Extended Post-Harvest Effect of Melatonin in Fresh-Cut Broccolini Plants (Bimi®)
Abstract
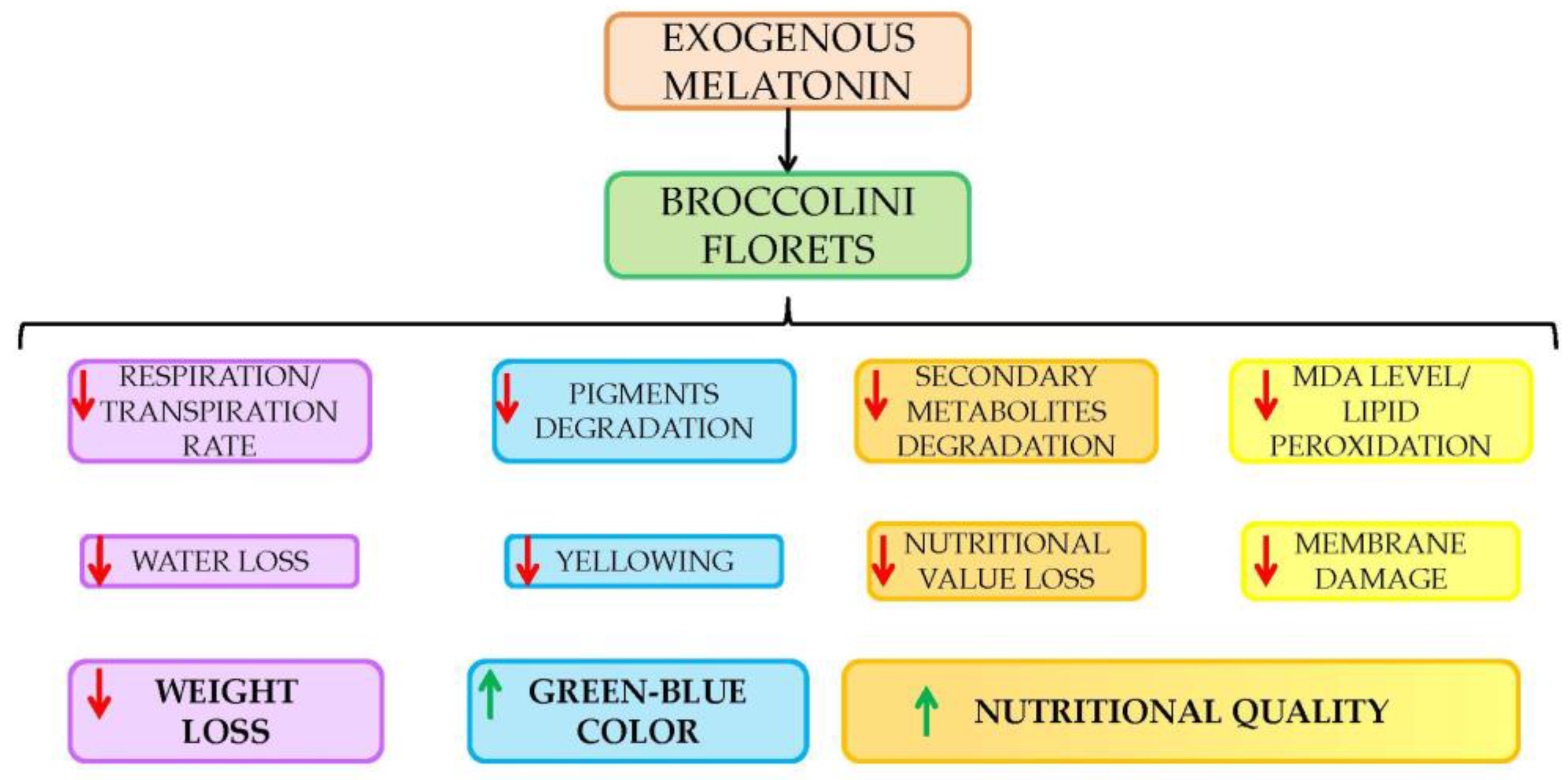
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Broccolini Treatments
2.4. Weight Loss
2.5. Color
2.6. Determination of Chlorophyll and Carotenoid Contents
2.7. Determination of Total Antioxidant Activity
2.8. Total Phenolic Content
