Abstract
Interspecific hybridization between commercial and wild canes followed by backcrossing may transfer favorable alleles responsible for drought tolerance in sugarcane. Our study aimed to assess the distribution of BC1 individuals on leaf anatomy and to classify them regarding heterosis values. Five BC1 populations were established using a commercial Saccharum spp. hybrid as a donor female and the F1 interspecific hybrids as recurrent males. Leaf anatomy included leaf thickness (LT), cuticle thickness (CT), the vertical length of bulliform cell (VBC), stomatal crypt depth (SCD), percent CT, percent VBC, and percent SCD. The anatomical traits of BC1 showed high phenotypic variations, and all populations can be divided into three groups based on their heterosis values. Heterosis seemed to be genotype and trait dependent as the estimates varied considerably across populations and observed traits, ranging from negative on LT to positive on VBC. Group I (BC1-1) showed positive heterosis on percent CT, percent VBC, and percent SCD. Dendrogram analysis revealed that some clones in population BC1-1 were promising regarding stalk weight and leaf anatomy, making them desirable for further clone selections. Backcrossing with commercial canes resulted in higher BC1 means than their mid-parents despite low heterosis on leaf anatomy.
1. Introduction
Sugarcane is an important vegetatively propagated crop in tropical and sub-tropical regions for dual purposes, namely sugar and energy [1]. While sugarcane (Saccharum officinarum L.) dominates the global sugar industry by accounting for 75%, it also contributes to the energy and fuel sectors by increasing the productions of ethanol and their derivatives, accounting for 40% of the world production [2]. In a global perspective, Thailand ranks fourth and second for sugar production and sugar export, respectively [3]. In domestic perspective, sugarcane ranks third, after rice and natural rubber, among 11 major crops for total harvested areas by 2019 [4]. Although sugarcane plays important roles in Thai agriculture and the Thai economy, it does come with challenges. Pipitpukdee et al. [5] projected that future sugarcane yield and harvested area will drop by 33% and 3%, respectively, in Thailand during the period 2046–2055 due to climatic change and reduced agricultural land areas.
Plant growth and crop productivity can be adversely affected by various environmental stresses. Water deficit, due to unpredictable rainfall, has emerged as one of the most significant constraints for sugarcane growth, development, and biomass [6,7,8]. Drought stress limits the expansion of sugarcane leaves, photosynthesis, the levels of nitrogen uptake, and other related physiological processes, resulting in severe losses of sugar yield [9]. Several approaches are proposed to mitigate those adverse effects including genetic and agronomic improvements. Genetic improvements through conventional and molecular breeding are achievable if the targeted traits are heritable [10]. Through the breeding approach, modern sugarcane cultivars that are adaptive and high-yielding can be achieved and may appeal to the preferences of farmers and industries. In sugarcane, interspecific hybridization is commonly used to incorporate favorable alleles from the wild species into commercial sugarcane, and significant yield improvements have been noticed [11,12,13]. While Saccharum officinarum carries hardiness, disease resistance, and ratooning abilities, S. spontaneum has favorable sugar content [14]. In addition, the wild type is a potential genetic stock for drought-tolerant properties that are lacking in commercial sugarcane cultivars [15]. However, in some cases, the F1 hybrids derived from interspecific crosses between wild-type (S. spontaneum) and commercial sugarcane (S. officinarum) still produce low to moderate sugar content; therefore, backcrossing with commercial canes may enhance the genetic gains and phenotypic variabilities of sugarcane on yield and sugar [13,16]. Backcrossing allows breeders to transfer desirable traits from a donor parent into the favored genetic background of a recurrent parent [17].
Leaf structure has direct effects on leaf photosynthesis. It is essential to each phase, from the light interception to the metabolic fixation of carbon dioxide, wherein leaf anatomy is a key factor in determining leaf photosynthesis and gas exchange [18]. When abiotic stresses, such as drought or water deficit, occur, some parameters of leaf structure including cuticles, bulliform cells, and stomatal crypts will play essential roles to mitigate those unfavorable conditions. The cuticle layer is mainly composed of wax and cutin [19], developed at early embryo development, maintained during plant growth and development [20], and commonly concentrated in the aerial organs of plants [21]. It acts as a hydrophobic barrier against transpirational water loss [19]. While thicker cuticles lead to lower rates of water loss and improve the water use efficiency under drought conditions [22], thinner cuticles allow rapid releases of water vapor through the stomata and increase the transpiration rate [23]. Bulliform cells are a set of large, thin-walled, vacuolated cells and are located at vascular bundles adjacent to the midrib on the adaxial leaf [24]. Under water deficit conditions, there is a loss of turgor pressure, and the bulliform cells are deflating, inducing leaf rolling as a plant mechanism to avoid excessive sunlight exposure, stomatal conductance, transpiration rate, and water loss [25]. When the drought is relieved, the bulliform cells are expanded to flatten the leaves by maintaining turgor pressure [26]. Stomata facilitates gas exchange between leaves and the atmosphere through depressed epidermal areas called stomatal crypts [27]. Stomata crypts play crucial roles in water retention and storage, transpiration, and photosynthesis, especially in plants with high stomatal conductance [27,28].
The properties of leaf anatomy may depend upon sugarcane genetic backgrounds, making them useful in line selections for stress-tolerant cultivars [29]. Previous studies reported that anatomical responses to drought were associated with increases in bulliform cells, lamina thickness [29,30], stomatal crypt depths, and cuticle thickness [31]. Jumkudling et al. [31] investigated the diversity and distribution of leaf anatomy among F1 individuals derived from interspecific hybridization between Saccharum spp. hybrid and S. spontaneum. They found that approximately 47%, 39%, and 39% of 23 F1 clones had high stomatal crypt depths, small bulliform cells, and high cuticle thickness, respectively [31]. Those anatomical properties can be applied in breeding programs to select drought-tolerant parents for their plasticity under drought stress conditions [29]. In our present study, we performed a backcross (BC1) between S. spontaneum × S. officinarum F1 hybrids and S. officinarum parent. It is interesting to investigate whether the backcrossing method is effective to increase the diversity of BC1 individuals within populations and the relative performance of BC1 individuals to their respective parents on given leaf anatomical traits. While the presence of heterosis or hybrid vigor has been well documented on cross-pollinated crops like maize [32,33,34], only few studies are available on sugarcane [35]. Therefore, this study aimed to assess the diversity and distribution of BC1 individuals within each population on anatomical traits and to classify each backcrossing population regarding their heterosis values on given traits. The information obtained in this study may help breeders to apply a backcrossing method in developing superior sugarcane genotypes with high adaptability and yield in targeted environments.
2. Materials and Methods
2.1. Plant Materials
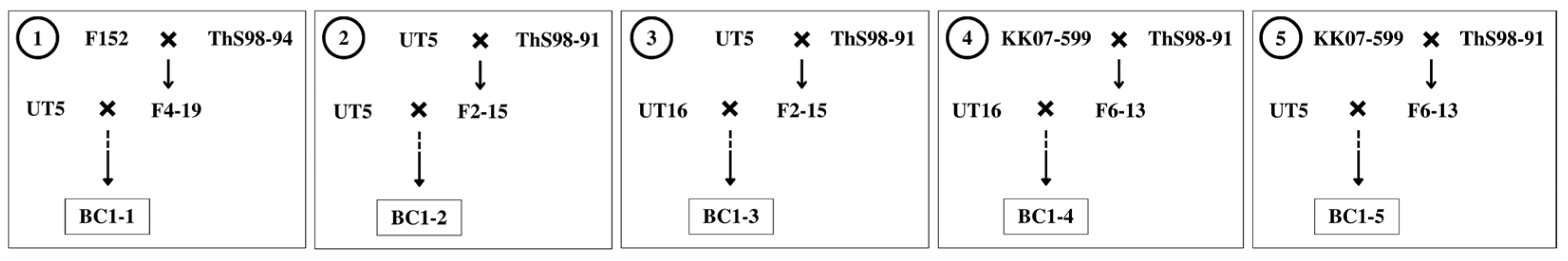
Two commercial cane genotypes UT5 and UT16 were assigned as donor parents because they possessed high sugar content and yield (Table 1). Five populations of interspecific hybrid backcross 1 (BC1) used in this study were labelled as BC1-1, BC1-2, BC1-3, BC1-4, and BC1-5 (Figure 1). While three populations, BC1-1, BC1-2, and BC1-5, had genotype UT5 as a donor female parent, the other two populations, BC1-3 and BC1-4, had genotype UT16 as the donor female. The F1 hybrids were previously developed by the Department of Agriculture (DOA) Thailand utilizing two S. spontaneum genotypes ThS98-91 and ThS98-94 as founder male parents. Those wild types showed high fiber, tiller number, and stalks per clump, but they had low sweetness and tiny stalk diameter.

Table 1.
