Multi-Traits Selection of Maize Hybrids under Sole-Crop and Multiple-Crops with Soybean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Data Collection
2.4. Data Analysis
3. Results
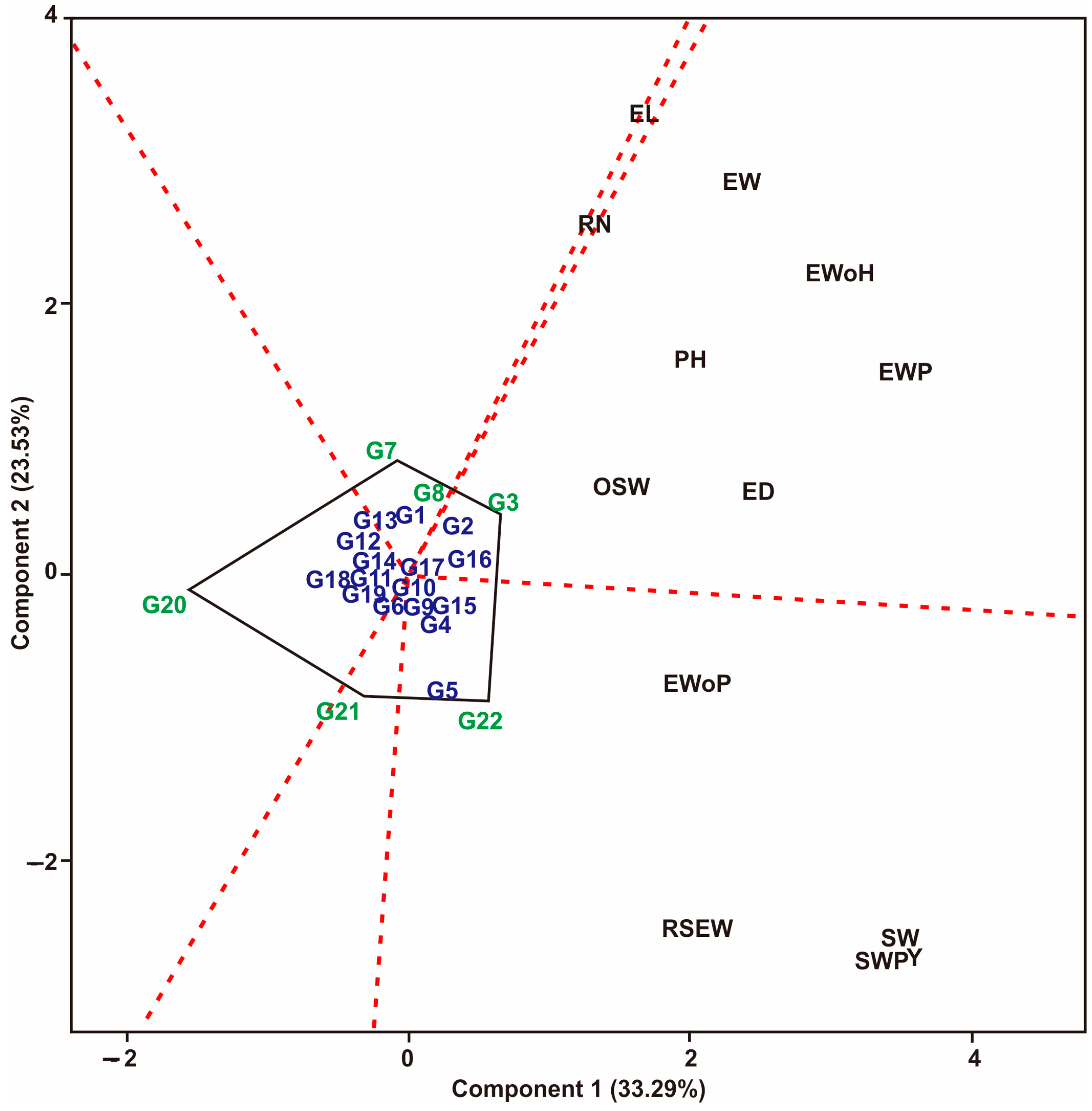
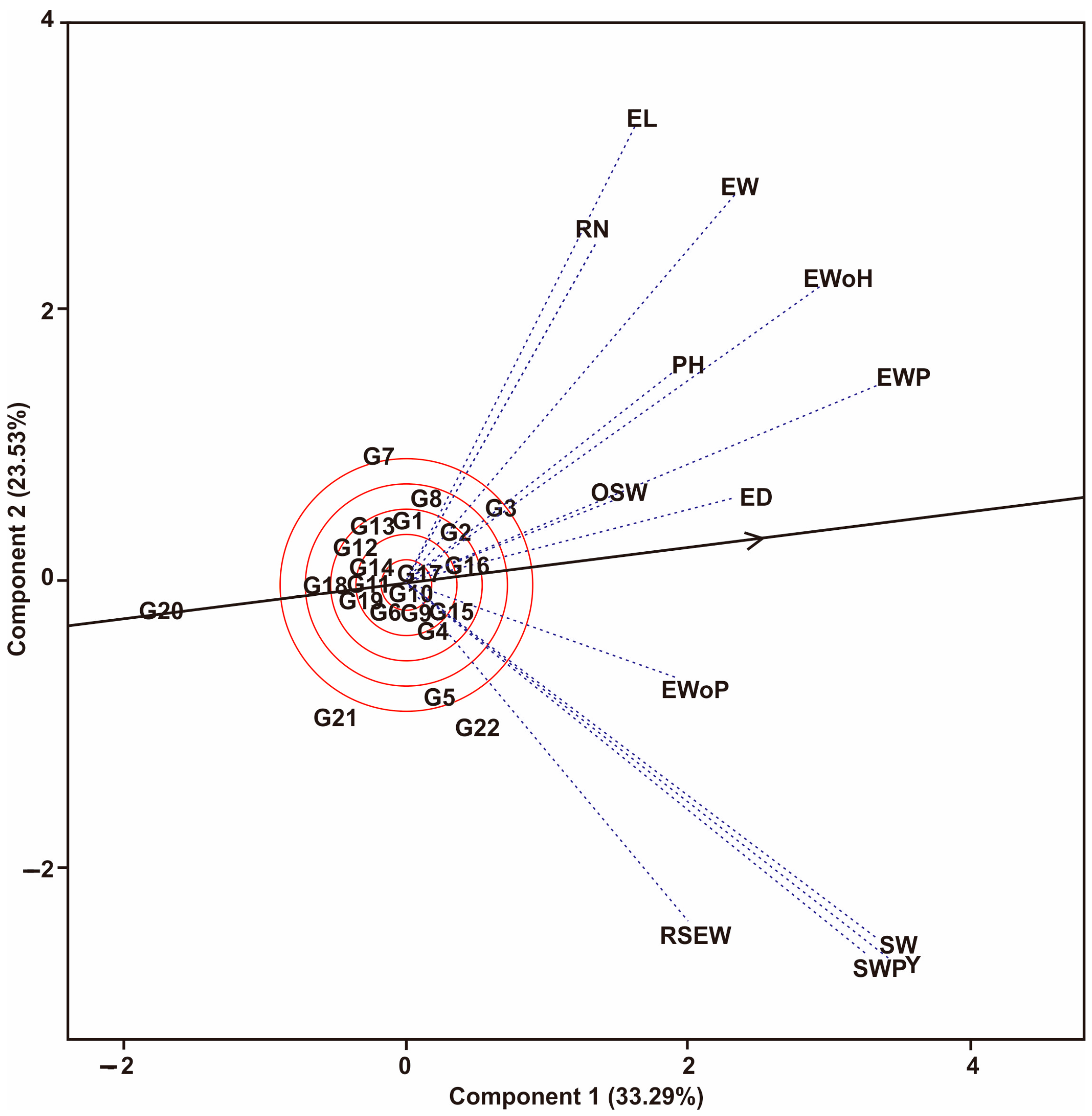
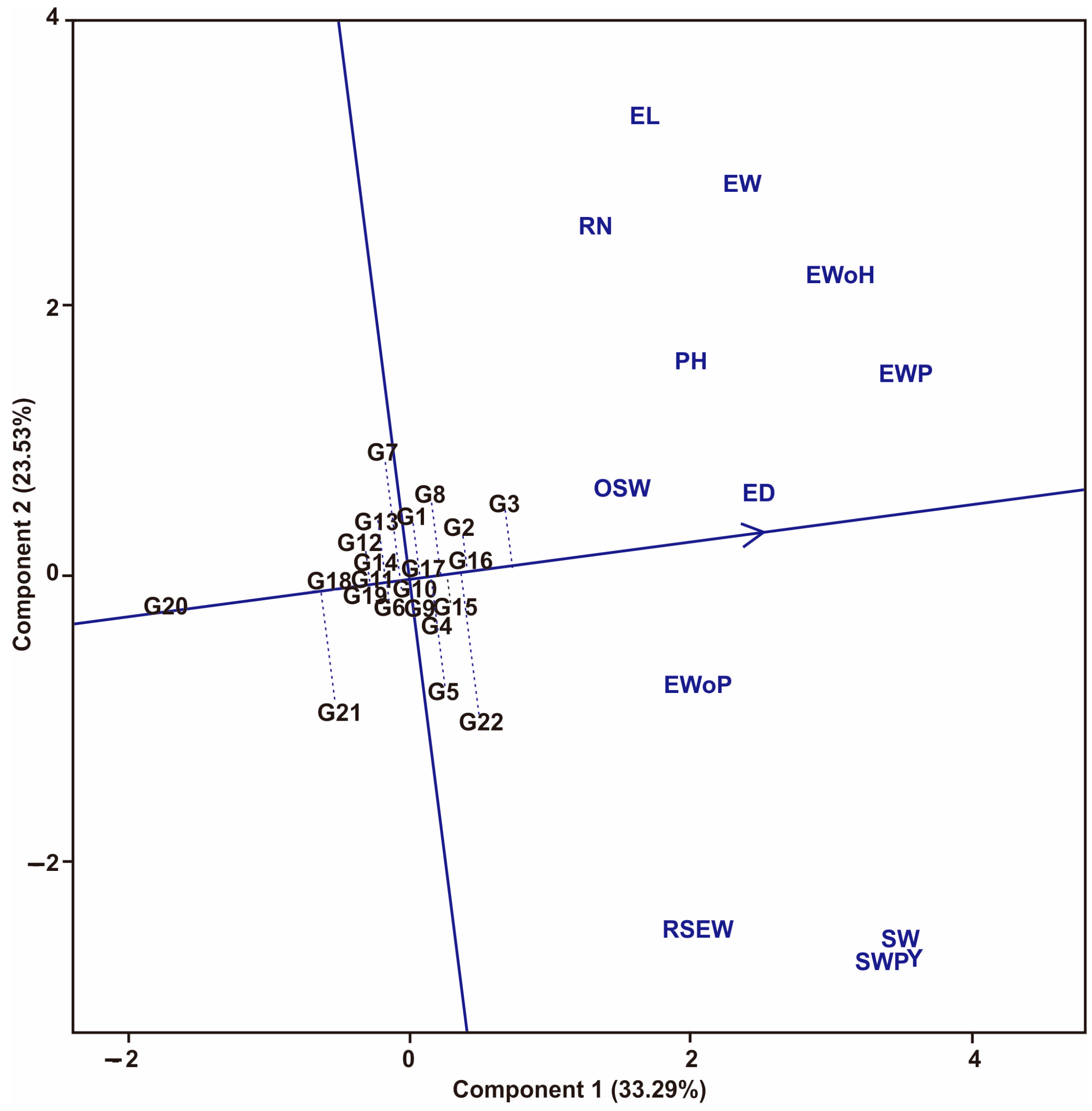
3.1. Multi-Trait Selection and Relationships between Traits Tested against Maize Hybrid Populations Genotype by Traits (GT) Biplot
3.2. Genotype-by-Environment Interactions (GEIs) on Maize Hybrid Yields
3.3. Yield Stability Based on Combined Stability Measurements on Maize Hybrid Populations
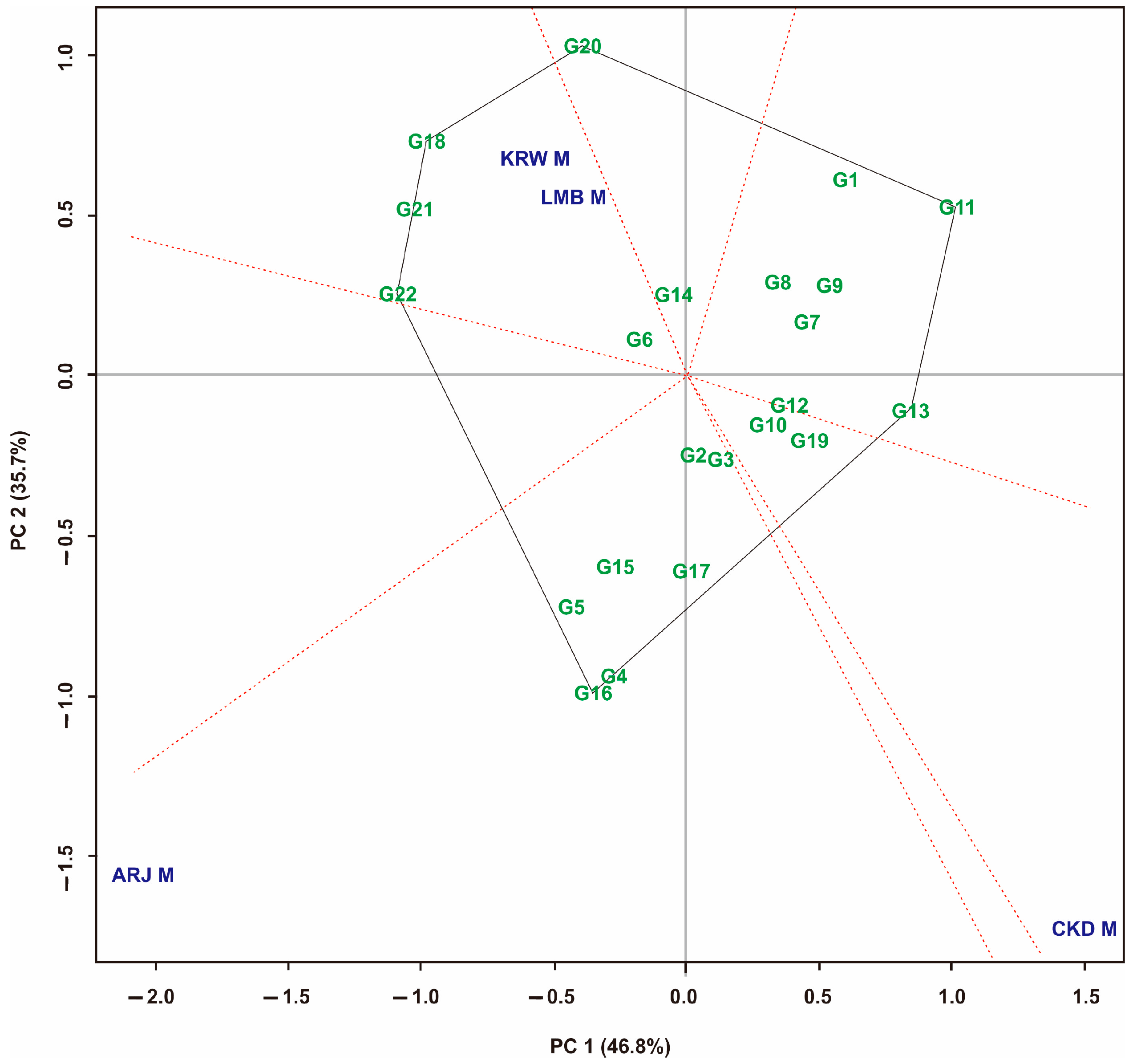
3.4. Yield Stability Based on GGE Biplot on Maize Hybrid Populations
3.5. Yield Stability Based on GGE Biplot on Maize Hybrid Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troyer, A.F.; Wellin, E.J. Heterosis decreasing in hybrids: Yield test inbreds. Crop Sci. 2009, 49, 1969–1976. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Bari, M.A.A.; Carena, M.J.; Messias, G. Identification of heterotic patterns between expired proprietary, NDSU, and industry short- season maize inbred lines. Crop Breed. Appl. Biotechnol. 2016, 16, 274–281. [Google Scholar] [CrossRef]
- Murdia, L.K.; Wadhwani, R.; Wadhawan, N.; Bajpai, P.; Shekhawat, S. Maize utilization in India: An overview. Am. J. Food Nutr. 2016, 4, 169–176. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Mushayi, M.; Shimelis, H.; Derera, J.; Shayanowako, A.I.T. Multi-environmental evaluation of maize hybrids developed from tropical and temperate lines. Euphytica 2020, 216, 84. [Google Scholar] [CrossRef]
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Setimela, P.S.; Magorokosho, C.; Lunduka, R.; Gasura, E.; Makumbi, D.; Tarekegne, A.; Cairns, J.E.; Ndhlela, T.; Erenstein, O.; Mwangi, W. On-farm yield gains with stress-tolerant maize in eastern and Southern Africa. Agron. J. 2017, 109, 406–417. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 1–17. [Google Scholar] [CrossRef]
- Maulana, H.; Maxiselly, Y.; Yuwariah, Y.; Dedi, R. Heritability and selection using GGE biplots and the Sustainability Index (SI) of maize mutants under different cropping systems in upland. Sustainability 2023, 15, 6824. [Google Scholar] [CrossRef]
- Nyoni, R.S.; Magorokosho, C.; Kamutando, C.N. Potential of temperate, tropical, and sub-tropical exotic maize germplasm for increased gains in yield performance in sub-tropical breeding programs. Agronomy 2023, 13, 1605. [Google Scholar] [CrossRef]
- Fan, X.M.; Yin, X.F.; Zhang, Y.D.; Bi, Y.Q.; Liu, L.; Chen, H.M.; Kang, M.S. Combining ability estimation for grain yield of maize exotic germplasm using testers from three heterotic groups. Crop Sci. 2016, 56, 2527–2535. [Google Scholar] [CrossRef]
- Habiba, R.M.M.; El-Diasty, M.Z.; Aly, R.S.H. Combining abilities and genetic parameters for grain yield and some agronomic traits in maize (Zea mays L.). Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 108. [Google Scholar] [CrossRef]