2.9. Total Flavonoid Content
2.10. Determination of Malondialdehyde Content
2.11. Measurement of the Antioxidant Activity of Enzymes
2.12. Statistical Analysis
3. Results and Discussion
3.1. Effect of Melatonin on Weight Loss
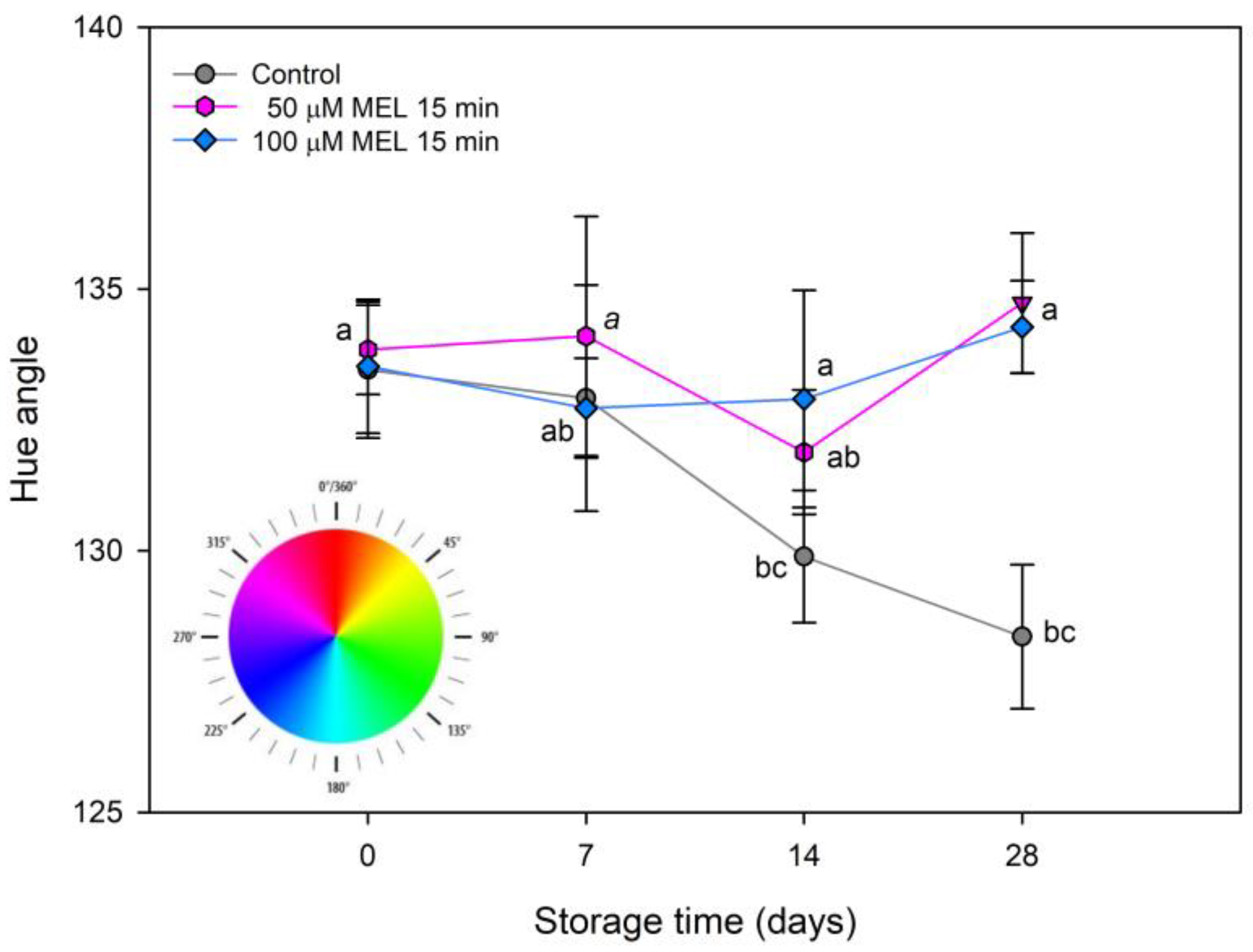
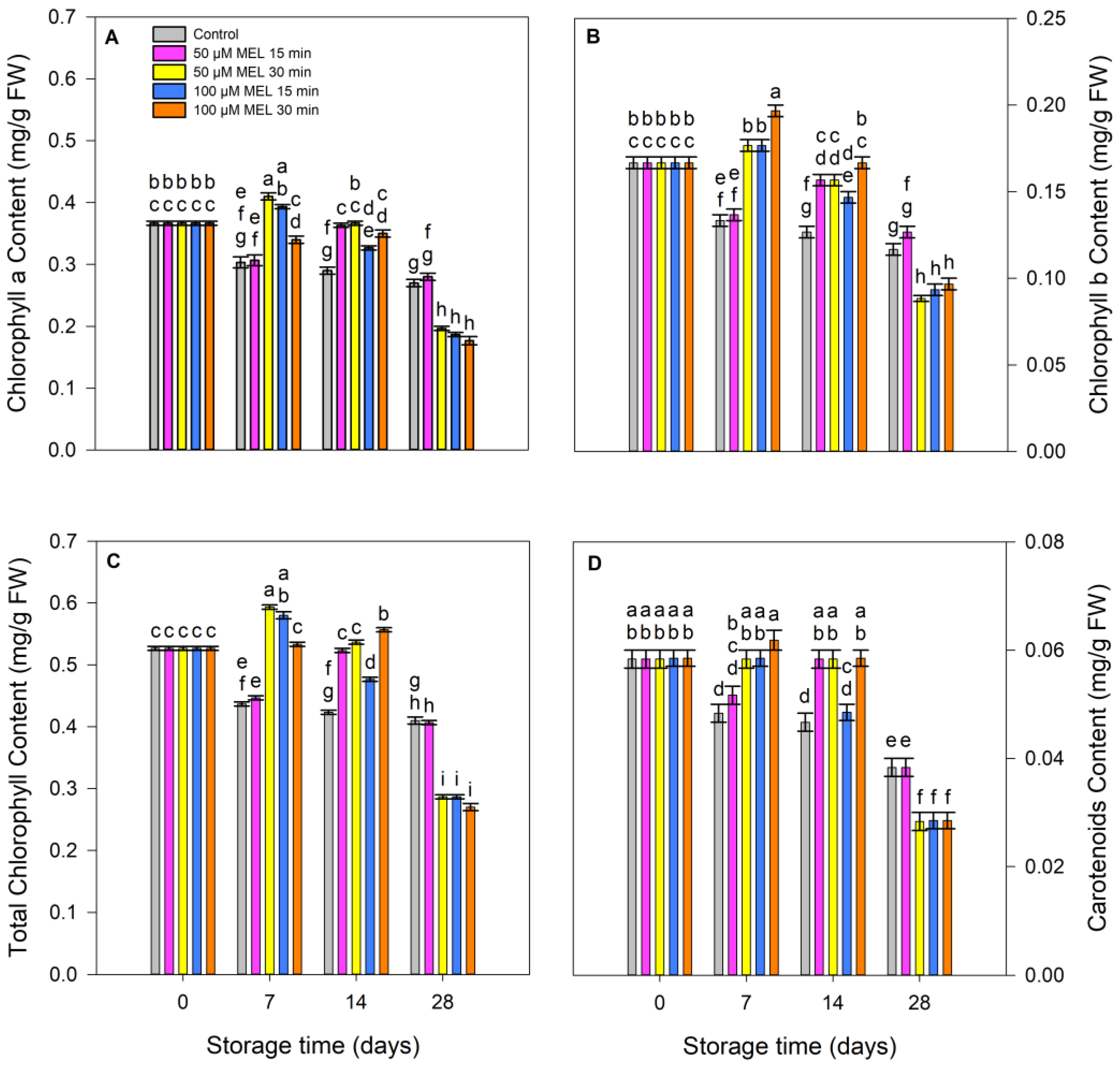
3.2. Effect of Melatonin on Color and Pigments
