Mixed Fermentations of Yeasts and Lactic Acid Bacteria as Sustainable Processes to Enhance the Chemical Composition of Cider Made of Topaz and Red Topaz Apple Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Glucides and Organic Acids Using HPLC
2.3. Volatile Compounds Using GC/MS
2.4. Amino Acids Using Gas-Chromatography
2.5. Analysis of Phenolic Compound Using HPLC-DAD ESI+
2.6. Sensory Analysis
2.7. Data Analysis
3. Results and Discussions
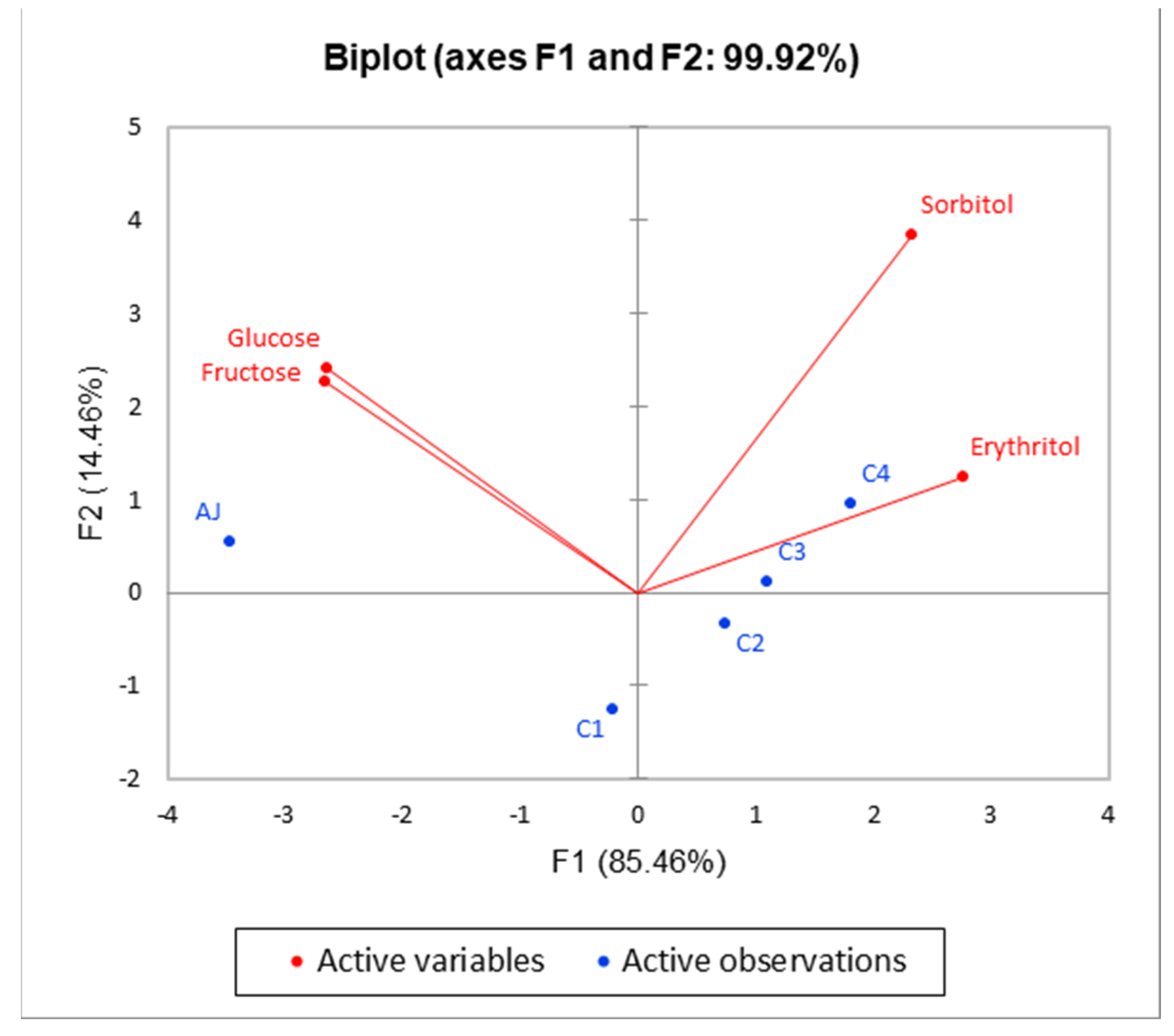
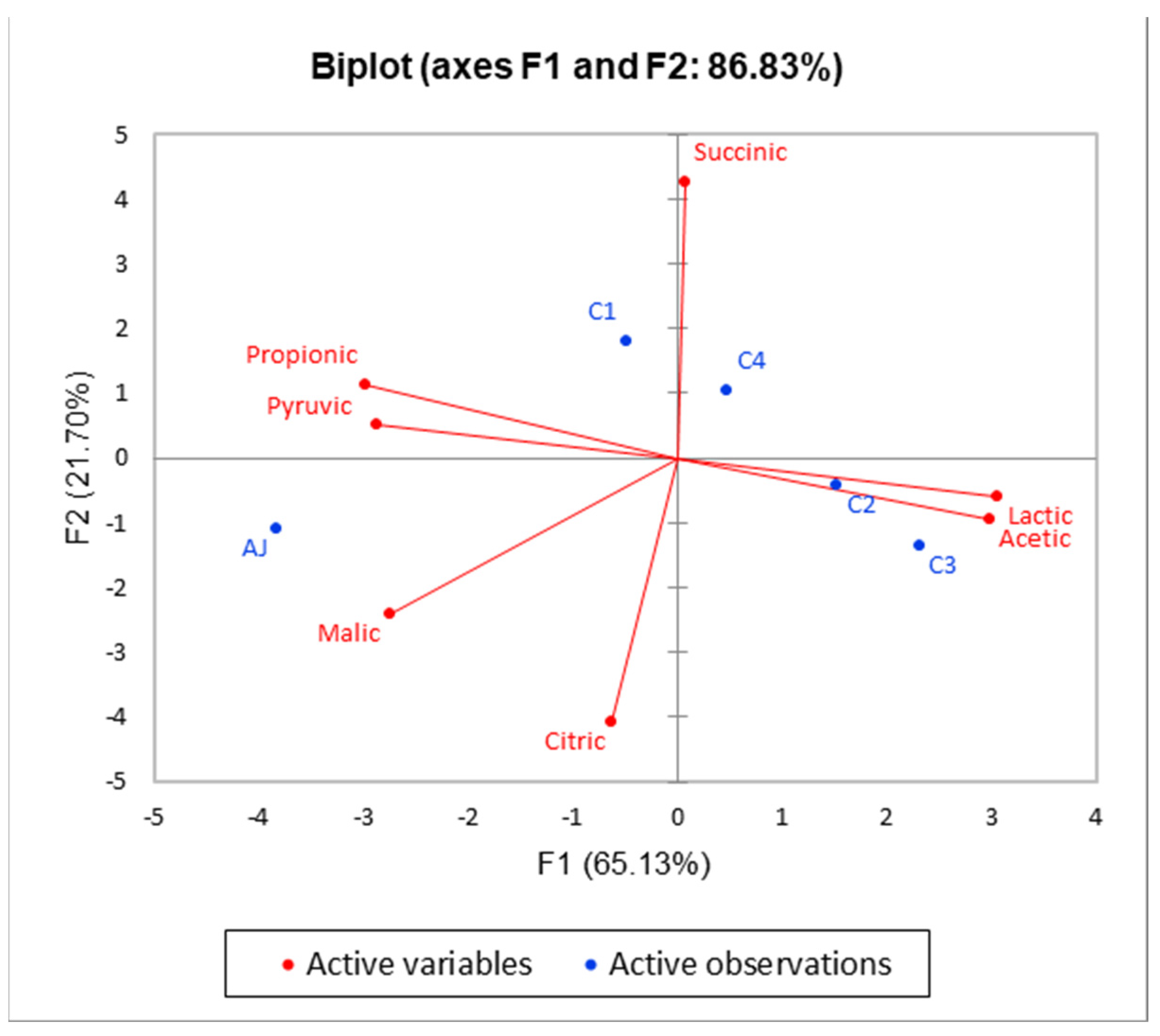
3.1. Determination of Sugars and Organic Acids
3.2. Volatile Compounds Analysis
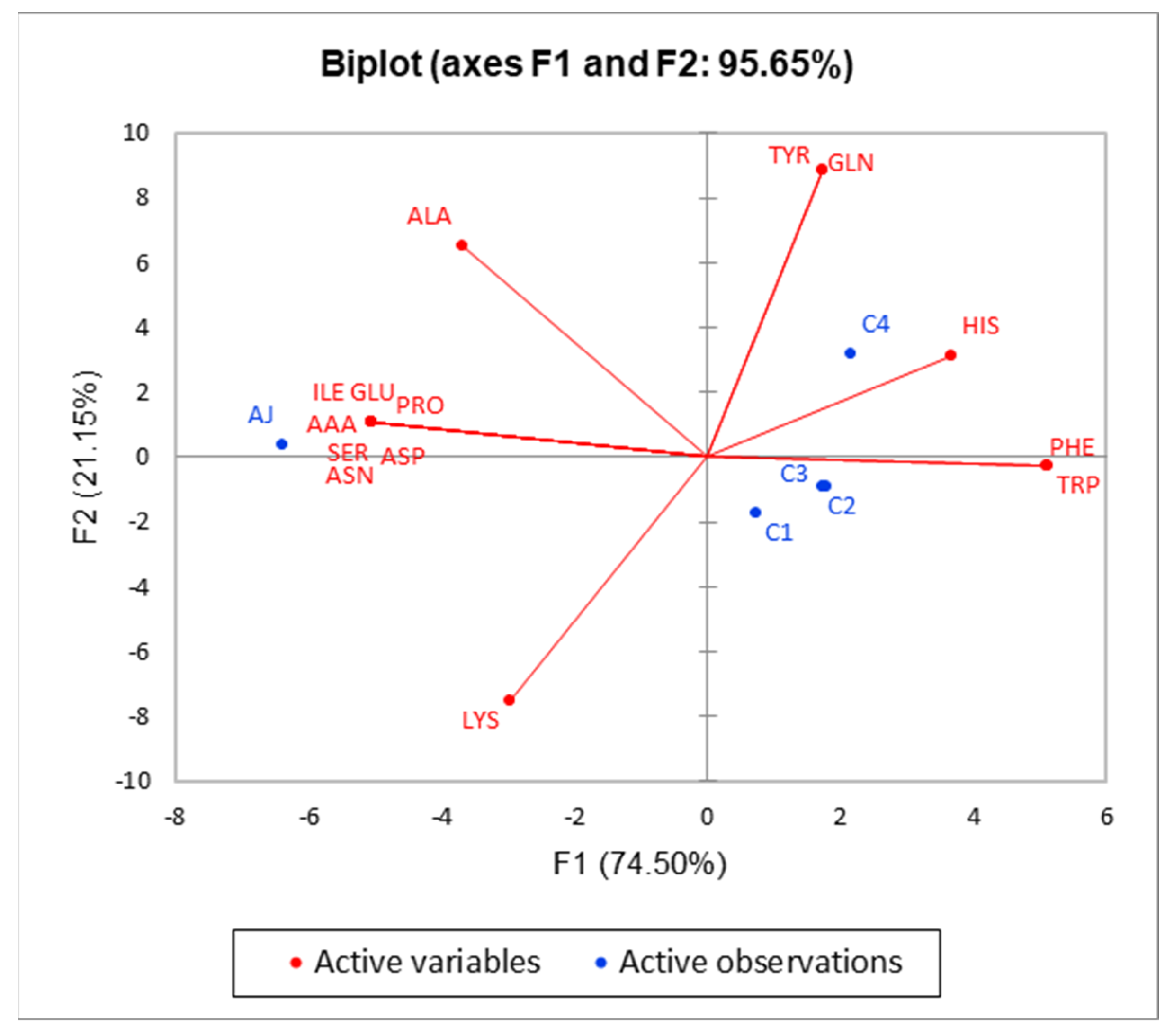
3.3. Amino Acids Profiles of the Ciders and Apple Juice
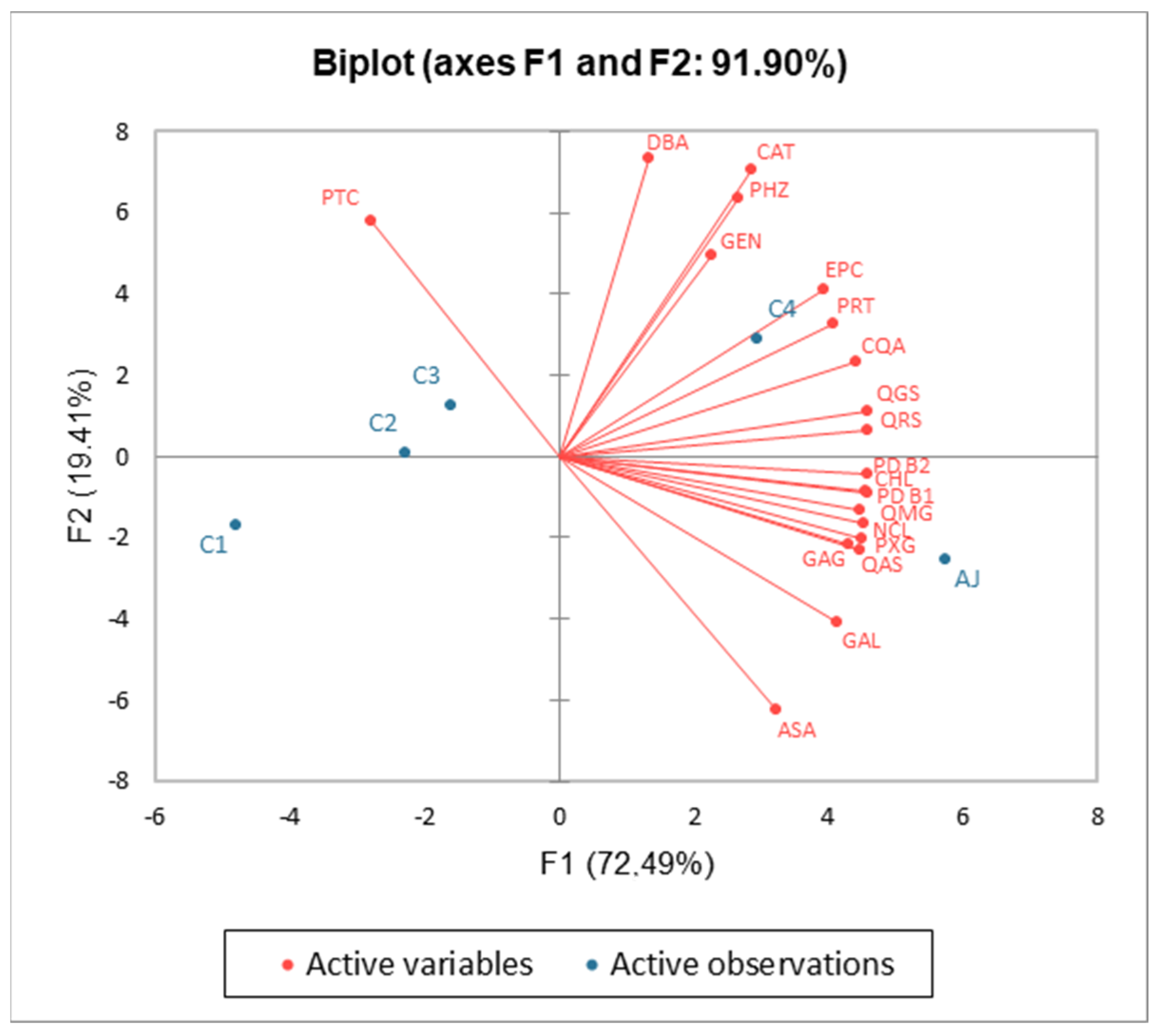
3.4. Phenolic Compounds Analysis
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumby, K.M.; Bartle, L.; Grbin, P.R.; Jiranek, V. Measures to Improve Wine Malolactic Fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 2033–2051. [Google Scholar] [CrossRef] [PubMed]
- Calugar, P.C.; Coldea, T.E.; Salanță, L.C.; Pop, C.R.; Pasqualone, A.; Burja-Udrea, C.; Zhao, H.; Mudura, E. An Overview of the Factors Influencing Apple Cider Sensory and Microbial Quality from Raw Materials to Emerging Processing Technologies. Processes 2021, 9, 502. [Google Scholar] [CrossRef]