- Ruswandi, D.; Syafii, M.; Maulana, H.; Ariyanti, M.; Indriani, N.P.; Yuwariah, Y. GGE biplot analysis for stability and adaptability of maize hybrids in Western Region of Indonesia. Int. J. Agron. 2021, 2021, 2166022. [Google Scholar] [CrossRef]
- Elisha, I.; Lawrence, E.U.; Idoma, K. Adaptation to Climate Change through Multiple Cropping Systems in Sokoto Adaptation to Climate Change through Multiple Cropping Systems in Sokoto State. Savanna 2018, 24, 314–322. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.J.; Cano, M.E.C.; Parkes, B. The deployment of intercropping and agroforestry as adaptation to climate change. Crop Environ. 2022, 1, 145–160. [Google Scholar] [CrossRef]
- Liu, L.; Xu, X.; Zhuang, D.; Chen, X.; Li, S. Changes in the Potential Multiple Cropping System in Response to Climate Change in China from 1960–2010. PLoS ONE 2013, 8, e80990. [Google Scholar] [CrossRef]
- Wolde, L.; Keno, T.; Tadesse, B.; Bogale, G.; T/Wold, A.; Abebe, B. Mega-environment targeting of maize varieties using AMMI and GGE bi-plot analysis in Ethiopia. Ethiop. J. Agric. Sci. 2018, 28, 65–84. [Google Scholar]
- Pramitha, J.L.; Joel, J.; Rajasekaran, R.; Uma, D.; Vinothana, K.; Balakrishnan, M.; Sathyasheela, K.R.V.; Muthurajan, R.; Hossain, F. Stability Analysis and Heterotic Studies in Maize (Zea mays L.) Inbreds to Develop Hybrids With Low Phytic Acid and High-Quality Protein. Front. Plant Sci. 2022, 12, 781469. [Google Scholar] [CrossRef]
- Andrade, M.I.; Naico, A.; Ricardo, J.; Eyzaguirre, R.; Makunde, G.S.; Ortiz, R.; Gruneberg, W.J. Genotype x environment interaction and selection for drought adaptation in sweetpotato (Ipomoea batatas [L.] Lam.) in Mozambique. Euphytica 2016, 209, 261–280. [Google Scholar] [CrossRef]
- Kiliç, H.; Akçura, M.; Aktaş, H. Assessment of parametric and non-parametric methods for selecting stable and adapted durum wheat genotypes in multi-environments. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 271–279. [Google Scholar] [CrossRef]
- Gauch, H.G. A Simple Protocol for AMMI Analysis of Yield Trials. Crop Sci. 2013, 53, 1860–1869. [Google Scholar] [CrossRef]
- Yan, W. Biplot analysis of incomplete two-way data. Crop Sci. 2013, 53, 48–57. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Goksoy, A.T.; Sincik, M.; Erdogmus, M.; Ergin, M.; Aytac, S.; Gumuscu, G.; Gunduz, O.; Keles, R.; Bayram, G.; Senyingit, E. The parametric and non-parametric stability analyses for interpreting genotype by environment interaction of some soybean genotypes. Turkish J. F. Crop. 2019, 24, 28–38. [Google Scholar] [CrossRef]
- Cubukcu, P.; Kocaturk, M.; Ilker, E.; Kadiroglu, A.; Vurarak, Y.; Sahin, Y.; Karakus, M.; Yildirim, U.A.; Goksoy, A.T.; Sincik, M. Stability analysis of some soybean genotypes using parametric and non parametric methods in multi-environments. Turkish J. F. Crop 2021, 26, 262–271. [Google Scholar] [CrossRef]
- Abate, F.; Mekbib, F.; Dessalegn, Y. Association of Different Parametric and Non parametric Stability Models in Durum Wheat (Triticum turgidum Desf.) Genotypes. Int. J. Plant Soil Sci. 2015, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Akçura, M.; Kaya, Y.; Taner, S. Evaluation of durum wheat genotypes using parametric and nonparametric stability statistics. Turkish J. F. Crop. 2009, 14, 111–122. [Google Scholar]
