, E.-S.; Lee, Y.-Y. Physicochemical and antioxidative properties of ultrasound-assisted extraction of mahua (Madhuca longifolia) seed oil in comparison with conventional Soxhlet and mechanical extractions. Ultrason. Sonochem. 2023, 92, 106280. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Elsaid, M.B.; Farag, M.A. Phytochemical and biological diversity of triterpenoid saponins from family Sapotaceae: A comprehensive review. Phytochemistry 2022, 202, 113345. [Google Scholar] [CrossRef] [PubMed]
- Bopitiya, D.; Madhujith, T. Antioxidant Activity and Total Phenolic Content of Mee (Madhuca sp.) Oil; Book of Abstracts of the Peradeniya; University Research Sessions: Nugegoda, Sri Lanka, 2012; Volume 17. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Madhujith, T.; Bopitiya, D.; Sivakanthan, S.; Jayawardana, N.W.I.A.; Walallawita, W.K.U.S. Comparison of oxidative stability of sesame (Sesamum indicum), soybean (Glycine max) and mahua (mee) (Madhuca longifolia) oils against photo-oxidation and autoxidation. Procedia Food Sci. 2016, 6, 204–207. [Google Scholar]
- Issaoui, M.; Delgado, A.M. Grading, labeling and standardization of edible oils. In Fruit Oils: Chemistry and Functionality; Ramadan, M., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Nayak, S.; Sahoo, U.K. Changes in Madhuca latifolia Macbride seed oil content and quality upon storage at different duration and condition. Vegetos 2021, 34, 422–431. [Google Scholar] [CrossRef]
- Behera, S.; Swain, M.R. A survey of post-harvest spoilage of mahua (Madhuca latifolia L.) flowers. J. Agric. Technol. 2013, 9, 227–235. [Google Scholar]
- Varghese, B.; Naithani, R.; Dulbo, M.S.; Naithani, S.C. Seed storage behavior of Madhuca indica J.P. Gmel. Seed Sci. Technol. 2002, 30, 107–117. [Google Scholar]
- Baskar, G.; Naveenkumar, R.; Mohanapriya, N.; Nivetha, S.R.; Aiswarya, R. Optimization and kinetics of ultrasonic assisted bio oil extraction from Madhuca indica seeds. Ind. Crops Prod. 2018, 124, 954–959. [Google Scholar] [CrossRef]
- Piseskul, J.; Suttisansanee, U.; Chupeerach, C.; Khemthong, C.; Thangsiri, S.; Temviriyanukul, P.; Sahasakul, Y.; Santivarangkna, C.; Chamchan, R.; Aursalung, A.; et al. Optimization of Enzyme-Assisted Mechanical Extraction Process of Hodgsonia heteroclita Oilseeds and Physical, Chemical, and Nutritional Properties of the Oils. Foods 2023, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Wijerathna, A.A.K.; Walgama, K.S.; Shanthini, R. Mathematical Modelling and Simulation of the Temperature of Sesame oil extracted in Sekku—A Traditional oil extraction Technology. In Proceedings of the 4th International Conference of Multidisciplinary Approaches (iCMA), Faculty of Graduate Studies, University of Sri Jayewardenepura, Nugegoda, Sri Lanka, 20–22 September 2017. [Google Scholar]
- Shashikumar, C.; Pradhan, R.C.; Mishra, S. Characterisation of Madhuca longifolia seed in relation to processing and design of equipment. Qual. Assur. Saf. Crops Foods 2018, 10, 215–221. [Google Scholar] [CrossRef]
- Keneni, Y.G.; Bahiru, L.A.; Marchetti, J.M. Effects of Different Extraction Solvents on Oil Extracted from Jatropha Seeds and the Potential of Seed Residues as a Heat Provider. BioEnergy Res. 2020, 14, 1207–1222. [Google Scholar] [CrossRef]
- Halal Research Council. What Is Halal? 2023. Available online: https://www.halalrc.org/glossary.php (accessed on 7 September 2023).