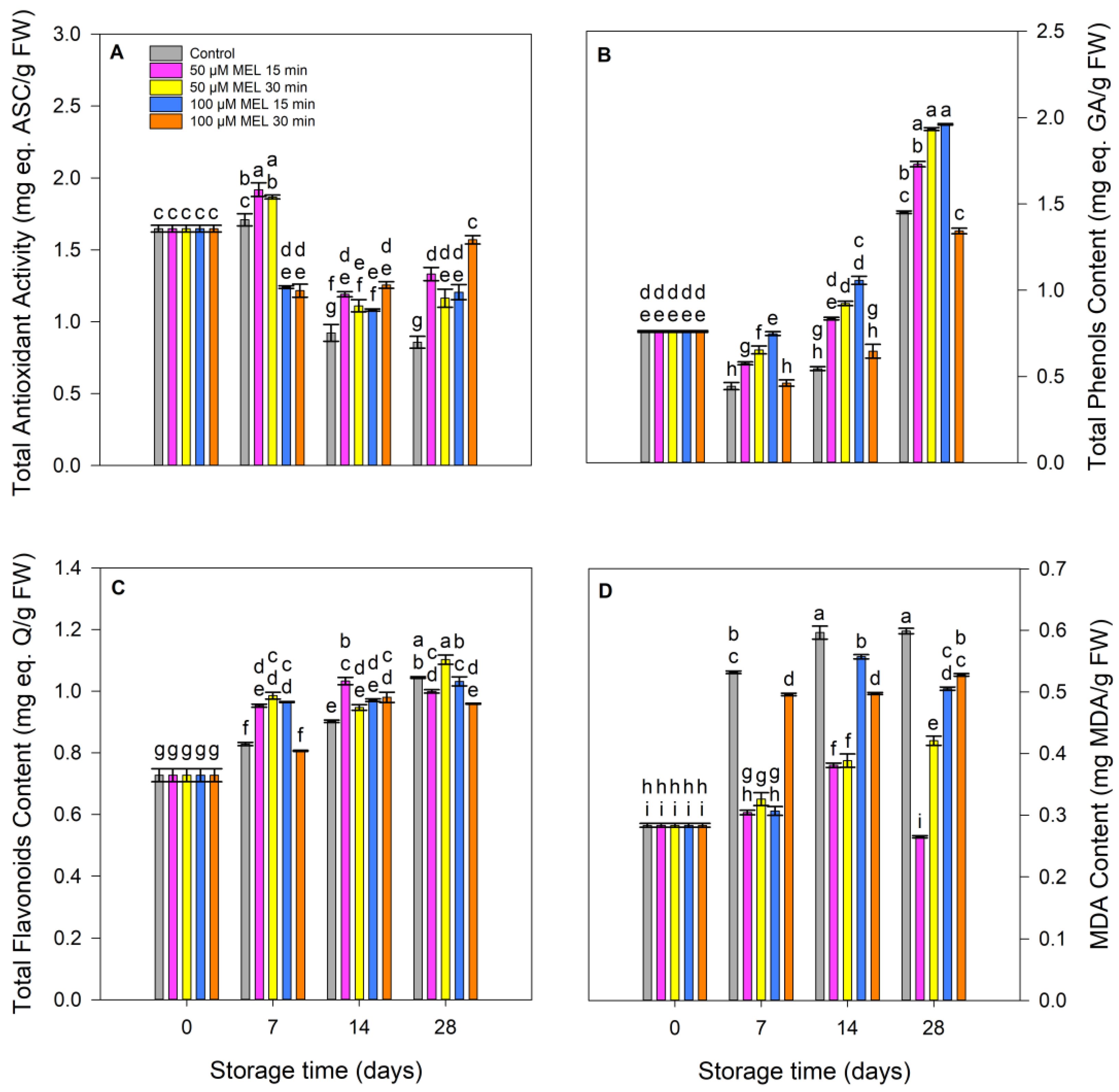
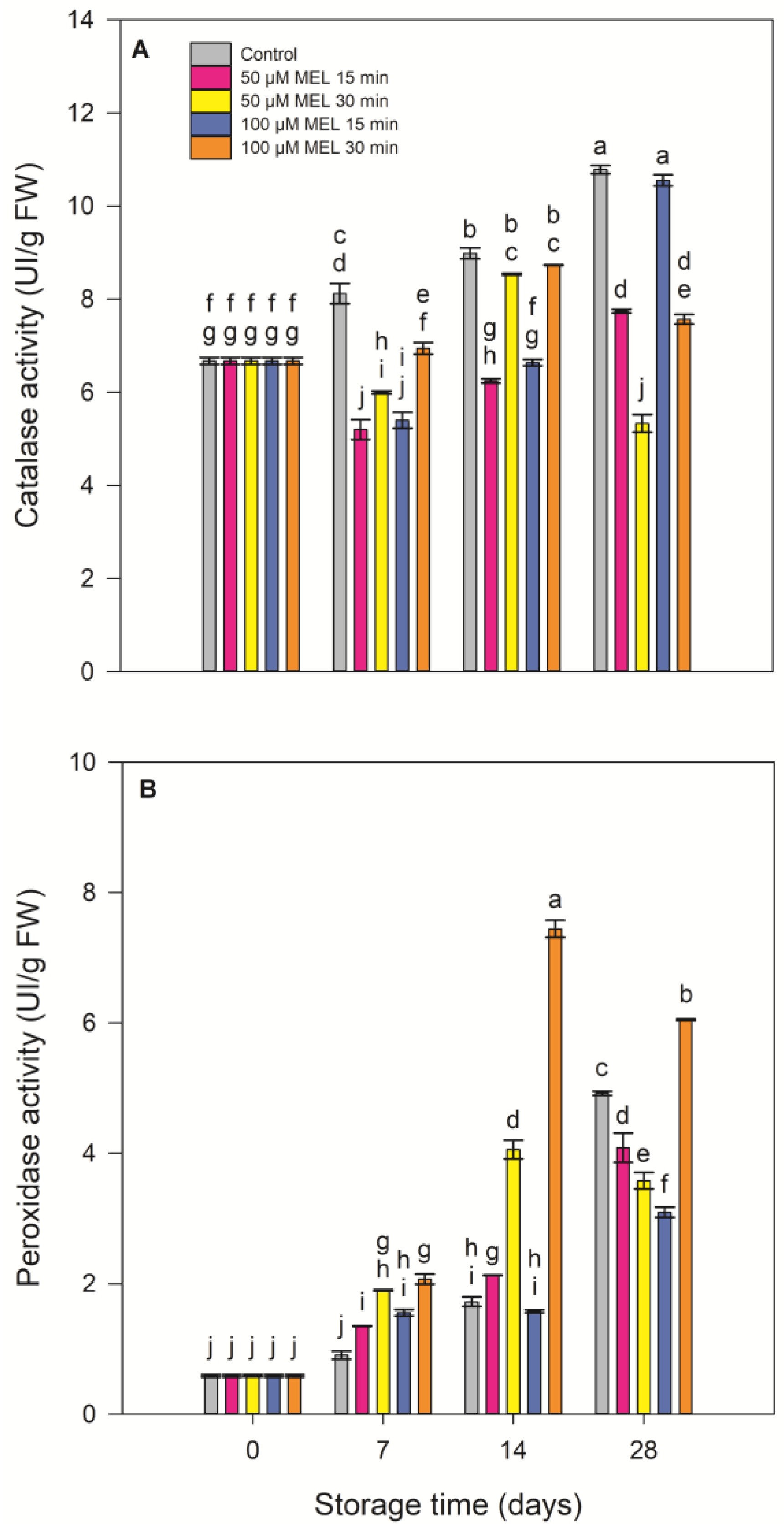
3.3. Effect of Melatonin on Antioxidative Status: Antioxidant Activity, Total Phenolic, Flavonoid, Malondialdehyde Contents and Enzymes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mann Pack Co. Broccolini. US Patent 75446875, 11 July 2000. [Google Scholar]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in Plant Protection and Human Health—Influences of Climate, Environment and Agronomic Practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kopeć, A.; Piatkowska, E.; Borczak, B.; Leszczyńska, T. The Beneficial Effects of Brassica Vegetables on Human Health. Rocz. Panstw. Zakl. Hig. 2012, 63, 389–395. [Google Scholar]
- Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 July 2022).
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T.; et al. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention. CA A Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef]
- The American Heart Association Diet and Lifestyle Recommendations. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations (accessed on 16 March 2023).