- Maulana, H.; Nafi’ah, H.H.; Solihin, E.; Ruswandi, D.; Arifin, M.; Amien, S.; Karuniawan, A. Combined stability analysis to select stable and high yielding sweet potato genotypes in multi-environmental trials in West Java, Indonesia. Agric. Nat. Resour. 2022, 56, 761–772. [Google Scholar] [CrossRef]
- Khan, M.M.H.; Rafi, M.; Ramlee, S.I.; Jusoh, M.; Mamun, M.A. AMMI and GGE biplot analysis for yield performance and stability assessment of selected Bambara groundnut (Vigna subterranea L. Verdc.) genotypes under the multi-environmental trials (METs). Sci. Rep. 2021, 11, 22791. [Google Scholar] [CrossRef]
- Kebede, G.; Worku, W.; Jifar, H.; Feyissa, F. Grain yield stability analysis using parametric and nonparametricstatistics in oat (Avena sativa L.) genotypes in Ethiopia. Grass. Res. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Wicaksana, N.; Maulana, H.; Yuwariah, Y.; Ismail, A.; Ruswandi, Y.A.R.; Ruswandi, D. Selection of high yield and stable maize hybrids in mega-environments of Java island, Indonesia. Agronomy 2022, 12, 2923. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G.; Ierna, A. Dissecting the genotype × environment interaction for potato tuber yield and components. Agronomy 2023, 13, 101. [Google Scholar] [CrossRef]
- Yan, W.; Mitchell-Fetch, J.; Beattie, A.; Nilsen, K.T.; Pageau, D.; DeHaan, B.; Hayes, M.; Mountain, N.; Cummiskey, A.; MacEachern, D. Oat mega-environments in Canada. Crop Sci. 2021, 61, 1141–1153. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Majidi, M.M.; Saeidi, G.; Mirlohi, A.; Amiri, R.; Sorkhilalehloo, B. Application of GGE biplot to analyse stability of Iranian tall fescue (Lolium arundinaceum) genotypes. Crop Pasture Sci. 2015, 66, 963–972. [Google Scholar] [CrossRef]
- Yan, W.; Rajcan, I. Biplot analysis of test sites and trait relations of soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef]
- Eberhart, S.A.; Russell, W.A. Stability parameters for comparing varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Plaisted, R.L.; Peterson, L.C. A technique for evaluating the ability of selection to yield consistently in different locations or seasons. Am. Potato J. 1959, 36, 381–385. [Google Scholar] [CrossRef]
- Plaisted, R.L. A shorter method for evaluating the ability of selections to yield consistently over locations. Am. Potato J. 1960, 37, 166–172. [Google Scholar] [CrossRef]
- Wricke, G. Übereine Methode zur Erfassung der ökologischen Streubreite in Feldversuchen. Z. Für Pflanz. 1962, 47, 92–96. [Google Scholar]
- Shukla, G.K. Some statistical aspects of partitioning genotype-environmental components of variability. Heredity 1972, 29, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Francis, T.R.; Kannenberg, L.W. Yield stability studies in short-season maize: I. A descriptive method for grouping genotypes. Can. J. Plant Sci. 1978, 5, 1029–1034. [Google Scholar] [CrossRef]