- Marikkar, J.M.N.; Yanty, N.A.M. Seed fat from Madhuca longifolia as raw material for Halal alternative fats. Borneo Sci. 2012, 31, 97–106. [Google Scholar]
- Yanty, N.A.M.; Dollah, S.; Marikkar, J.M.N.; Miskandar, M.S.; Desa, M.N.M.; Nusantoro, B.P. Physicochemical Properties and Thermal Behavior of Binary Blends of Madhuca longifolia Seed Fat and Palm Oil as a Lard Substitute. J. Adv. Agric. Technol. 2018, 5, 202–208. [Google Scholar] [CrossRef]
- Tambun, R.; Ferani, D.G.; Afrina, A.; Tambun, J.A.A.; Tarigan, I.A.A. Fatty Acid Direct Production from Palm Kernel Oil. In Proceedings of the 1st International Conference on Industrial and Manufacturing Engineering, Medan City North Sumatera, Indonesia, 16 October 2018. [Google Scholar] [CrossRef]
- Medeiros de Azevedo, W.; Ferreira Ribeiro de Oliveira, L.; Alves Alcântara, M.; Tribuzy de Magalhães Cordeiro, A.M.; da Silva, F.; Chaves Damasceno, K.S.; Kelly de Araújo, N. Physicochemical characterization, fatty acid profile, antioxidant activity and antibacterial potential of cacay oil, coconut oil and cacay butter. PLoS ONE 2020, 15, e0232224. [Google Scholar] [CrossRef]
- Bisht, V.; Neeraj; Solanki, V.K.; Dalal, N. Mahua an important Indian species: A review. J. Pharmacogn. Phytochem. 2018, 7, 3414–3418. [Google Scholar]
- Dalvi, T.S.; Kumbhar, U.J.; Shah, N. Madhuca longifolia: Ethanobotanical, phytochemical studies, pharmacological aspects with future prospects. Interdiscip. J. Appl. Basic Subj. 2022, 2, 1–9. [Google Scholar]
- Naik, B.; Kumar, V. Cocoa Butter and Its Alternatives: A Review. J. Bioresour. Eng. Technol. 2014, 1, 7–17. [Google Scholar]
- Mahajan, U.N.; Mahapatra, D.K.; Mahajan, N.M.; Kazi, F.S.; Baghel, N. Exploring the role of Mahua oil as potent emulsifier in cream formulations. Int. J. Herb. Med. 2017, 5, 93–97. [Google Scholar]
- Pawar, M.S.; Kadam, A.S.; Yemul, S.O. Development of polyether amide based corrosion protective polyurethane coating from mahua oil. Prog. Org. Coat. 2015, 89, 143–149. [Google Scholar] [CrossRef]
- Gandhi, S.S.; Gogate, P.R. Process intensification of fatty acid ester production using esterification followed by transesterification of high acid value mahua (lluppai ennai) oil: Comparison of the ultrasonic reactors. Fuel 2021, 294, 120560. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Keneni, Y.G. Review Oil extraction from plant seeds for biodiesel production. AIMS Energy 2017, 5, 316–340. [Google Scholar] [CrossRef]
- Kayode, B.; Hart, A. An overview of transesterification methods for producing biodiesel from waste vegetable oils. Biofuels 2017, 10, 419–437. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, V.; Sharma, Y.C. Methyl transesterification of waste cooking oil using a laboratory synthesized reusable heterogeneous base catalyst: Process optimization and homogeneity study of catalyst. Energy Convers. Manag. 2017, 148, 1438–1452. [Google Scholar] [CrossRef]
- Kumar, M.V.; Babu, A.V.; Kumar, P.R. Experimental investigation on mee methyl ester blended with diesel fuel in a compression ignition diesel engine. Int. J. Ambient. Energy 2017, 40, 304–316. [Google Scholar] [CrossRef]