List of sugarcane genotypes used in this study.
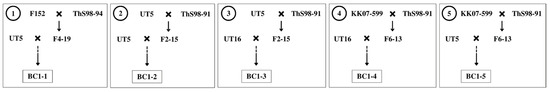
Figure 1.
Mating schemes of five sugarcane interspecific hybrids backcross 1 (BC1) populations between commercial cane (Saccharum officinarum) and wild type (Saccharum spontaneum).
2.2. Experimental Design and Cultural Practices
The experiment was conducted under field conditions at the Agronomy Research Station, Khon Kaen University, Thailand (16°28′ N, 102°48′ E, 200 m above sea level) during July of 2021 to March of 2022. Sugarcane seedlings were prepared in plastic bags. Only healthy seedlings without off-types were selected and transplanted into the field plot at 45 days after planting (DAP). The soil type was Yasothon series (fine-loamy; siliceous, isohypothermic, Oxic Paleustults). The planting plot was prepared by digging the planting holes with the plant spacing of 1.5 m between rows and 0.5 m between plants [36]. The water regime was maintained at the normal level using a drip irrigation system, especially during seedling and early vegetative stages to ensure optimum growth. A mixed chemical fertilizer with the formula 47 kg N, 47 kg P2O5, and 47 kg K2O ha−1 was applied twice, namely before planting and at 3 months after planting (MAP). At early vegetative stage, weed controls were carried out through two methods including a small, multi-purpose soil tillage machine and free-hand weeding. Both pests and diseases were controlled if only exceeding the economic injury level (EIL) [37].
2.3. Data Collection
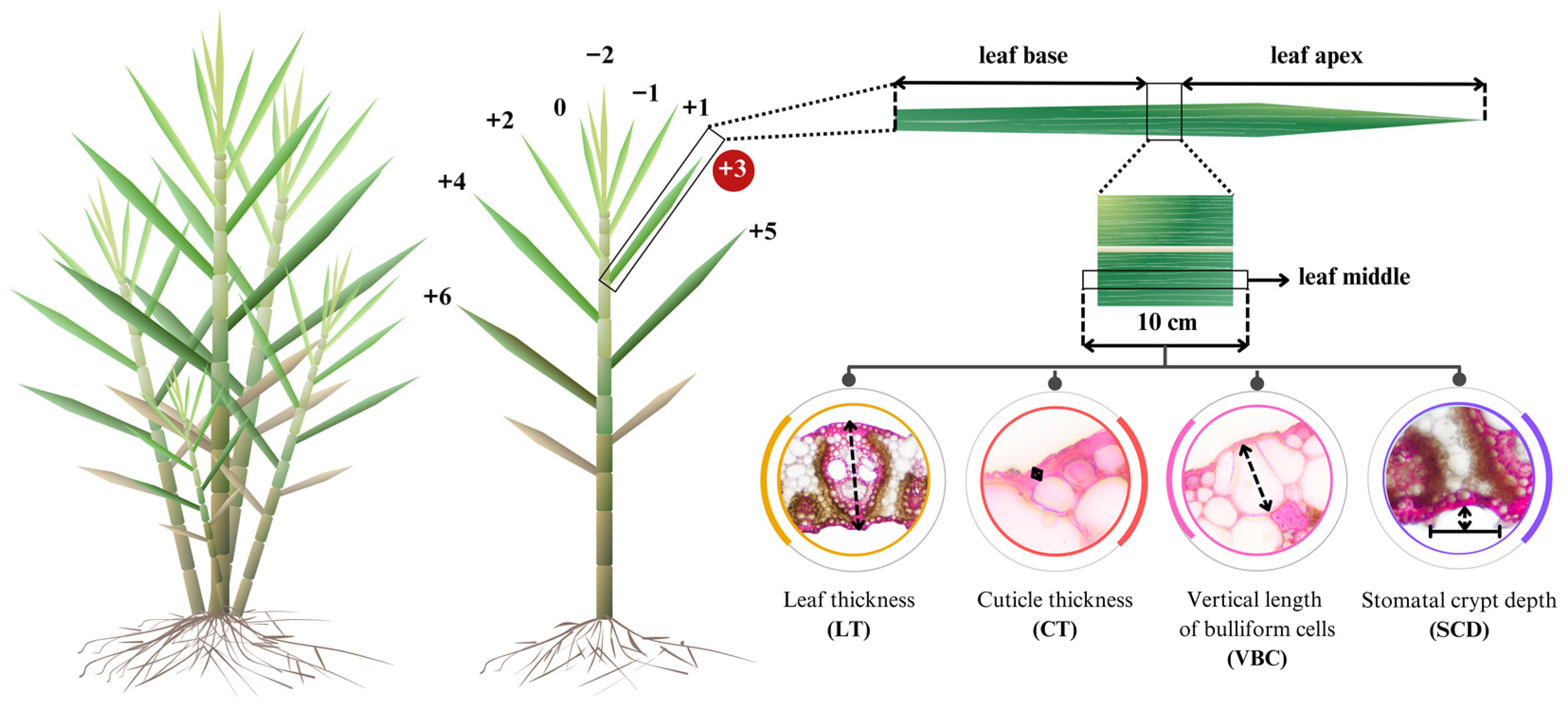
The procedures of sample preparation and phenotyping for anatomical traits followed Taratima et al. [29]. The third leaf of the main shoot, the first tiller, and the second tiller were collected at 7 MAP (Figure 2). Three replicates per sample were performed. The leaf length was measured. Each leaf sample was cut in the middle part (10 cm), and the samples were then immediately soaked in 100 mL of 70% ethyl alcohol for 48 h to maintain and stabilize the cells for anatomical studies. Then, the middle part of each leaf sample was selected and dissected into a cross-section as thin as possible. After that, the tissue was placed on the slide and dyed with 1% (w/v) Safranin O before dehydration by serial ethyl alcohol and xylene and mounted using DePeX. Anatomical properties were observed and measured using a light compound microscope (Olympus BH-2, Olympus America Inc., Melville, NY, USA) and a Zeiss 540214-0000004 using the MB2004 configuration AxioVision program (Carl Zeiss Microimaging Inc., Thornwood, NY, USA) with a magnification of 10×. The measurements included leaf thickness (LT), cuticle thickness (CT), the vertical length of bulliform cell (VBC), and stomatal crypt depth (SCD). In addition, a relative proportion of CT, VBC, and SCD to the leaf thickness was calculated according to Jumkudling et al. [31] and presented in percentage unit, as follows:
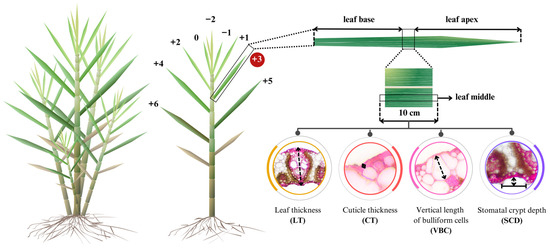
Figure 2.
Leaf sampling and measurement position in each tiller of each sugarcane genotype.
Stalk weight was observed at 10 months after planting by harvesting all stalks taken from each clone and weighing those stalks per clone. The measurement was performed for both BC1 individuals and their respective parents.
2.4. Statistical Analysis
The histogram of each BC1 population including their parents for each anatomical trait was performed and visualised using the Sigma Plot 10.0 software.
Mid-parent heterosis (MPH) was calculated and expressed in percentages using the means of mid-parent (MP) and BC1 hybrids. The MP value derived from the average of two respective parents (P1 and P2). Hence, MPH can be calculated by following Sobhakumari and Mathew [38].
where BC1 is the means of hybrid performance; MP is the mid-parent value given by (P1 + P2)/2; P1 and P2 are the means of P1 (female parent) and P2 (male parent) of respective BC1 hybrids, respectively.
Hierarchical agglomerative clustering was performed to construct dendrogram by assigning leaf thickness, cuticle thickness, the ratio of cuticle thickness/leaf thickness, the vertical length ratio of bulliform cell/leaf thickness, the ratio of stomatal crypt depth/leaf thickness, and stalk weight for the Ward criteria. This analysis was performed using the JMP Pro software (version 10.0, SAS Institute Inc., Chicago, IL, USA).