- Nassar, R.; Huhn, M. Studies on Estimation of Phenotypic Stability: Tests of Significance for Nonparametric Measures of Phenotypic Stability. Biometrics 1987, 43, 45–53. [Google Scholar] [CrossRef]
- Huehn, M. Nonparametric measures of phenotypic stability. Part 1: Theory. Euphytica 1990, 47, 189–194. [Google Scholar] [CrossRef]
- Thennarasu, K. On Certain Non-Parametric Procedures for Studying Genotype-Environment Interactions and Yield Stability. Ph.D. Thesis, PJ School, IARI, New Delhi, India, 1995. [Google Scholar]
- Kang, M.S. A rank-sum method for selecting high-yielding, stable corn genotypes. Cereal Res. Commun. 1988, 16, 113–115. [Google Scholar]
- Pour-aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Poczai, P.; Siddique, K.H.M. STABILITYSOFT: A new online program to calculate parametric and non-parametric stability statistics for crop traits. Apllications Plant Sci. 2019, 7, 1–6. [Google Scholar] [CrossRef]
- Amien, S.; Maulana, H.; Ruswandi, D.; Nurjanah, S. Genetic gain and relationship of yield and yield attributes of mutant and cross-bred stevia (Stevia rebaudiana) genotypes. Biodiversitas 2021, 22, 3119–3126. [Google Scholar] [CrossRef]
- Kendal, E. Comparing durum wheat cultivars by genotype × yield × trait and genotype × trait biplot method. Chil. J. Agric. Res. 2019, 79, 512–522. [Google Scholar] [CrossRef]
- Yan, W.; Frégeau-reid, J. Genotype by yield*trait (GYT) biplot: A novel approach for genotype selection based on multiple traits. Sci. Rep. 2018, 8, 8242. [Google Scholar] [CrossRef]
- Mohammadi, R. Genotype by yield*trait biplot for genotype evaluation and trait profiles in durum wheat. Cereal Res. Commun. 2019, 47, 541–551. [Google Scholar] [CrossRef]
- Van Esse, H.P.; Reuber, T.L.; van der Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2020, 225, 70–86. [Google Scholar] [CrossRef]
- Oyekunle, M.; Haruna, A.; Badu-Apraku, B.; Usman, I.S.; Mani, H.; Ado, S.G.; Olaoye, G.; Obeng-Antwi, K.; Abdulmalik, R.O.; Ahmed, H.O. Assessment of early-maturing maize hybrids and testing sites using GGE biplot analysis. Crop Sci. 2017, 57, 2942–2950. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Bankole, F.A.; Ajayo, B.S.; Fakorede, M.A.B.; Akinwale, R.O.; Talabi, A.O.; Bandyopadhyay, R.; Ortega-Beltran, A. Identification of early and extra-early maturing tropical maize inbred lines resistant to Exserohilum turcicum in sub-Saharan Africa. Crop Prot. 2021, 139, 105386. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.E.; Akinwale, R.O.; Michel, S.; Burstmayr, H. Grain yield and stability of tropical maize hybrids developed from elite cultivars in contrasting environments under a rainforest agroecology. Euphytica 2020, 216, 89. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Magaji, U.; Miah, G.; Hussin, G.; Ramli, A. Genotype × environment interaction and stability analyses of yield and yield components of established and mutant rice genotypes tested in multiple locations in Malaysia. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 590–606. [Google Scholar] [CrossRef]