- Manjunath, H.; Omprakash, H.B.; Hemachandra, R.K. Process optimization for biodiesel production from simarouba, mahua, and waste cooking oils. Int. J. Green. Energy 2015, 12, 424–430. [Google Scholar]
- Hegde, H.T.; Gunaga, R.P.; Thakur, N.S. Current Trends and Future Prospects for Utilization of Mahua Resources; Tropical Forest Research Institute: Padariya, India, 2019; Volume 6. [Google Scholar]
- Shrirao, A.V.; Koch, N.I.; Chandewar, A.V. Madhuca longifolia (Sapotaceae): A Review of its Traditional Uses and Phyto-Pharmacological Profile. Res. Chron. Health Sci. 2017, 3, 45–50. [Google Scholar]
- Reddy, I.S. Madhuca indica: An untapped forest tree for its medicinal uses. Pharma Innov. J. 2022, 11, 1747–1751. [Google Scholar]
- Janghel, V.; Chandel, S.S.; Sahu, J.; Patel, P.K. Madhuca indica (Mahua)—Pharmaceutical, Nutraceutical and Economical Importance for Tribal People of Chhattisgarh State. Int. J. Pharm. Phytopharm. Res. 2019, 9, 16–28. [Google Scholar]
- Ramchandra, D.; Gaikwad, M.D.; Liyaqat, A.M.D.; Swamy, S.K.P. Anti-inflammatory activity of Madhuca longifolia seeds saponin mixture. Pharm. Biol. 2009, 47, 592–597. [Google Scholar]
- Bhaumik, A.; Kumar, M.U.; Khan, K.A.; Srinivas, C.H. The Bioactive Compounds Obtained from the Fruit Seeds of Madhuca longifolia (L.) act as potential anticancer agents. Sch. J. App. Med. Sci. 2014, 2, 1235–1238. [Google Scholar]
- Withana, W.V.E.; Hapuarachchi, N.S.; Cooray, R.; Dissanayake, Y.; Warnakula, L. Importance of Genetic Diversity and Phytochemical Assessment of Madhuca spp. in Sri Lanka: A Review. In Proceedings of the International Research Conference, Uva Wellassa University, Badulla, Sri Lanka, 7–9 February 2019. [Google Scholar]









| Type of Oil/Fat | Saponification Value (mg KOH/g) | Iodine Value (gI2/100 g) | Acid Value (mg KOH/g) | Peroxide Value (meq/kg) | Melting Point °C | Smoke Point °C | Specific Gravity (at 25 °C) | Refractive Index (at 40 °C) |
|---|---|---|---|---|---|---|---|---|
| Mee | 181–184 | 56–57 | 4 | 3 | 33–34 | 168–171 | 0.9272 | 1.4672 |
| Coconut | 250–268 | 6–11 | 0.2 | 3 | 24 | 177 | 0.918 | 1.4486 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hippola, A.; Jayakodi, Y.; Gamage, A.; Madhujith, T.; Merah, O. Nutritional, Functional Properties and Applications of Mee (Madhuca longifolia) Seed Fat. Agronomy 2023, 13, 2445. https://doi.org/10.3390/agronomy13102445
Hippola A, Jayakodi Y, Gamage A, Madhujith T, Merah O. Nutritional, Functional Properties and Applications of Mee (Madhuca longifolia) Seed Fat. Agronomy. 2023; 13(10):2445. https://doi.org/10.3390/agronomy13102445
Chicago/Turabian StyleHippola, Asanthi, Yasasvi Jayakodi, Ashoka Gamage, Terrence Madhujith, and Othmane Merah. 2023. "Nutritional, Functional Properties and Applications of Mee (Madhuca longifolia) Seed Fat" Agronomy 13, no. 10: 2445. https://doi.org/10.3390/agronomy13102445
APA StyleHippola, A., Jayakodi, Y., Gamage, A., Madhujith, T., & Merah, O. (2023). Nutritional, Functional Properties and Applications of Mee (Madhuca longifolia) Seed Fat. Agronomy, 13(10), 2445. https://doi.org/10.3390/agronomy13102445