3. Results
3.1. Distribution and Diversity of Sugarcane Interspecific Hybrid Backcross 1 (BC1) within Populations Regarding Leaf Anatomy
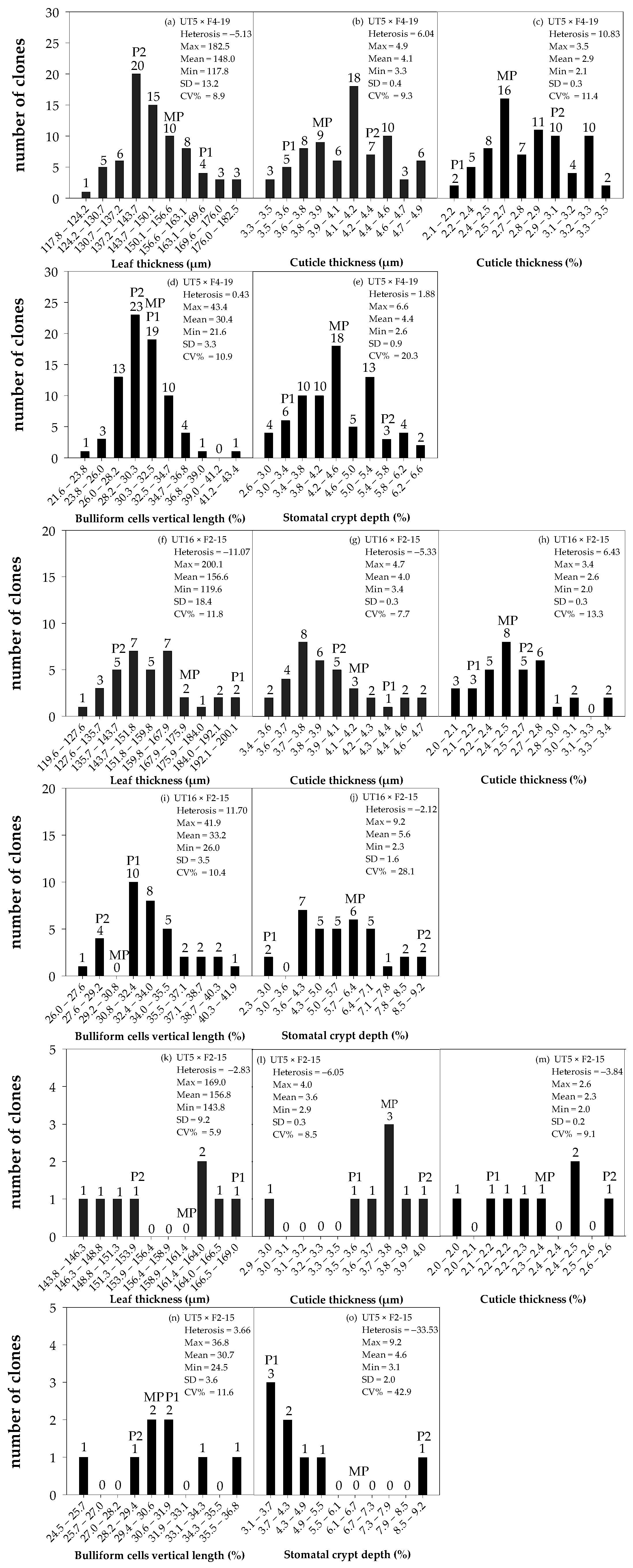
Overall, five sugarcane interspecific hybrid backcross 1 (BC1) populations were diverse regarding anatomical traits and can be divided into three groups based on their heterosis values (Figure 3a–o). Group I comprised a population BC1-1 and had negative heterosis on leaf thickness (LT) but positive heterosis on cuticle thickness (CT), percent CT, percent vertical length of bulliform cells (VBC), and percent stomatal crypt depth (SCD) (Figure 3a–e). Group II comprised three populations, BC1-3, BC1-4, and BC1-5, and had positive heterosis on percent CT and percent VBC (Figure 3f–j and Figure S1a–j). Group III comprised a population BC1-2 and had positive heterosis on percent VBC (Figure 3k–o). Considering all parameters of leaf anatomy observed, ‘Group I’ was superior to other groups.
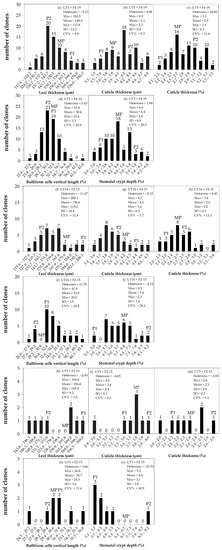
Figure 3.
Frequency distribution of leaf anatomy in three populations of interspecific backcross 1: BC1-1 (UT5 × F4-19) (N = 73) (a–e); BC1-3 (UT16 × F2-15) (N = 35) (f–j); and BC1-2 (UT5 × F2-15) (N = 8) (k–o). MP, P1, and P2 are mid-parent, female parent, and male parent, respectively. Numbers above the bars are the number of clones in each class interval. SD is standard deviation. CV% is coefficient of variation on given traits, calculated as (SD/mean) × 100.
The interspecific hybrid backcross 1 population 1 (BC1-1) composed of 73 clones and was derived from a commercial genotype ‘UT5’ as a female and the F1 interspecific hybrid ‘F4-19’ as a male. Those parents differed in LT (m), CT (m), percent CT (%), percent VBC (%), and percent SCD (%). The BC1 clones were distributed between the male and female parents (Figure 3a–e). The female parent ‘UT5’ had high LT but low percent SCD and percent CT, whereas the male parent ‘F4-19’ had low LT but high percent SCD and percent CT (Figure 3a–e). Both percent CT and percent SCD of the progenies were spread between their two parents (Figure 3c,e). Six clones in this population revealed high means and positive heterosis on percent SCD (Figure 3e). All BC1-1 clones were spread evenly for percent CT, where 22 of them (or 30%) had means from 2.96% to 3.50%, which were higher than the male parent, representing 10.83% of heterosis (Figure 3c). About 20 of 73 BC1-1 (or 27%) had percent VBC values from 31.5% to 43.4%, which were higher than the female parent, whereas other BC1-1 clones had lower means (21.60–29.06%) than the male parent on that trait (Figure 3d). The percent SCD values of all ‘BC1-1’ clones were higher than the mid-parent values (Figure 3e).
The interspecific hybrid backcross 1 population 3 (BC1-3) was derived from a commercial genotype ‘UT16’ as a female and the F1 interspecific hybrid ‘F2-15’ as a male. Those parents differed in LT (m), CT (m), percent CT, percent VBC, and percent SCD. The BC1 clones were distributed between the male and female parents (Figure 3f–j). While the female parent ‘UT16’ had high LT but a low percent SCD and percent CT, the male parent had low LT but a high percent SCD and percent CT (Figure 3f–j). Both the percent CT and percent SCD of BC1 clones were spread between the female and male parents (Figure 3g,j). All BC1-3 clones were spread evenly for percent CT, where 11 and 14 of 35 clones had means between mid-parent and male parent and between female and mid-parent, respectively (Figure 3h). However, 21 of 35 BC1-3 clones had a higher percent VBC (31.50–41.9%) than the female parent, whereas 2 of 35 clones had a lower means (26.0–28.36%) than the male parent, and the remaining clones were distributed between the female and male parents (Figure 3i). Population BC1-3 demonstrated negative heterosis for percent SCD (Figure 3j). All clones in this population had a lower percent SCD than the mid-parent values and were distributed between the mid-parent and female parent.
The interspecific hybrid backcross 1 population 2 (BC1-2) in Group III was derived from UT5 × F2-15 crosses. The female parent ‘UT5’ had high LT but a low percent SCD and percent CT, whereas the male parent ‘F2-15’ had low LT but high percent SCD and percent CT (Figure 3k–o). All clones in this population had negative heterosis for most leaf anatomy, and the most negative heterosis was found on percent SCD (−33.53%) (Figure 3o). Two of eight clones (or 25% of the population) had a higher percent VBC (31.1–36.8%) than the female parent, whereas one clone had a lower mean (24.5–25.7%) than the male parent, and the remaining clones were distributed between the female and male parents (Figure 3n). All clones were close to the mid-parent and were distributed between the female and male parents for CT (Figure 3l), percent CT (Figure 3m), and percent SCD (Figure 3n).