- Ikmal, A.M.; Noraziyah, A.A.S.; Ellina, Z.P.D.; Riana, T.A.T.N.A.; Amira, I.; Wickneswari, R.; Aisyah, Z.S. Genotype-by-environment interaction and stability snalysis of qDTYs pyramided rice (Oryza sativa) lines under water-Limited environments. Int. J. Agric. Biol. 2020, 24, 1835–1844. [Google Scholar] [CrossRef]
- Habtegebriel, M.H. Adaptability and stability for soybean yield by AMMI and GGE models in Ethiopia. Front. Plant Sci. 2022, 13, 950992. [Google Scholar] [CrossRef] [PubMed]
- Zdziarski, A.D.; Woyann, L.G.; Milioli, A.S.; Zanella, R.; Dallacorte, L.V.; Panho, M.C.; Benin, G. Mega-environment identification for soybean (Glycine max) breeding and production in Brazilian Midwest region. Plant Breed. 2019, 138, 336–347. [Google Scholar] [CrossRef]
- Vaezi, B.; Pour-Aboughadareh, A.; Mohammadi, R.; Mehraban, A.; Pour-Hossein, T.; Koohkan, E.; Gasemi, S.; Moradkhani, H.; Siddique, K.H.M. Integrating different stability models to investigate genotype x environment interactions and identify stable and high-yielding barley genotypes. Euphytica 2019, 215, 63. [Google Scholar] [CrossRef]
- Romão, R.F.; Nuvunga, J.J.; Silva, C.P.; Oliveira, L.A.; Mendes, C.T.E.; Balestre, M. Predictive ability of AMMI and factorial analytical models in the study of unbalanced multi-environment data. Genet. Mol. Res. 2019, 18, GMR18176. [Google Scholar] [CrossRef]
- Pham, H.; Reisner, J.; Swift, A.; Olafsson, S.; Vardeman, S. Crop phenotype prediction using biclustering to explain genotype-by-environment interactions. Front. Plant Sci. 2022, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.R.; Majidi, M.M.; Mirlohi, A.; Saeidi, G. Integrating parametric and non-parametric measures to investigate genotype 3 environment interactions in tall fescue. Euphytica 2016, 208, 583–596. [Google Scholar] [CrossRef]
- Rad, M.R.N.; Kadir, M.A.; Rafii, M.Y.; Jaafar, H.Z.E.; Naghavi, M.R.; Ahmadi, F. Genotype × environment interaction by AMMI and GGE biplot analysis in three consecutive generations of wheat (Triticum aestivum) under normal and drought stress conditions. Aust. J. Crop Sci. 2013, 7, 956–961. [Google Scholar]
- Tabu, H.I.; Tshiabukole, J.P.K.; Kankolongo, A.M.; Lubobo, A.K.; Kimuni, L.N. Yield stability and agronomic performances of provitamin A maize (Zea mays L.) genotypes in South-East of DR Congo. Open Agric. 2023, 8, 1–12. [Google Scholar] [CrossRef]
- Pobkhunthod, N.; Authapun, J.; Chotchutima, S.; Rungmekarat, S.; Kittipadakul, P.; Duangpatra, J.; Chaisan, T. Multilocation yield trials and yield stability evaluation by GGE biplot analysis of promising large-seeded peanut lines. Front. Genet. 2022, 13, 876763. [Google Scholar] [CrossRef]


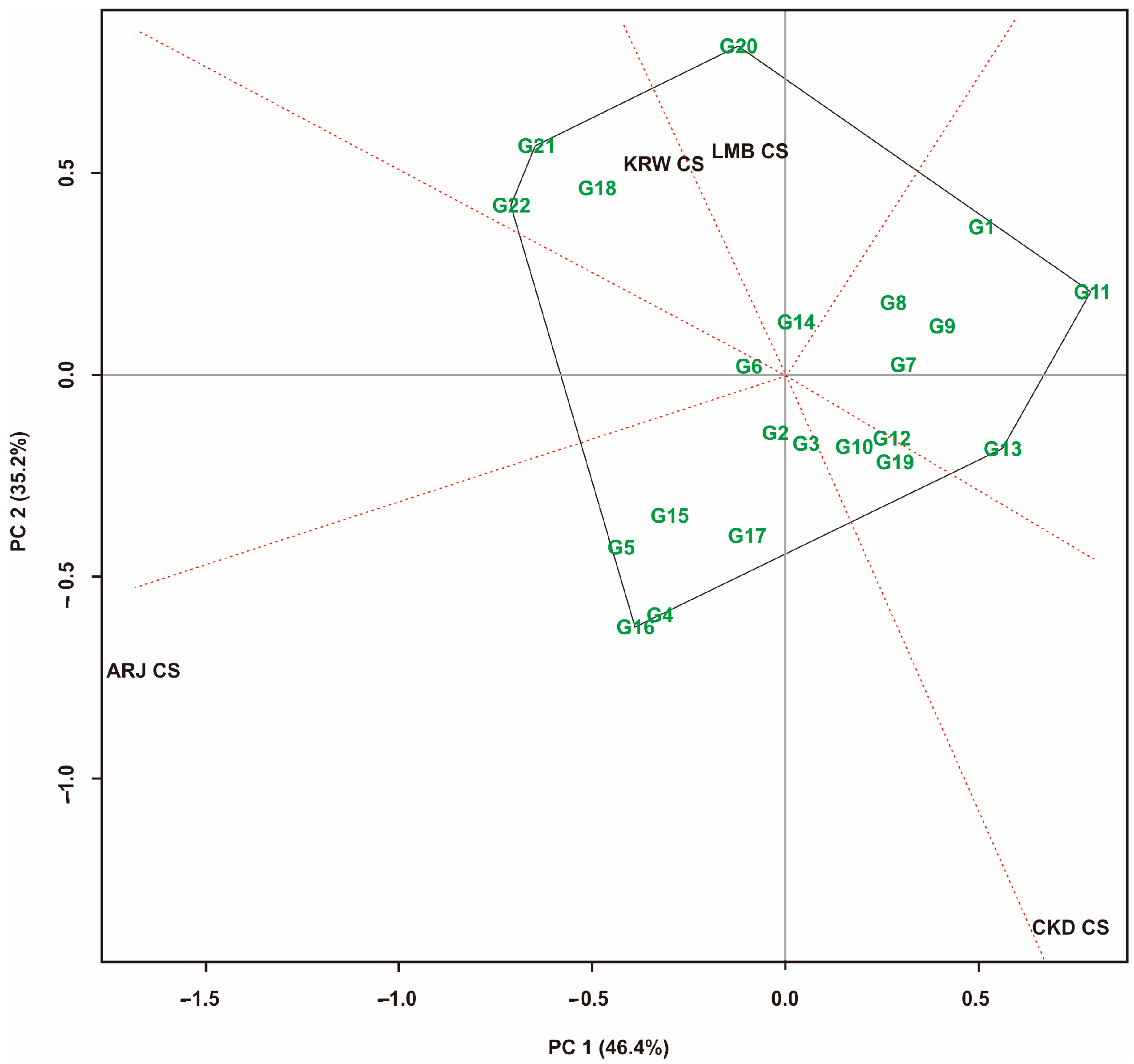
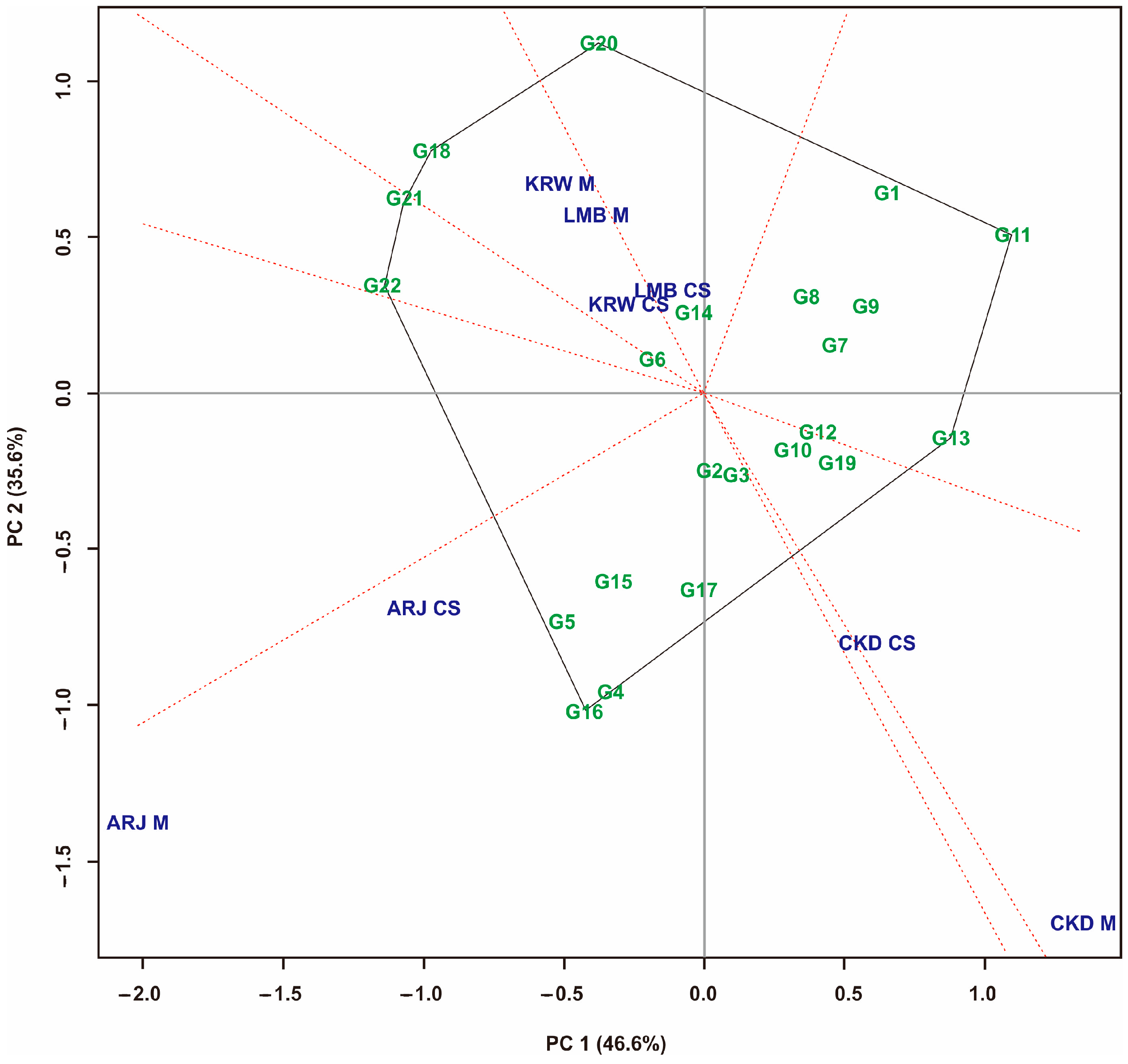
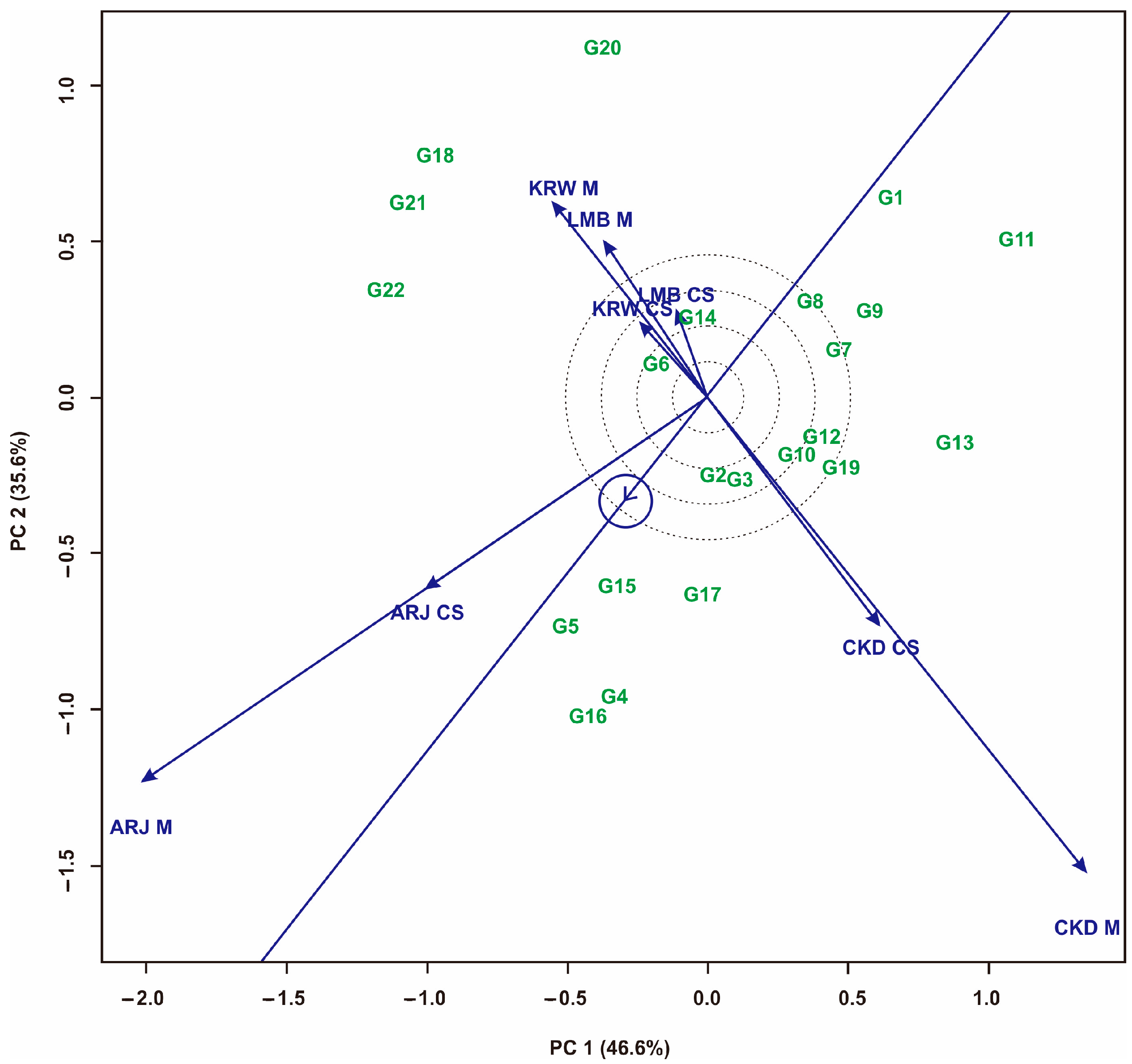
| No | Code | Maize Hybrids | Pedigree |
|---|---|---|---|
| 1 | G1 | DR 4 × MDR 7.2.3 | Female is a downy mildew resistant (DMR) line; male is a mutant line derived from DMR line |
| 2 | G2 | DR 4 × MDR 16.6.14 | Female is a DMR line; male is a mutant line derived from a high protein line |
| 3 | G3 | DR 5 × MDR 18.8.1 | Female is a DMR line; male is a mutant line derived from a high protein line |