- Chen, X.; Lin, M.; Hu, L.; Xu, T.; Xiong, D.; Li, L.; Zhao, Z. Research on the Effect of Simultaneous and Sequential Fermentation with Saccharomyces Cerevisiae and Lactobacillus Plantarum on Antioxidant Activity and Flavor of Apple Cider. Fermentation 2023, 9, 102. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non- Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Zamora, A.; Gutiérrez-Avendaño, D.O.; Arellano-Plaza, M.; De la Torre González, F.J.; Barrera-Martínez, I.; Gschaedler Mathis, A.; Casas-Godoy, L. The Non- Saccharomyces Yeast Pichia Kluyveri for the Production of Aromatic Volatile Compounds in Alcoholic Fermentation. FEMS Yeast Res. 2021, 20, foaa067. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia Kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Changes in the Concentration of Yeast-Derived Volatile Compounds of Red Wine during Malolactic Fermentation with Four Commercial Starter Cultures of Oenococcus Oeni. J. Agric. Food Chem. 2005, 53, 10134–10139. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X. Sen The Effects of Simultaneous and Sequential Inoculation of Yeast and Autochthonous Oenococcus Oeni on the Chemical Composition of Red-Fleshed Apple Cider. LWT 2020, 124, 109184. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Chuat, V.; Dalmasso, M.; Laplace, J.-M.; Cretenet, M. Multiplex PCR for Rapid Identification of Major Lactic Acid Bacteria Genera in Cider and Other Fermented Foods. Int. J. Food Microbiol. 2019, 291, 17–24. [Google Scholar] [CrossRef]
- Kristof, I.; Ledesma, S.C.; Apud, G.R.; Vera, N.R.; Aredes Fernández, P.A. Oenococcus Oeni Allows the Increase of Antihypertensive and Antioxidant Activities in Apple Cider. Heliyon 2023, 9, e16806. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Barroca, M.J.; Coldea, T.E.; Bartkiene, E.; Anjos, O. Apple Fermented Products: An Overview of Technology, Properties and Health Effects. Processes 2021, 9, 223. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and Technological Potential of Lactobacillus Plantarum Bacteria Suitable for Wine Malolactic Fermentation and Grape Aroma Release. LWT 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Balmaseda, A.; Bordons, A.; Reguant, C.; Bautista-Gallego, J. Non-Saccharomyces in Wine: Effect Upon Oenococcus Oeni and Malolactic Fermentation. Front. Microbiol. 2018, 9, 534. [Google Scholar] [CrossRef]