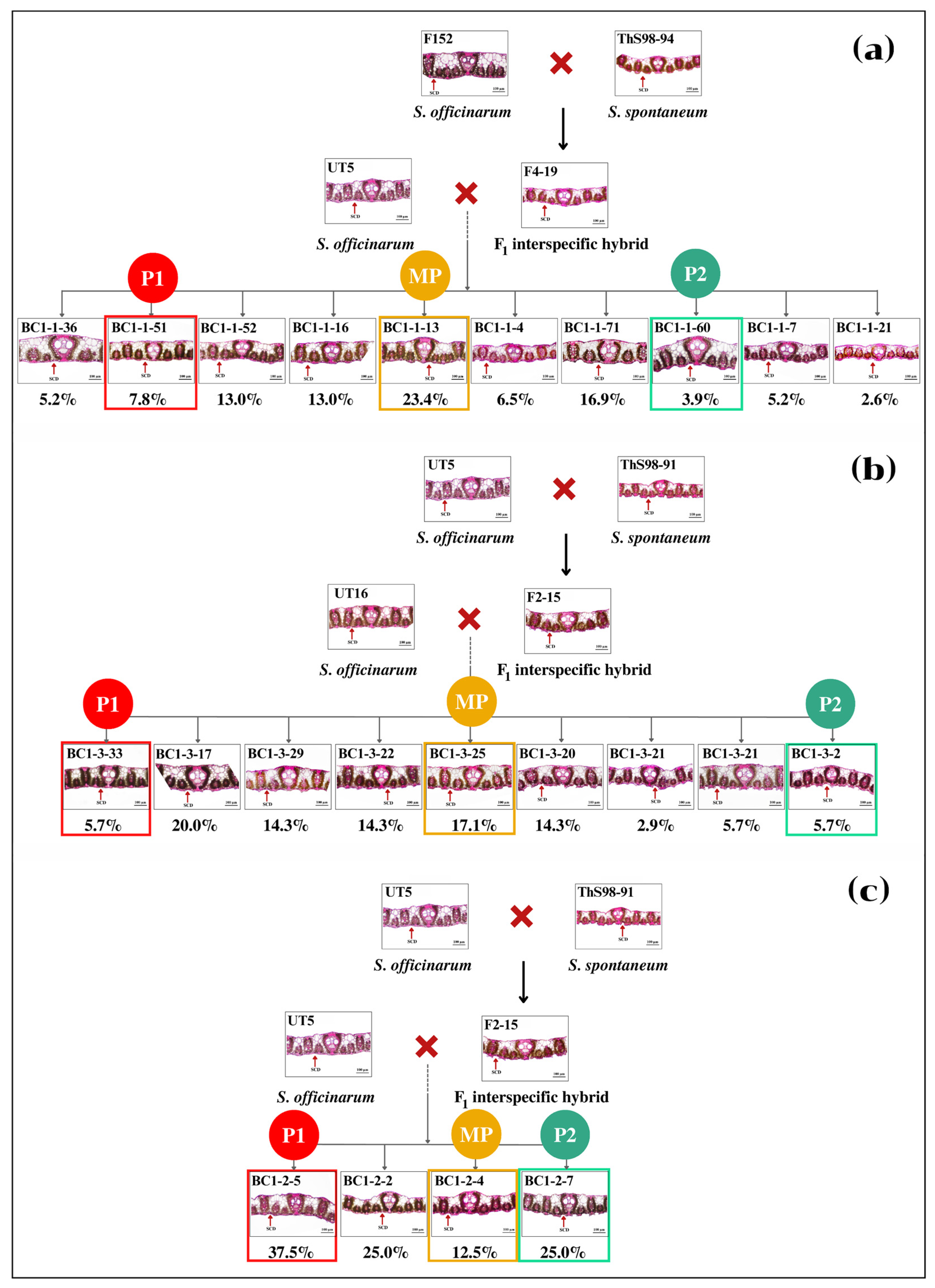
In population BC1-1, the percent SCD of male and female parents were different, where the male had high SCD but the female showed an unclear SCD. We found that six clones BC1-1-7, BC1-1-21, BC1-1-62, BC1-1-74, BC1-1-81, and BC1-1-88 (5.6–6.6%) had a higher percent SCD than the male parent, whereas 31 clones and the remaining clones had a similar percent SCD to the male and female parents, respectively (Figure 4a). In population BC1-3, the SCD of both parents was also obviously different, where the male had a higher SCD than the female. All BC1-3 clones had SCD variations. While clone BC1-3-2 had a similar SCD to the male parent, the remaining clones were like the female parent (Figure 4b). Likewise, in populations BC1-4 and BC1-5, the male had higher SCD than the female, but the distributions of progenies between those two BC1 populations were slightly different (Figure S2). In population BC1-2, both parents had distinct SCD, where the male had high SCD, but the female had low SCD. Clone BC1-2-7 had similar SCD to the male parent, whereas three clones were like the female parent (Figure 4c). The remaining clones had values between the female and the mid-parent.
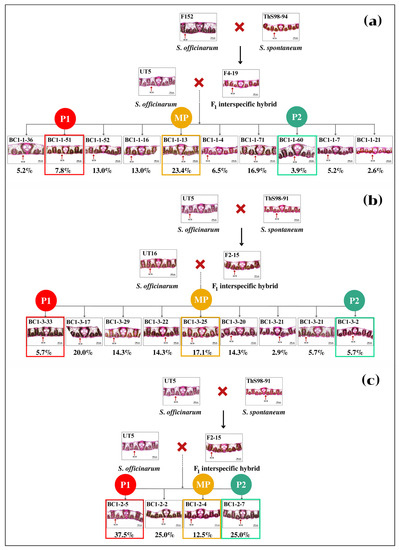
Figure 4.
Stomatal crypt depth (SCD) of sugarcane leaves of three populations of interspecific backcross 1: BC1-1 (UT5 × F4-19) (a); BC1-3 (UT16 × F2-15) (b); and BC1-2 (UT5 × F2-15) (c). MP, P1, and P2 are mid-parent, female parent, and male parent, respectively. Scale = 100 µm.
3.2. Performance of BC1 Clones on Stalk Weight and Leaf Anatomy
An ideal type of sugarcane was high-yielding and drought-tolerant. That ideotype can be achieved by selecting sugarcane genotypes having low LT and VBC but high CT, SCD, and yield. In our study, yield was represented by stalk weight.
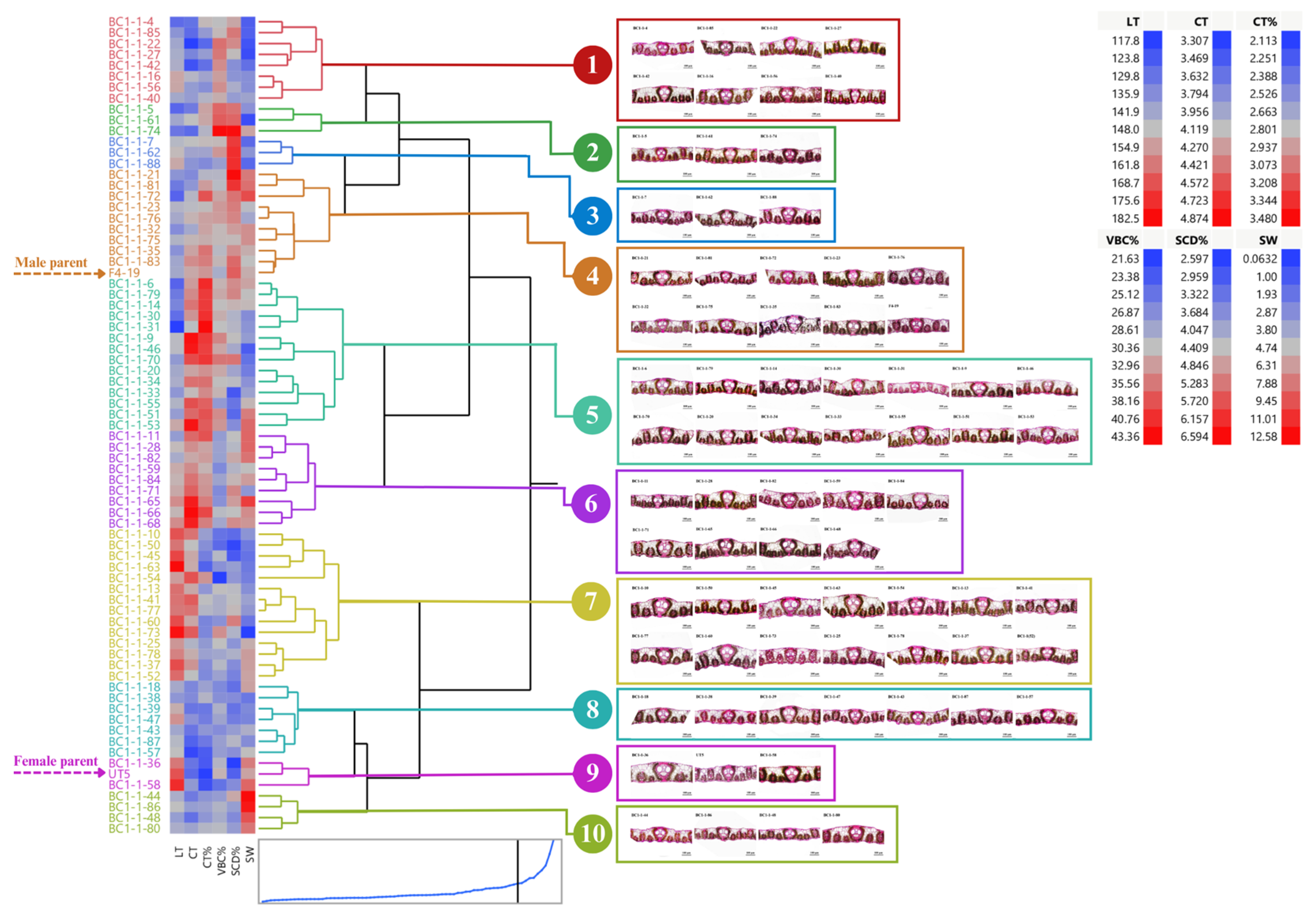
BC1-1 and their parents can be clustered into 10 groups (average R2 = 0.69) through cluster analysis using stalk weight and leaf anatomy according to Ward’s criteria (Figure 5). Among all clones in BC1-1, clones in groups 4 and 6 appealed to those selection criteria. Group 4 consisted of nine clones including BC1-1-21, BC1-1-23, BC1-1-32, BC1-1-35, BC1-1-72, BC1-1-75, BC1-1-76, BC1-1-81, BC1-1-83, and the male parent. Group 6 had nine clones including BC1-1-11, BC1-1-28, BC1-1-82, BC1-1-59, BC1-1-84, BC1-1-71, BC1-1-65, BC1-1-66, and BC1-1-68. Clones in group 6 had higher CT but lower SCD than clones in group 4. Two clones in group 6, BC1-1-11 and BC1-1-82, had the potential to be drought-tolerant genotypes based on those anatomical traits, whereas the other two clones, BC1-1-65 and BC1-1-68, had a high stalk weight and acceptable leaf anatomy (Figure 5).