| 4 | G4 | DR 6 × DR 7 | Female and male are DMR lines |
| 5 | G5 | DR 7 × DR 8 | Female is a DMR line; male is a high protein line |
| 6 | G6 | DR 8 × MDR 18.8.1 | Female is a high protein line; male is a mutant line derived from a high protein line |
| 7 | G7 | DR 8 × DR 9 | Female is a high protein line; male is a DMR line |
| 8 | G8 | DR 8 × MDR 1.1.3 | Female is a high protein line; male is a shading tolerant mutant |
| 9 | G9 | DR 10 × MDR 9.1.3 | Female is a high protein line; male is a shading tolerant mutant |
| 10 | G10 | DR 11 × DR 16 | Female is a DMR line; male is a high protein line |
| 11 | G11 | DR 14 × DR 18 | Female is a DMR line; male is a high protein line |
| 12 | G12 | DR 19 × DR 20 | Both female and male are high protein lines |
| 13 | G13 | MDR 3.1.4 × MDR 18.5.1 | Both female and male are shading tolerant mutants |
| 14 | G14 | MDR 3.1.2 × MDR 153.14.1 | Both female and male are shading tolerant mutants |
| 15 | G15 | MDR 7.4.3 × DR 18 | Female is a shading tolerant mutant and male is a high protein line |
| 16 | G16 | MDR 7.4.3 × MDR 18.8.1 | Female is a shading tolerant mutant; male is a mutant line derived from a high protein line |
| 17 | G17 | MDR 7.4.3 × MDR 1.1.3 | Both female and male are shading tolerant mutants |
| 18 | G18 | MDR 9.1.3 × MDR 1.1.3 | Both female and male are shading tolerant mutants |
| 19 | G19 | MDR 18.8.1 × MDR 7.1.9 | Both female and male are shading tolerant mutants |
| 20 | G20 | MDR 153.3.2 × MDR 8.5.3 | Both female and male are shading tolerant mutants |
| 21 | G21 | BR 154 × MDR 18.8.1 | Female is a DMR line; male is a high protein mutant |
| 22 | G22 | BR 154 × MDR 153.3.2 | Female is a DMR line; male is shading tolerant mutant |
| Arjasari | Cikajang | Karawang | Cikande | |
|---|---|---|---|---|
| Rainfall (mm) | 169.8 | 297.38 | 252.5 | 237.28 |
| Temperature (°C) | 24.58 | - | - | 24.23 |
| Humidity (%) | 91.25 | - | 83.7 | 78.53 |
| Source | df | SS | MS | %SS | |
|---|---|---|---|---|---|
| Genotypes | 21 | 61.40 | 2.92 | ** | 2.24 |
| Environments | 7 | 2348.70 | 335.53 | ** | 85.63 |
| Block | 16 | 24.70 | 1.54 | ** | |
| Interactions | 147 | 332.70 | 2.26 | ** | 12.13 |
| Total | 527 | 2858.40 | 5.42 | ||
| Error | 336 | 90.80 | 0.27 | ||
| Min (t·ha−1) | 1.37 | ||||
| Max (t·ha−1) | 11.11 | ||||
| Mean (t·ha−1) | 5.27 | ||||
| SD | 2.33 | ||||
| CV (%) | 9.87 |
| Hybrids | Y | S(1) | S(2) | S(3) | S(6) | NP(1) | NP(2) | NP(3) | NP(4) | Wi2 | σi2 | S²di | bi | CVi | θ(i) | θi | KR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | 4.30 | 8.79 | 54.79 | 35.67 | 4.28 | 6.50 | 0.62 | 0.69 | 0.82 | 3.50 | 0.53 | 0.49 | 0.94 | 36.08 | 0.34 | 0.45 | 37 |
| G2 | 4.54 | 3.93 | 11.43 | 7.62 | 2.00 | 2.25 | 0.22 | 0.27 | 0.37 | 0.36 | 0.04 | 0.05 | 0.98 | 32.33 | 0.37 | 0.21 | 10 |
| G3 | 4.53 | 6.04 | 26.41 | 14.36 | 2.56 | 5.63 | 0.29 | 0.48 | 0.47 | 0.90 | 0.12 | 0.11 | 1.09 | 36.41 | 0.36 | 0.25 | 14 |
| G4 | 4.75 | 9.50 | 65.93 | 36.20 | 4.55 | 7.25 | 0.59 | 0.63 | 0.75 | 3.82 | 0.58 | 0.51 | 1.12 | 37.90 | 0.34 | 0.47 | 23 |
| G5 | 4.96 | 4.25 | 18.13 | 7.52 | 1.27 | 4.00 | 0.51 | 0.30 | 0.25 | 1.20 | 0.17 | 0.15 | 1.10 | 33.58 | 0.36 | 0.28 | 10 |