- Nehme, N.; Mathieu, F.; Taillandier, P. Impact of the Co-Culture of Saccharomyces Cerevisiae–Oenococcus Oeni on Malolactic Fermentation and Partial Characterization of a Yeast-Derived Inhibitory Peptidic Fraction. Food Microbiol. 2010, 27, 150–157. [Google Scholar] [CrossRef]
- du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures—An Overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.M.; de Souza Oliveira, R.P.; Domínguez, J.M. Potential of Lees from Wine, Beer and Cider Manufacturing as a Source of Economic Nutrients: An Overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Malvoni, M.; Congedo, P.M.; Laforgia, D. Analysis of Energy Consumption: A Case Study of an Italian Winery. Energy Procedia 2017, 126, 227–233. [Google Scholar] [CrossRef]
- Jussier, D.; Dubé Morneau, A.; Mira de Orduña, R. Effect of Simultaneous Inoculation with Yeast and Bacteria on Fermentation Kinetics and Key Wine Parameters of Cool-Climate Chardonnay. Appl. Environ. Microbiol. 2006, 72, 221–227. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Aung, M.T.; Lee, P.-R.; Yu, B. Yeast and Volatile Evolution in Cider Co-Fermentation with Saccharomyces Cerevisiae and Williopsis Saturnus. Ann. Microbiol. 2016, 66, 307–315. [Google Scholar] [CrossRef]