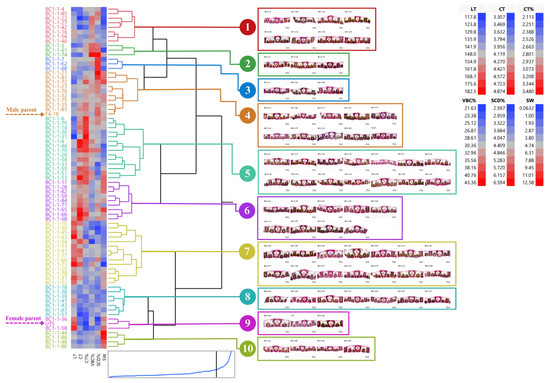
Figure 5.
Dendrogram showing genetic relationship among 73 sugarcane genotypes (71 BC1-1 clones and 2 parents) regarding stalk weight and leaf anatomical traits. Ward’s clustering method was based on leaf thickness, cuticle thickness, percent cuticle thickness, percent vertical length of bulliform cells, percent stomatal crypt depth, and stalk weight (scale: distance scale).
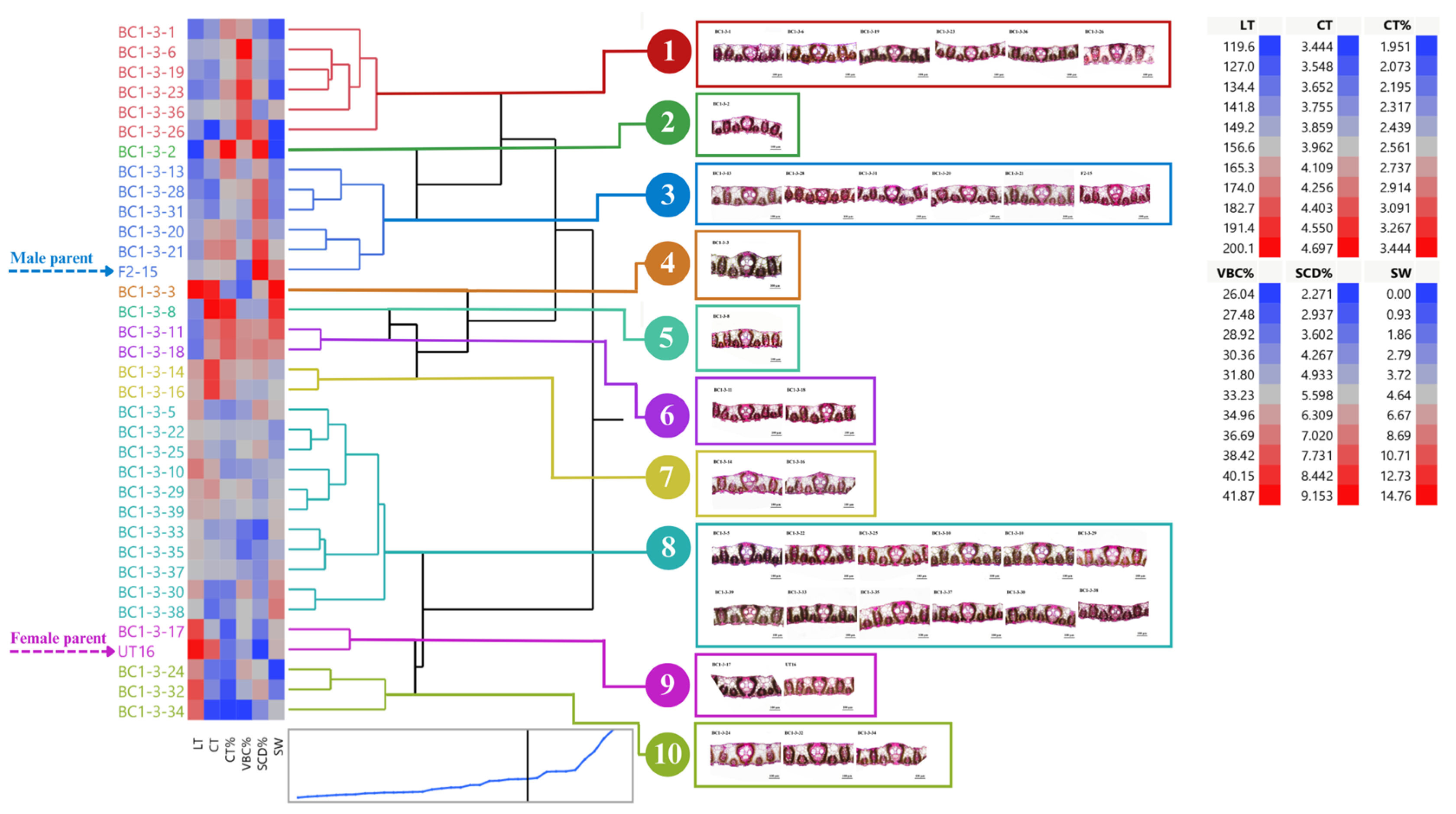
BC1-3 and their parents can be clustered into ten groups (average R2 = 0.79) (Figure 6). We found that two clones, BC1-3-20 and BC1-3-21, in group 3 appealed to selection criteria previously mentioned. Clone BC1-3-8 in group 5 and two clones, BC1-3-11 and BC1-3-18, in group 6 were also favorable as they had high stalk weight and acceptable leaf anatomy. In contrast, most clones in group 10 had low means for most leaf anatomy observed except for LT (Figure 6).
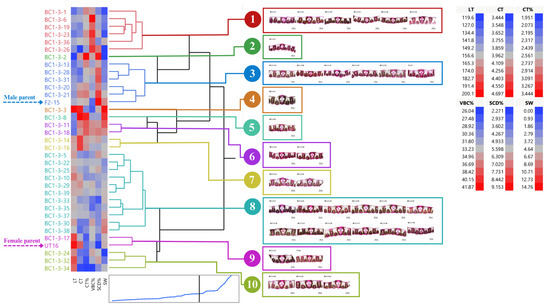
Figure 6.
Dendrogram showing genetic relationship among 35 sugarcane genotypes (33 BC1-3 clones and 2 parents) regarding stalk weight and leaf anatomical traits. Ward’s clustering method was based on leaf thickness, cuticle thickness, percent cuticle thickness, percent vertical length of bulliform cells, percent stomatal crypt depth, and stalk weight (scale: distance scale).
BC1-4 including their parents can be clustered into four groups (Figure S3). In this population, small phenotypic variations existed, and no promising clones appealed to our selection criteria. Two clones BC1-4-11 and BC1-4-13 seemed to be the closest to appeal our criteria with thicker cuticle layers, smaller bulliform cells, and deeper stomatal crypts, but they had poor stalk weight.
BC1-5 including their parents can be clustered into three groups (average R2 = 0.79) (Figure S4). Group 2 was the best among the other groups in this population. In this group, clone BC1-5-30 was the most favorable as it showed thicker cuticles, smaller bulliform cells, higher stalk weight, and moderate stomatal crypt depth.