| G6 | 4.47 | 5.25 | 19.27 | 13.32 | 2.67 | 3.38 | 0.32 | 0.41 | 0.52 | 0.34 | 0.04 | 0.05 | 0.99 | 32.94 | 0.37 | 0.21 | 13 |
| G7 | 4.08 | 6.21 | 30.21 | 31.33 | 4.89 | 6.25 | 1.14 | 1.04 | 0.92 | 1.78 | 0.26 | 0.12 | 0.75 | 28.34 | 0.36 | 0.32 | 33 |
| G8 | 4.38 | 4.82 | 16.27 | 10.98 | 2.60 | 4.25 | 0.33 | 0.48 | 0.46 | 0.94 | 0.13 | 0.12 | 0.92 | 31.89 | 0.36 | 0.26 | 24 |
| G9 | 4.36 | 6.00 | 25.93 | 18.62 | 3.08 | 4.63 | 0.47 | 0.58 | 0.62 | 1.69 | 0.25 | 0.19 | 0.85 | 30.47 | 0.36 | 0.31 | 29 |
| G10 | 4.42 | 3.29 | 8.79 | 7.03 | 2.11 | 2.63 | 0.23 | 0.40 | 0.38 | 0.32 | 0.03 | 0.05 | 1.01 | 33.95 | 0.37 | 0.21 | 14 |
| G11 | 4.48 | 8.93 | 68.29 | 34.14 | 3.71 | 5.50 | 0.34 | 0.54 | 0.64 | 4.71 | 0.72 | 0.65 | 0.90 | 34.77 | 0.34 | 0.54 | 30 |
| G12 | 4.39 | 5.36 | 21.64 | 14.09 | 2.93 | 4.63 | 0.47 | 0.50 | 0.50 | 0.94 | 0.13 | 0.13 | 1.00 | 34.75 | 0.36 | 0.26 | 24 |
| G13 | 4.34 | 9.00 | 64.50 | 46.31 | 5.28 | 6.25 | 0.98 | 0.76 | 0.92 | 3.27 | 0.50 | 0.47 | 0.98 | 37.01 | 0.35 | 0.43 | 34 |
| G14 | 4.47 | 4.86 | 19.07 | 12.42 | 2.33 | 3.38 | 0.33 | 0.40 | 0.45 | 0.49 | 0.06 | 0.07 | 1.04 | 34.69 | 0.37 | 0.22 | 16 |
| G15 | 4.77 | 6.46 | 30.84 | 17.44 | 2.61 | 4.75 | 0.47 | 0.51 | 0.52 | 1.91 | 0.28 | 0.27 | 1.04 | 34.15 | 0.36 | 0.33 | 17 |
| G16 | 4.79 | 9.79 | 77.14 | 37.24 | 3.79 | 7.25 | 0.50 | 0.56 | 0.67 | 6.48 | 1.00 | 0.74 | 1.29 | 43.72 | 0.32 | 0.67 | 24 |
| G17 | 4.49 | 6.61 | 30.98 | 21.42 | 3.85 | 5.38 | 0.44 | 0.54 | 0.65 | 1.24 | 0.18 | 0.18 | 1.02 | 34.73 | 0.36 | 0.28 | 19 |
| G18 | 4.42 | 9.86 | 72.29 | 37.48 | 3.93 | 6.63 | 0.45 | 0.60 | 0.73 | 6.52 | 1.01 | 0.93 | 0.95 | 38.56 | 0.32 | 0.67 | 36 |
| G19 | 4.41 | 6.18 | 27.98 | 20.35 | 3.43 | 5.38 | 0.41 | 0.61 | 0.64 | 0.86 | 0.12 | 0.12 | 1.05 | 35.90 | 0.36 | 0.25 | 20 |
| G20 | 3.90 | 6.00 | 27.36 | 28.37 | 4.74 | 6.00 | 1.41 | 1.01 | 0.89 | 3.41 | 0.52 | 0.47 | 0.91 | 38.78 | 0.35 | 0.44 | 38 |
| G21 | 4.62 | 8.07 | 57.43 | 26.80 | 3.20 | 4.50 | 0.29 | 0.47 | 0.54 | 4.14 | 0.63 | 0.59 | 0.98 | 35.45 | 0.34 | 0.50 | 25 |
| G22 | 4.89 | 8.00 | 57.14 | 25.00 | 3.00 | 4.38 | 0.37 | 0.35 | 0.50 | 3.11 | 0.47 | 0.42 | 1.10 | 35.65 | 0.35 | 0.42 | 16 |
| Genotype | Y | S(1) | S(2) | S(3) | S(6) | NP(1) | NP(2) | NP(3) | NP(4) | Wi2 | σi2 | S²di | bi | CVi | θ(i) | θi | KR | SR | AR | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | 20 | 17 | 15 | 18 | 18 | 19 | 19 | 19 | 19 | 17 | 17 | 17 | 12 | 16 | 17 | 6 | 21 | 287 | 16.88 | 3.38 |
| G2 | 7 | 2 | 2 | 3 | 2 | 1 | 1 | 1 | 2 | 3 | 3 | 3 | 6 | 4 | 3 | 20 | 1 | 64 | 3.76 | 4.37 |
| G3 | 8 | 10 | 9 | 8 | 5 | 15 | 4 | 9 | 6 | 6 | 6 | 5 | 15 | 17 | 6 | 17 | 4 | 150 | 8.82 | 4.34 |
| G4 | 5 | 20 | 19 | 19 | 19 | 21 | 18 | 18 | 18 | 18 | 18 | 18 | 19 | 19 | 18 | 5 | 11 | 283 | 16.65 | 4.69 |
| G5 | 1 | 3 | 4 | 2 | 1 | 5 | 17 | 2 | 1 | 9 | 9 | 10 | 17 | 6 | 9 | 14 | 1 | 111 | 6.53 | 5.37 |
| G6 | 11 | 6 | 6 | 6 | 8 | 3 | 5 | 6 | 9 | 2 | 2 | 2 | 3 | 5 | 2 | 21 | 3 | 100 | 5.88 | 4.57 |
| G7 | 21 | 12 | 12 | 16 | 21 | 17 | 21 | 22 | 21 | 12 | 12 | 6 | 21 | 1 | 12 | 11 | 18 | 256 | 15.06 | 5.85 |