- Rask, L.; Andréasson, E.; Ekbom, B.; Eriksson, S.; Pontoppidan, B.; Meijer, J. Myrosinase: Gene Family Evolution and Herbivore Defense in Brassicaceae. Plant Mol. Biol. 2000, 42, 93–114. [Google Scholar] [CrossRef]
- Bones, A.; Rossiter, J. The Enzymic and Chemically Induced Decomposition of Glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The Myrosinase-Glucosinolate System, Its Organisation and Biochemistry. Physiol. Plant 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of Glucosinolates—Gene Discovery and Beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Kirsh, V.A.; Peters, U.; Mayne, S.T.; Subar, A.F.; Chatterjee, N.; Johnson, C.C.; Hayes, R.B.; on behalf of the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Prospective Study of Fruit and Vegetable Intake and Risk of Prostate Cancer. JNCI J. Natl. Cancer Inst. 2007, 99, 1200–1209. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, X.-O.; Xiang, Y.-B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.-T.; Zheng, W. Cruciferous Vegetable Consumption Is Associated with a Reduced Risk of Total and Cardiovascular Disease Mortality. Am. J. Clin. Nutr. 2011, 94, 240–246. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Tohidi, M.; Nazeri, P.; Mehran, M.; Azizi, F.; Mirmiran, P. Effect of Broccoli Sprouts on Insulin Resistance in Type 2 Diabetic Patients: A Randomized Double-Blind Clinical Trial. Int. J. Food Sci. Nutr. 2012, 63, 767–771. [Google Scholar] [CrossRef]
- Gudiño, I.; Martín, A.; Casquete, R.; Prieto, M.H.; Ayuso, M.C.; Córdoba, M.G. Evaluation of Broccoli (Brassica oleracea Var. Italica) Crop by-Products as Sources of Bioactive Compounds. Sci. Hortic. 2022, 304, 111284. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X. In Vitro Antitumor Activity of Broccolini Seeds Extracts. Scanning 2011, 33, 402–404. [Google Scholar] [CrossRef]
- Granado, F.; Olmedilla, B.; Herrero, C.; Pérez-Sacristán, B.; Blanco, I.; Blázquez, S. Bioavailability of Carotenoids and Tocopherols from Broccoli: In Vivo and in Vitro Assessment. Exp. Biol. Med. 2006, 231, 1733–1738. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of the Phenolic Components of Collard Greens, Kale, and Chinese Broccoli. J. Agric. Food Chem. 2009, 57, 7401–7408. [Google Scholar] [CrossRef]
- Podse˛dek, A.; Sosnowska, D.; Redzynia, M.; Anders, B. Antioxidant Capacity and Content of Brassica oleracea Dietary Antioxidants. Int. J. Food Sci. Tech. 2006, 41, 49–58. [Google Scholar] [CrossRef]
- Young, J.E.; Zhao, X.; Carey, E.E.; Welti, R.; Yang, S.-S.; Wang, W. Phytochemical Phenolics in Organically Grown Vegetables. Mol. Nutr. Food Res. 2005, 49, 1136–1142. [Google Scholar] [CrossRef]
- Miao, H.; Zeng, W.; Zhao, M.; Wang, J.; Wang, Q. Effect of Melatonin Treatment on Visual Quality and Health-Promoting Properties of Broccoli Florets under Room Temperature. Food Chem. 2020, 319, 126498. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Flowering, Fruit Set and Fruit Ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef]
- Wu, C.; Cao, S.; Xie, K.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Zhang, C.; Xu, F.; et al. Melatonin Delays Yellowing of Broccoli during Storage by Regulating Chlorophyll Catabolism and Maintaining Chloroplast Ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Tian, M.S.; Downs, C.G.; Lill, R.E.; King, G.A. A Role for Ethylene in the Yellowing of Broccoli after Harvest. J. Am. Soc. Hortic. Sci. 1994, 119, 276–281. [Google Scholar] [CrossRef]
- Guzman, I.; Yousef, G.G.; Brown, A.F. Simultaneous Extraction and Quantitation of Carotenoids, Chlorophylls, and Tocopherols in Brassica Vegetables. J. Agric. Food Chem. 2012, 60, 7238–7244. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Heinzelman, R.V. Structure of melatonin. J. Am. Chem. Soc. 1959, 81, 6084–6085. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by High Performance Liquid Chromatography-Mass Spectrometry. J. Pineal. Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol. Rhythm. Res. 1995, 26, 406–409. [Google Scholar]