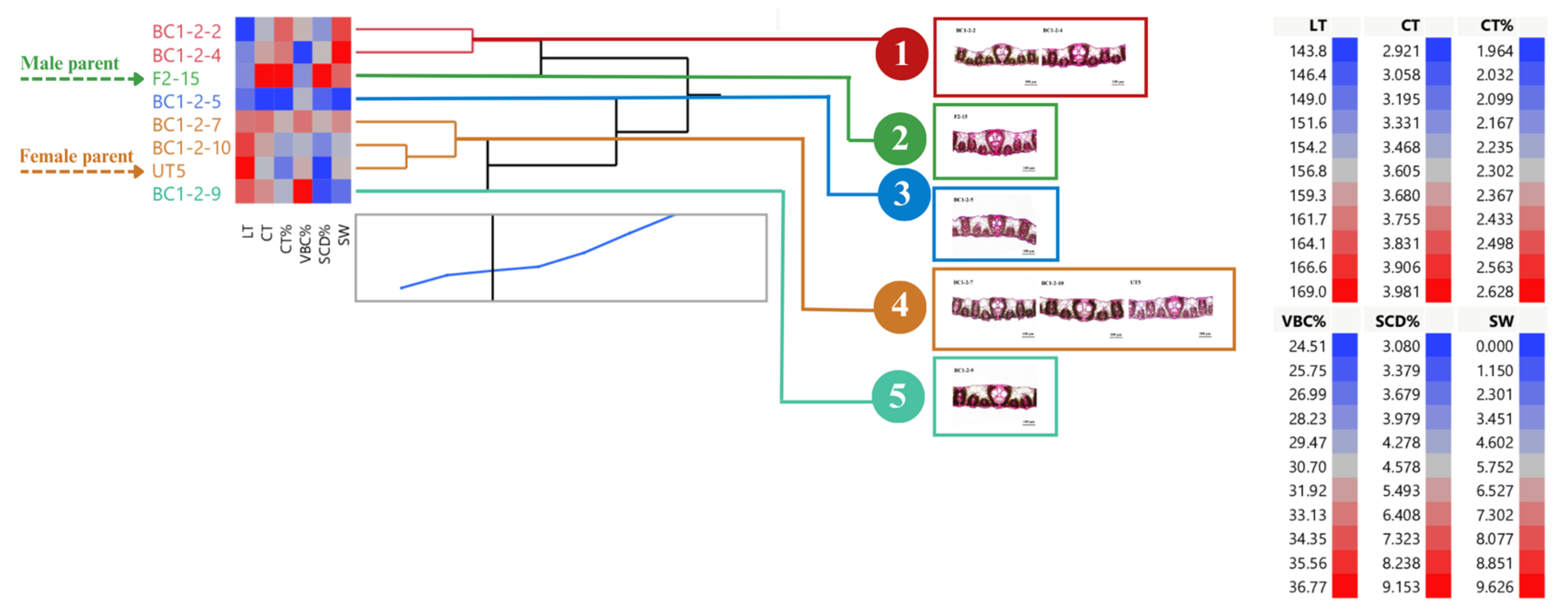
BC1-2 and their parents can be clustered into five groups (average R2 = 0.90) (Figure 7). Groups 1 and 2 had low LT, whereas high LT was found in group 4 (three clones: BC1-2-7, BC1-2-10, and the female parent) and group 5 (BC1-2-9). A low percent VBC was found in groups 1, 2, and 3. Groups 1 and 2 showed high percent SCD and stalk weight. We only noticed one clone, BC1-2-4, that can appeal to the selection criteria (Figure 7).
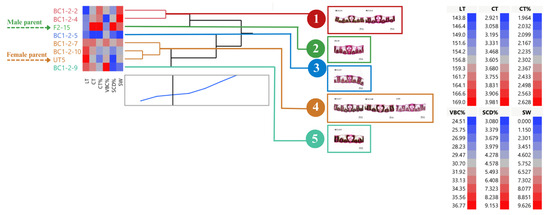
Figure 7.
Dendrogram showing genetic relationship among 8 sugarcane genotypes (6 BC1-2 clones and 2 parents) regarding stalk weight and leaf anatomical traits. Ward’s clustering method was based on leaf thickness, cuticle thickness, percent cuticle thickness, percent vertical length of bulliform cells, percent stomatal crypt depth, and stalk weight (scale: distance scale).
4. Discussion
Interspecific hybridization between S. officinarum (2n = 8x = 80; x = 10) and S. spontaneum (2n = 5x–16x = 40–128; x = 8) produced modern sugarcane cultivars [39]. By intercrossing with S. spontaneum, the hybrids will accumulate higher doses of biotic and abiotic tolerance genes including hexokinase (HXK), pathogenesis-related protein (PR1), coronatine-insensitive protein (COI-1), and jasmonate ZIM domain-containing protein (JAZ), and serine/threonine protein kinase 2 (SnRK2) [40]. Approximately 70–80% of the genome comes from S. officinarum, while 10–20% originates from S. spontaneum, and the remaining 10% derives from interspecific recombination [41]. While a recombination between S. officinarum × S. spontaneum will result in F1 progenies with 2n = 136 chromosomes, a backcrossing with S. officinarum assigned as female parent will not alter the chromosome number [42]. Backcrossing with S. officinarum was performed to recover the agronomic performance of F1 hybrids after experiencing interspecific hybridizations. This process led to a decrease in the percentage of S. spontaneum genomes, accounting for 15–25% in modern cultivars [43,44]. Our current study shows that BC1 clones within each population were highly diverse for four leaf anatomy traits, namely LT, CT, VBC, and SCD. It may be that alleles responsible for given traits were segregated among genotypes in addition to the unbalanced chromosome pairing.
Several methods are available and have been applied to evaluate the diversity of sugarcane genotypes including morphological, physiological, anatomical, and molecular markers. We utilized leaf anatomy for diversity analysis since it is practical and cost-saving despite time-consuming and laborious. In our study, hybrids in group II had relatively lower phenotypic variations on given traits than hybrids in other groups because those hybrids in group II derived from the same male parent. Meanwhile, hybrids in group III had a low frequency of the distribution and number of clones within crosses due to intraspecific hybridizations. Three backcrosses, BC1-1, BC1-2, and BC1-3, performed distinct leaf anatomy that involved a drought-tolerant mechanism including stomatal crypt depth, bulliform cell shapes, and cuticle thickness. Once backcrossing was complete, it produced offspring that were highly divergent. An unusual genome transmission is often observed in crosses between female S. officinarum and male S. spontaneum. The diploid, somatic complement (2n) of the female is retained along with the haploid, gametic complement (1n) of the male, known as 2n + n transmission and results in considerable complexity in the genomes of commercial clones [45]. Our five BC1 populations can be divided into three groups regarding per se and heterosis values. In each population, dendrogram analysis was used to cluster the individuals. ‘Group I’ or ‘BC1-1’ had two promising clones, BC1-1-11 and BC1-1-82, which had favorable means for drought tolerance parameters such as thicker cuticle layers, deeper stomatal crypts, and higher stalk weight, while other two clones, BC1-1-65 and BC1-1-68, had favorable means for stalk weight and moderate means for leaf anatomy, i.e., leaf thickness, cuticle thickness, and stomatal crypt depth.
Heterosis is estimated to compare the relative performance of offspring with the performance of parents. In sugarcane, the presence of heterosis has been reported on cane yield, yield components, sugar, and juicy parameters. Zhou [46] found that crosses from different heterotic groups resulted in 24 to 42% higher cane yield than those derived from within heterotic groups. Verma and Singh [47] noticed high and positive heterosis on the number of millable canes per clump (60–80%), the number of internodes per cane (30–50%), stalk weight (50–70%), kg-brix (up to 250%), and commercial cane sugar (40–90%). However, they found low and positive heterosis for stalk girth (5–16%) and sucrose percent in juice (below 9%) and negative heterosis for invert sugar (−38–−2%) and fiber percent (−13–−4%). It seemed that the magnitudes of heterosis highly varied from negative to positive estimates depending upon genotypes and traits observed. Heterosis estimates on leaf anatomy are still lacking in previous reports. We first reported that the estimates had high variations from negative on leaf thickness (−11–−2%) to positive on bulliform cells vertical length (0.43–11.70%) across five BC1 populations. Dermail et al. [32] and Dermail et al. [33] argued that heterosis can indicate parental adaptations in addition to hybrid performance; however, the estimates might be biased due to environmental conditions. They found that heterosis was inflated when both progenies and parents were evaluated under sub-optimum conditions since the parents showed lower plant standing due to long-term inbreeding depression. In our study, relatively low heterosis (<50%) on leaf anatomy can be explained by three factors: (1) narrow genetic distance between donor and recipient parents crossed; (2) the occurrence of natural self-pollination (<20%) [48]; and (3) optimum growing conditions under normal water regime. Both BC1-2 and BC1-3 had low heterosis estimates because their founder female parents possessed narrow genetic bases in Thailand. In contrast, BC1-1 had overall positive heterosis as it had genotype F152 as the founder female parent which is an exotic germplasm.