| G8 | 17 | 4 | 3 | 4 | 6 | 6 | 7 | 8 | 5 | 7 | 7 | 8 | 13 | 3 | 7 | 16 | 12 | 133 | 7.82 | 4.12 |
| G9 | 18 | 8 | 8 | 10 | 11 | 9 | 15 | 15 | 12 | 11 | 11 | 12 | 20 | 2 | 11 | 12 | 16 | 201 | 11.82 | 4.08 |
| G10 | 13 | 1 | 1 | 1 | 3 | 2 | 2 | 5 | 3 | 1 | 1 | 1 | 2 | 7 | 1 | 22 | 4 | 70 | 4.12 | 5.38 |
| G11 | 10 | 18 | 20 | 17 | 14 | 14 | 8 | 12 | 13 | 20 | 20 | 20 | 18 | 12 | 20 | 3 | 17 | 256 | 15.06 | 4.81 |
| G12 | 16 | 7 | 7 | 7 | 9 | 9 | 13 | 10 | 7 | 8 | 8 | 9 | 1 | 11 | 8 | 15 | 12 | 157 | 9.24 | 3.40 |
| G13 | 19 | 19 | 18 | 22 | 22 | 17 | 20 | 20 | 22 | 15 | 15 | 15 | 4 | 18 | 15 | 8 | 19 | 288 | 16.94 | 4.68 |
| G14 | 12 | 5 | 5 | 5 | 4 | 3 | 6 | 4 | 4 | 4 | 4 | 4 | 8 | 9 | 4 | 19 | 6 | 106 | 6.24 | 3.89 |
| G15 | 4 | 13 | 13 | 9 | 7 | 11 | 14 | 11 | 10 | 13 | 13 | 13 | 9 | 8 | 13 | 10 | 8 | 179 | 10.53 | 2.68 |
| G16 | 3 | 21 | 22 | 20 | 15 | 21 | 16 | 14 | 16 | 21 | 21 | 21 | 22 | 22 | 21 | 2 | 12 | 290 | 17.06 | 6.14 |
| G17 | 9 | 14 | 14 | 12 | 16 | 12 | 11 | 13 | 15 | 10 | 10 | 11 | 5 | 10 | 10 | 13 | 9 | 194 | 11.41 | 2.59 |
| G18 | 14 | 22 | 21 | 21 | 17 | 20 | 12 | 16 | 17 | 22 | 22 | 22 | 11 | 20 | 22 | 1 | 20 | 300 | 17.65 | 5.44 |
| G19 | 15 | 11 | 11 | 11 | 13 | 12 | 10 | 17 | 14 | 5 | 5 | 7 | 10 | 15 | 5 | 18 | 10 | 189 | 11.12 | 3.89 |
| G20 | 22 | 8 | 10 | 15 | 20 | 16 | 22 | 21 | 20 | 16 | 16 | 16 | 14 | 21 | 16 | 7 | 22 | 282 | 16.59 | 4.67 |
| G21 | 6 | 16 | 17 | 14 | 12 | 8 | 3 | 7 | 11 | 19 | 19 | 19 | 7 | 13 | 19 | 4 | 15 | 209 | 12.29 | 5.41 |
| G22 | 2 | 15 | 16 | 13 | 10 | 7 | 9 | 3 | 8 | 14 | 14 | 14 | 16 | 14 | 14 | 9 | 6 | 184 | 10.82 | 4.33 |
| Stability Measurements | Stable High Yield | Unstable High Yield |
|---|---|---|
| Combined stability | G2, G5 | G22, G17, G15, G19, G3, G16, G4 |
| GGE biplot | G2, G3, G10, G12, G14 | G4, G16, G18, G20 |
| Slice | G2 | G4, G16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruswandi, D.; Maulana, H.; Karuniawan, A.; Mansyur; Ismail, A.; Maxiselly, Y.; Fauzan, M.R.; Abdullah, M.A.; Yuwariah, Y. Multi-Traits Selection of Maize Hybrids under Sole-Crop and Multiple-Crops with Soybean. Agronomy 2023, 13, 2448. https://doi.org/10.3390/agronomy13102448
Ruswandi D, Maulana H, Karuniawan A, Mansyur, Ismail A, Maxiselly Y, Fauzan MR, Abdullah MA, Yuwariah Y. Multi-Traits Selection of Maize Hybrids under Sole-Crop and Multiple-Crops with Soybean. Agronomy. 2023; 13(10):2448. https://doi.org/10.3390/agronomy13102448
Chicago/Turabian StyleRuswandi, Dedi, Haris Maulana, Agung Karuniawan, Mansyur, Ade Ismail, Yudithia Maxiselly, Mochamad Rafi Fauzan, Muhammad Ali Abdullah, and Yuyun Yuwariah. 2023. "Multi-Traits Selection of Maize Hybrids under Sole-Crop and Multiple-Crops with Soybean" Agronomy 13, no. 10: 2448. https://doi.org/10.3390/agronomy13102448
APA StyleRuswandi, D., Maulana, H., Karuniawan, A., Mansyur, Ismail, A., Maxiselly, Y., Fauzan, M. R., Abdullah, M. A., & Yuwariah, Y. (2023). Multi-Traits Selection of Maize Hybrids under Sole-Crop and Multiple-Crops with Soybean. Agronomy, 13(10), 2448. https://doi.org/10.3390/agronomy13102448