- Arnao, M.B. Phytomelatonin: Discovery, Content, and Role in Plants. Adv. Bot. 2014, 2014, e815769. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of Melatonin in Plants: A Review. J. Pineal. Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Its Relationship to Plant Hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An Overview of the Importance and Mediating Functions of Melatonin against Environmental Stresses. Physiol. Plant 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, pH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Ahmad, N.; Naeem, M.; Ali, H.; Alabbosh, K.F.; Hussain, H.; Khan, I.; Siddiqui, S.A.; Khan, A.A.; Iqbal, B. From Challenges to Solutions: The Impact of Melatonin on Abiotic Stress Synergies in Horticultural Plants via Redox Regulation and Epigenetic Signaling. Sci. Hortic. 2023, 321, 112369. [Google Scholar] [CrossRef]
- Iqbal, B.; Kong, F.; Ullah, I.; Ali, S.; Li, H.; Wang, J.; Khattak, W.A.; Zhou, Z. Phosphorus Application Improves the Cotton Yield by Enhancing Reproductive Organ Biomass and Nutrient Accumulation in Two Cotton Cultivars with Different Phosphorus Sensitivity. Agronomy 2020, 10, 153. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The Role of Melatonin in Plant Growth and Metabolism, and Its Interplay with Nitric Oxide and Auxin in Plants under Different Types of Abiotic Stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Ali, S.; Gill, R.A.; Ulhassan, Z.; Zhang, N.; Hussain, S.; Zhang, K.; Huang, Q.; Sagir, M.; Tahir, M.B.; Gill, M.B.; et al. Exogenously Applied Melatonin Enhanced the Tolerance of Brassica Napus against Cobalt Toxicity by Modulating Antioxidant Defense, Osmotic Adjustment, and Expression of Stress Response Genes. Ecotoxicol. Environ. Saf. 2023, 252, 114624. [Google Scholar] [CrossRef]
- Altaf, M.A.; Sharma, N.; Srivastava, D.; Mandal, S.; Adavi, S.; Jena, R.; Bairwa, R.K.; Gopalakrishnan, A.V.; Kumar, A.; Dey, A.; et al. Deciphering the Melatonin-Mediated Response and Signalling in the Regulation of Heavy Metal Stress in Plants. Planta 2023, 257, 115. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Effects of Melatonin Treatment on the Enzymatic Browning and Nutritional Quality of Fresh-Cut Pear Fruit. Food Chem. 2019, 299, 125116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huber, D.J.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Delay of Postharvest Browning in Litchi Fruit by Melatonin via the Enhancing of Antioxidative Processes and Oxidation Repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.-Y. Melatonin Attenuates Postharvest Physiological Deterioration of Cassava Storage Roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef]
- Tan, X.; Fan, Z.; Kuang, J.; Lu, W.; Reiter, R.J.; Lakshmanan, P.; Su, X.; Zhou, J.; Chen, J.; Shan, W. Melatonin Delays Leaf Senescence of Chinese Flowering Cabbage by Suppressing ABFs-mediated Abscisic Acid Biosynthesis and Chlorophyll Degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin Promotes Ripening and Improves Quality of Tomato Fruit during Postharvest Life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Wei, L.; Liu, C.; Zheng, H.; Zheng, L. Melatonin Treatment Affects the Glucoraphanin-Sulforaphane System in Postharvest Fresh-Cut Broccoli (Brassica oleracea L.). Food Chem. 2020, 307, 125562. [Google Scholar] [CrossRef]
- Wei, L.; Liu, C.; Wang, J.; Younas, S.; Zheng, H.; Zheng, L. Melatonin Immersion Affects the Quality of Fresh-Cut Broccoli (Brassica oleracea L.) during Cold Storage: Focus on the Antioxidant System. J. Food Process Preserv. 2020, 44, e14691. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Ruiz-Cano, D.; Giraldo-Acosta, M.; Cano, A.; Arnao, M.B. Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals. Molecules 2022, 27, 1523. [Google Scholar] [CrossRef]
- Hu, H.; Luo, S.; An, R.; Li, P. Endogenous Melatonin Delays Sepal Senescence and Extends the Storage Life of Broccoli Florets by Decreasing Ethylene Biosynthesis. Postharvest Biol. Technol. 2022, 188, 111894. [Google Scholar] [CrossRef]