Regarding leaf anatomy, the sugarcane ideotype of drought-tolerant cultivars should have high cuticle thickness and stomatal crypt depth [29,49]. Drought conditions may adversely reduce the bulliform cell area in sugarcane, and drought-tolerant cultivars possessed smaller bulliform cells than susceptible cultivars [31,49]. Nevertheless, the correlations among leaf anatomical traits may not be persistent due to several factors such as genetic backgrounds, developmental stages, climatic changes, and environmental managements [29]. Our previous study found that the F1 interspecific hybrids had the averaged means of percent stomatal crypt depth, percent bulliform cell shapes, and percent cuticle thickness of 47%, 39%, and 39%, respectively [31]. In our current study, one cycle of backcrossing of the F1 interspecific hybrids with commercial canes resulted in slightly higher means on leaf anatomical traits related to drought tolerance including percent stomatal crypt depths, percent bulliform cells, and percent cuticle thicknesses, accounting for 60%, 47%, and 35%, respectively. It indicated that the anatomical properties of wild species tolerant to water deficit were successfully introgressed into the F1 hybrids and eventually to the BC1 populations. We have learned that leaf anatomy showed great sensitivities and promising markers for drought-tolerant screening when different genetic backgrounds of sugarcane were tested under different regimes of drought [29]. Future studies are encouraged to evaluate the representative clones of each sugarcane BC1 population derived from our current study under different stress conditions regarding agronomic performance and final yields to confirm whether our backcrossing scheme is effective. If not, additional cycles of backcrossing or even further recombination with exotic germplasm are required to expand the genetic distance of our germplasm.
5. Conclusions
This is the first report investigating the diversity and heterosis of leaf anatomy of sugarcane populations derived from interspecific hybridization between S. spp. and S. spontaneum and followed by one cycle of backcross. Diverse ranges of both per se and heterosis values were observed among five populations and across traits. Five populations can be divided into three groups. Regarding heterosis, Group I (‘BC1-1’ hybrids) showed positive heterosis on leaf thickness, cuticle thickness, the vertical length of the bulliform cell, and stomatal crypt percentage. The BC1-1 progenies performed better than their respective parents and the other progenies of Group II (BC1-3) and Group III (BC1-2). Regarding the distribution pattern within each population, most clones in Group I were similar to their male parents, which still retained 50% favorable alleles for drought tolerance, and so did most clones in Group II for leaf thickness and percent cuticle thickness. In contrast, large proportions of clones in Group III were equal to their mid-parents. Two clones in populations BC1-1, BC1-1-11 and BC1-1-82 were identified as potential genetic stock for drought tolerance parameters like thicker cuticles, deeper stomatal crypts, and higher stalk weight. The other two clones from the same population, BC1-1-65 and BC1-1-68, were also promising for high stalk weight and moderate drought tolerance. Heterosis seemed to be genotype and trait dependent in sugarcane. Backcrossing with a commercial cane resulted in slightly higher means of BC1 than those of their mid-parents, and the low heterosis on leaf anatomy noticed in this study might be due to the superior donor female parents used, namely UT5 and UT6.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13102457/s1, Figure S1. Frequency distribution of leaf anatomy in two populations of interspecific backcross 1: BC1-4 (UT16 × F6-13) (N = 21) (a–e) and BC1-5 (UT5 × F6-13) (N = 26) (f–j). MP, P1, and P2 are mid-parent, female parent, and male parent, respectively. Numbers above the bars are the number of clones in each class interval. SD is standard deviation. CV% is coefficient of variation on given traits, calculated as (SD/mean) × 100. Figure S2. Stomatal crypt depth (SCD) of sugarcane leaves of two populations of interspecific backcross 1: BC1-4 (UT16 × F6-13) (a) and BC1-5 (UT5 × F6-13) (b). MP, P1, and P2 are mid-parent, female parent, and male parent, respectively. Scale = 100 µm. Figure S3. Dendrogram showing genetic relationship among 21 sugarcane genotypes (19 BC1-4 clones and 2 parents) regarding stalk weight and leaf anatomical traits. Ward’s clustering method was based on leaf thickness, cuticle thickness, percent cuticle thickness, percent vertical length of bulliform cells, percent stomatal crypt depth, and stalk weight (scale: distance scale). Figure S4. Dendrogram showing genetic relationship among 26 sugarcane genotypes (24 BC1-5 clones and 2 parents) regarding stalk weight and leaf anatomical traits. Ward’s clustering method was based on leaf thickness, cuticle thickness, percent cuticle thickness, percent vertical length of bulliform cells, percent stomatal crypt depth, and stalk weight (scale: distance scale).
Author Contributions
Conceptualization, N.J. and P.S.; methodology, N.P., N.J. and P.S.; validation, N.J. and P.S.; formal analysis, K.W.; investigation, K.W., N.J. and P.S.; resources, N.J. and P.S.; data curation, K.W.; writing—original draft preparation, K.W. and N.J.; writing—review and editing, K.W., A.D., N.J. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Fund of Khon Kaen University, grant number 65A103000128 which has received funding support from the National Science, Research, and Innovation Fund (NSRF). The partial funding was also supported by the Northeast Thailand Cane and Sugar Research Center (NECS), Khon Kaen University (PR65-4-002).
Data Availability Statement
Data are available within this article.
Acknowledgments
Grateful acknowledgment is made to the Fundamental Fund of Khon Kaen University. Assistance was also received from the Northeast Thailand Cane and Sugar Research Center, Faculty of Agriculture, Khon Kaen University. Acknowledgment is extended to the Faculty of Interdisciplinary Science, Khon Kaen University, Nong Khai Campus, Muang, Nong Khai, Thailand for facility support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Budeguer, F.; Enrique, R.; Perera, M.F.; Racedo, J.; Castagnaro, A.P.; Noguera, A.S.; Welin, B. Genetic transformation of sugarcane, current status and future prospects. Front. Plant Sci. 2021, 12, 768609. [Google Scholar] [CrossRef]
- Mohan, C. Genome editing in sugarcane: Challenges ahead. Front. Plant Sci. 2016, 7, 1542. [Google Scholar] [CrossRef]
- UN Comtrade. World Cane Sugar Export’s Statistics. 2019. Available online: https://comtrade.un.org/data/ (accessed on 25 August 2019).
- Office of the Cane and Sugar Board. Production Report. 2019. Available online: http://www.ocsb.go.th/th/home/index.php (accessed on 20 February 2020).