- Cano, A.; Giraldo-Acosta, M.; García-Sánchez, S.; Hernández-Ruiz, J.B.; Arnao, M.B. Effect of Melatonin in Broccoli Postharvest and Possible Melatonin Ingestion Level. Plants 2022, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Acosta, M.; Martínez-Andújar, C.; Martínez-Melgarejo, P.A.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Protective Effect (Safener) of Melatonin on Vigna radiata L. Seedlings in the Presence of the Fungicide Copper Oxychloride. J. Plant Growth Regul. 2023, 42, 4918–4934. [Google Scholar] [CrossRef]
- Mclellan, M.R.; Lind, L.R.; Kime, R.W. HUE Angle Determinations and Statistical Analysis for Multiquadrant Hunter L,a,b Data. J. Food Qual. 1995, 18, 235–240. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. Methods to Measure the Antioxidant Activity in Plant Material. A Comparative Discussion. Free Rad. Res. 1999, 31, 89–96. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apicult. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Niehaus, W.G., Jr.; Samuelsson, B. Formation of Malonaldehyde from Phospholipid Arachidonate during Microsomal Lipid Peroxidation. Eur. J. Biochem. 1968, 6, 126–130. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases: In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1955; Volume 2, pp. 764–775. [Google Scholar]
- Kaur, H.; Bhatla, S.C. Melatonin–Nitric Oxide Interaction Modulates Catalase Activity and Hydrogen Peroxide Homeostasis in Sunflower Seedling Cotyledons Accompanying NaCl Stress. J. Plant Growth Regul. 2022, 5, 1–2. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. ABTS/TEAC (2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid)/Trolox®-Equivalent Antioxidant Capacity) Radical Scavenging Mixed-Mode Assay. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 117–139. [Google Scholar]
- Serrano, M.; Martinez-Romero, D.; Guillén, F.; Castillo, S.; Valero, D. Maintenance of Broccoli Quality and Functional Properties during Cold Storage as Affected by Modified Atmosphere Packaging. Postharvest Biol. Technol. 2006, 39, 61–68. [Google Scholar] [CrossRef]
- Pintos, F.M.; Hasperué, J.H.; Ixtaina, P.; Vicente, A.R.; Lemoine, M.L.; Rodoni, L.M. Short Light Exposure Preserves Broccoli Head Quality and Nutrients during Refrigerated Storage. J. Food Process. Preserv. 2021, 45, e15801. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.; Zhang, J.; Shan, C.; Rengel, Z.; Song, Z.; Chen, Q. Phytomelatonin Receptor PMTR1-Mediated Signaling Regulates Stomatal Closure in Arabidopsis Thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Generozova, I.P.; Vasilyev, S.V.; Butsanets, P.A.; Shugaev, A.G. Combined Action of Melatonin and Water Deficiency on Growth and MDA Content of Hypocotyls and Roots of Lupine and Respiration of Mitochondria Isolated from These Organs. Russ. J. Plant Physiol. 2022, 69, 93. [Google Scholar] [CrossRef]
- Rodríguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of Antioxidant Enzymes: A Significant Role for Melatonin. J. Pineal. Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial Effects of Melatonin in Overcoming Drought Stress in Wheat Seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Siddiqui, H.M.; Alamri, S.; Al-Khaishany, Y.M.; Khan, N.M.; Al-Amri, A.; Ali, M.H.; Alaraidh, A.I.; Alsahli, A.A. Exogenous Melatonin Counteracts NaCl-Induced Damage by Regulating the Antioxidant System, Proline and Carbohydrates Metabolism in Tomato Seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo-Acosta, M.; Ruiz-Cano, D.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Extended Post-Harvest Effect of Melatonin in Fresh-Cut Broccolini Plants (Bimi®). Agronomy 2023, 13, 2459. https://doi.org/10.3390/agronomy13102459
Giraldo-Acosta M, Ruiz-Cano D, Cano A, Hernández-Ruiz J, Arnao MB. Extended Post-Harvest Effect of Melatonin in Fresh-Cut Broccolini Plants (Bimi®). Agronomy. 2023; 13(10):2459. https://doi.org/10.3390/agronomy13102459
Chicago/Turabian StyleGiraldo-Acosta, Manuela, Domingo Ruiz-Cano, Antonio Cano, Josefa Hernández-Ruiz, and Marino B. Arnao. 2023. "Extended Post-Harvest Effect of Melatonin in Fresh-Cut Broccolini Plants (Bimi®)" Agronomy 13, no. 10: 2459. https://doi.org/10.3390/agronomy13102459