- Pipitpukdee, S.; Attavanich, W.; Bejranonda, S. Climate Change Impacts on Sugarcane Production in Thailand. Atmosphere 2020, 11, 408. [Google Scholar] [CrossRef]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araújo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Hussain, B.; Khan, Z.; Ozturk, N.Z.; Ullah, N. From Genetics to Functional Genomics: Improvement in Drought Signaling and Tolerance in Wheat. Front. Plant Sci. 2015, 6, 1012. [Google Scholar] [CrossRef]
- Li, C.; Nong, Q.; Solanki, M.K.; Liang, Q.; Xie, J.; Liu, X.; Li, Y.; Wang, W.; Yang, L.; Li, Y. Differential expression profiles and pathways of genes in sugarcane leaf at elongation stage in response to drought stress. Sci. Rep. 2016, 6, 25698. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Filho, J.B.M.; Carena, M.J. Breeding Plans. In Quantitative Genetics in Maize Breeding, 3rd ed.; Carena, M.J., Hallauer, A.R., Filho, J.B.M., Eds.; Springer: New York, NY, USA, 2010; Volume 6, pp. 577–653. [Google Scholar] [CrossRef]
- Alwala, S.; Kimbeng, C.A.; Veremis, J.C.; Gravois, K.A. Identification of molecular markers associated with sugar-related traits in a Saccharum interspecific cross. Euphytica 2009, 167, 127–142. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Lin, Y.; Fu, C.; Liu, S.; Deng, Z. Unexpected inheritance pattern of Erianthus arundinaceus chromosomes in the intergeneric progeny between Saccharum spp. and Erianthus arundinaceus. PLoS ONE 2014, 9, e110390. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhao, X.; Chai, J.; Ding, X.; Li, X.; Huang, Y. Chromosome-specific painting unveils chromosomal fusions and distinct allopolyploid species in the Saccharum complex. New Phytol. 2022, 233, 1953–1965. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Khurshid, H.; Esh, A.; Wu, C.; Wang, Q.; Piperidis, N. Past and recent advances in sugarcane cytogenetics. Crop J. 2023, 11, 1–8. [Google Scholar] [CrossRef]
- Paterson, A.H.; Wang, X.; Li, J.; Tang, H. Ancient and recent polyploidy in monocots. In Polyploidy and Genome Evolution, 1st ed.; Soltis, P., Soltis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 93–108. [Google Scholar] [CrossRef]
- Bremer, G. Problems in breeding and cytology of sugar cane. Euphytica 1961, 10, 59–78. [Google Scholar] [CrossRef]
- Reyes-Valdés, M.H. A Model for Marker-Based Selection in Gene Introgression Breeding Programs. Crop Sci. 2000, 40, 91–98. [Google Scholar] [CrossRef]
- Chatterjee, J.; Dionora, J.; Elmido-Mabilangan, A.; Wanchana, S.; Thakur, V.; Bandyopadhyay, A.; Brar, D.S.; Quick, W.P. The evolutionary basis of naturally diverse rice leaves anatomy. PLoS ONE 2016, 11, e0164532. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Li, H.; Chang, C. Evolutionary insight of plant cuticle biosynthesis in bryophytes. Plant Signal. Behav. 2021, 16, 1943921. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The plant cuticle: Old challenges, new perspectives. J. Exp. Bot. 2017, 68, 5251–5255. [Google Scholar] [CrossRef]
- Zhou, X.; Jenks, M.A.; Liu, J.; Liu, A.; Zhang, X.; Xiang, J.; Zou, J.; Peng, Y.; Chen, X. Overexpression of transcription factor OsWR2 regulates wax and cutin biosynthesis in rice and enhances its tolerance to water deficit. Plant Mol. Biol. Rep. 2014, 32, 719–731. [Google Scholar] [CrossRef]
- Liu, L.L.; Deng, Y.Q.; Dong, X.X.; Wang, C.F.; Yuan, F.; Han, G.L.; Wang, B.S. ALDH2C4 regulates cuticle thickness and reduces water loss to promote drought tolerance. Plant Sci. 2022, 323, 111405. [Google Scholar] [CrossRef]
- Li, L.; Shi, Z.Y.; Li, L.; Shen, G.Z.; Wang, X.Q.; An, L.S.; Zhang, J.L. Overexpression of ACL1 (abaxially curled leaf 1) increased bulliform cells and induced abaxial curling of leaf blades in rice. Mol. Plant. 2010, 3, 807–817. [Google Scholar] [CrossRef]
- Matschi, S.; Vasquez, M.F.; Bourgault, R.; Steinbach, P.; Chamness, J.; Kaczmar, N.; Gore, M.A.; Molina, I.; Smith, L.G. Structure-function analysis of the maize bulliform cell cuticle and its potential role in dehydration and leaf rolling. Plant Direct. 2020, 4, e00282. [Google Scholar] [CrossRef]
- Latif, A.; Ying, S.; Cuixia, P.; Ali, N. Rice curled its leaves either adaxially or abaxially to combat drought stress. Rice Sci. 2023, 30, 405–416. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Hassiotou, F.; Veneklaas, E.J. Stomatal crypts have small effects on transpiration: A numerical model analysis. Plant Physiol. 2009, 151, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.R.; Cui, H.; Callahan, H.; Scoffoni, C.; John, G.P.; Bartlett, M.K.; Burge, D.O.; Sack, L. Evolution of leaf structure and drought tolerance in species of Californian Ceanothus. Am. J. Bot. 2018, 105, 1672–1687. [Google Scholar] [CrossRef] [PubMed]
- Taratima, W.; Ritmaha, T.; Jongrungklang, N.; Maneerattanarungroj, P.; Kunpratum, N. Effect of stress on the leaf anatomy of sugarcane cultivars with different drought tolerance (Saccharum officinarum, Poaceae). Rev. Biol. Trop. 2020, 68, 1159–1170. [Google Scholar] [CrossRef]
- Nawazish, S.; Hameed, M.; Naurin, S. Leaf anatomical adaptations of Cenchrus ciliaris L., from the Salt Range, Pakistan against drought stress. Pak. J. Bot. 2006, 38, 1723–1730. [Google Scholar]
- Jumkudling, S.; Songsri, P.; Taratima, W.; Jongrungklang, N. Diversity and distribution of anatomical characteristics involved with drought resistance of inter-specific (Saccharum spp. hybrid × S. spontaneum) sugarcane F1 hybrid population. Sugar Tech 2022, 24, 1342–1356. [Google Scholar] [CrossRef]
- Dermail, A.; Suriharn, B.; Chankaew, S.; Sanitchon, J.; Lertrat, K. Hybrid prediction based on SSR-genetic distance, heterosis and combining ability on agronomic traits and yields in sweet and waxy corn. Sci. Hortic. 2020, 259, 108817. [Google Scholar] [CrossRef]
- Dermail, A.; Lübberstedt, T.; Suwarno, W.B.; Chankaew, S.; Lertrat, K.; Ruanjaichon, V.; Suriharn, K. Combining ability of tropical × temperate maize inducers for haploid induction rate, R1-nj seed set, and agronomic traits. Front. Plant Sci. 2023, 14, 1154905. [Google Scholar] [CrossRef]
- Trentin, H.U.; Yavuz, R.; Dermail, A.; Frei, U.K.; Dutta, S.; Lübberstedt, T. A Comparison between Inbred and Hybrid Maize Haploid Inducers. Plants 2023, 12, 1095. [Google Scholar] [CrossRef]
- Mendes de Paula, T.O.; Brasileiro, B.P.; Cursi, D.E.; Freitas, E.G.; dos Santos, J.M.; Resende, M.D.V.; Kimbeng, C.; Pereira Barbosa, M.H. Establishment of gene pools for systematic heterosis exploitation in sugarcane breeding. Agron. J. 2020, 112, 3847–3858. [Google Scholar] [CrossRef]
- Khonghintaisong, J.; Songsri, P.; Jongrungklang, N. Understanding growth rate patterns among different drought resistant sugarcane cultivars during plant and ratoon crops encountered water deficit at early growth stage under natural field conditions. Agronomy 2021, 11, 2083. [Google Scholar] [CrossRef]
- White, W.H.; Viator, R.P.; Dufrene, E.O.; Dalley, C.D.; Richard, E.P.; Tew, T.L. Re-evaluation of sugarcane borer (Lepidoptera: Crambidae) bioeconomics in Louisiana. Crop Prot. 2008, 27, 1256–1261. [Google Scholar] [CrossRef]
- Sobhakumari, V.P.; Mathew, S.D. Effect of Hybrid Vigor on Callus Induction and Regeneration of Sugarcane. Cytologia 2009, 74, 71–77. [Google Scholar] [CrossRef]
- D’Hont, A.; Ison, D.; Alix, K.; Roux, C.; Glaszmann, J.C. Determination of basic chromosome numbers in the genus Saccharum by physical mapping of ribosomal RNA genes. Genome 1998, 41, 221–225. [Google Scholar] [CrossRef]
- Feng, M.; Zhao, J.; Li, S.; Wei, N.; Kuang, B.; Yang, X. Molecular genetic mechanisms of heterosis in sugarcane cultivars using a comparative transcriptome analysis of hybrids and ancestral parents. Agronomy 2023, 13, 348. [Google Scholar] [CrossRef]
- Garsmeur, O.; Droc, G.; Antonise, R.; Grimwood, J.; Potier, B.; Aitken, K.; Jenkins, J.; Martin, G.; Charron, C.; Hervouet, C.; et al. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat. Commun. 2018, 9, 2638. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, A.K.; Singh, A. Wide hybridization. In Plant Breeding and Cultivar Development, 3rd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 159–178. [Google Scholar] [CrossRef]
- Cuadrado, A.; Acevedo, R.; Moreno Diaz De La Espina, S.; Jouve, N.; De La Torre, C. Genome remodelling in three modern S. officinarum × S. spontaneum sugarcane cultivars. J. Exp. Bot. 2004, 55, 847–854. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Han, J.; Zhang, Y.; Ma, S.; Yu, G.; Wang, Z.; Wang, K. Species-specific abundant retrotransposons elucidate the genomic composition of modern sugarcane cultivars. Chromosoma 2020, 129, 45–55. [Google Scholar] [CrossRef]
- Ming, R.; Liu, S.C.; Moore, P.H.; Irvine, J.E.; Paterson, A.H. QTL analysis in a complex autopolyploid: Genetic control of sugar content in sugarcane. Genome Res. 2001, 11, 2075–2084. [Google Scholar] [CrossRef]
- Zhou, M. General and specific combining ability for cane yield and implications for sugarcane breeding. Crop Sci. 2021, 61, 539–551. [Google Scholar] [CrossRef]
- Verma, P.S.; Singh, S.B. Heterosis in relation to per-se performance and effects of general combining ability in sugarcane. Sugar Tech 2004, 6, 181–185. [Google Scholar] [CrossRef]
- McIntyre, C.; Jackson, P. Low level of selfing found in a sample of crosses in Australian sugarcane breeding programs. Euphytica 2001, 117, 245–249. [Google Scholar] [CrossRef]
- Zhang, F.J.; Zhang, K.K.; Du, C.Z.; Li, J.; Xing, Y.X.; Yang, L.T.; Li, Y.R. Effect of drought stress on anatomical structure and chloroplast ultrastructure in leaves of sugarcane. Sugar Tech 2015, 17, 41–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).