- Avîrvarei, A.-C.; Pop, C.R.; Mudura, E.; Ranga, F.; Hegheș, S.-C.; Gal, E.; Zhao, H.; Fărcaș, A.C.; Chiș, M.S.; Coldea, T.E. Contribution of Saccharomyces and Non-Saccharomyces Yeasts on the Volatile and Phenolic Profiles of Rosehip Mead. Antioxidants 2023, 12, 1457. [Google Scholar] [CrossRef]
- Picinelli, A.; Suárez, B.; Moreno, J.; Rodríguez, R.; Caso-García, L.M.; Mangas, J.J. Chemical Characterization of Asturian Cider. J. Agric. Food Chem. 2000, 48, 3997–4002. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Lemos, M.; Genisheva, Z.; Domingues, L.; Vilanova, M.; Oliveira, J.M. Validation of a LLME/GC-MS Methodology for Quantification of Volatile Compounds in Fermented Beverages. Molecules 2020, 25, 621. [Google Scholar] [CrossRef] [PubMed]
- Coldea, T.E.; Socaciu, C.; Mudura, E.; Socaci, S.A.; Ranga, F.; Pop, C.R.; Vriesekoop, F.; Pasqualone, A. Volatile and Phenolic Profiles of Traditional Romanian Apple Brandy after Rapid Ageing with Different Wood Chips. Food Chem. 2020, 320, 126643. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Januszek, M. Effect of Musts Oxygenation at Various Stages of Cider Production on Oenological Parameters, Antioxidant Activity, and Profile of Volatile Cider Compounds. Biomolecules 2020, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Bedriñana, R.P.; Alonso, J.J.M.; Valles, B.S. Evaluation of autochthonous Saccharomyces bayanus strains under stress conditions for making ice ciders. LWT—Food Sci. Technol. 2017, 81, 217–225. [Google Scholar] [CrossRef]
- Sánchez, A.; de Revel, G.; Antalick, G.; Herrero, M.; García, L.A.; Díaz, M. Influence of Controlled Inoculation of Malolactic Fermentation on the Sensory Properties of Industrial Cider. J. Ind. Microbiol. Biotechnol. 2014, 41, 853–867. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review. Molecules 2020, 25, 3698. [Google Scholar] [CrossRef]
- Zhang, D.; Lovitt, R.W. Strategies for Enhanced Malolactic Fermentation in Wine and Cider Maturation. J. Chem. Technol. Biotechnol. 2006, 81, 1130–1140. [Google Scholar] [CrossRef]
- Tracey, R.; Rooyen, T.J. Van Utilization of Glucose, Fructose and Malic Acid by Malolactic Bacteria: Effect of Ethanol and Formation of Mannitol and Volatile Acids. J. Appl. Bacteriol. 1988, 65, 113–118. [Google Scholar] [CrossRef]
- Tronchoni, J.; Gamero, A.; Arroyo-López, F.N.; Barrio, E.; Querol, A. Differences in the Glucose and Fructose Consumption Profiles in Diverse Saccharomyces Wine Species and Their Hybrids during Grape Juice Fermentation. Int. J. Food Microbiol. 2009, 134, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.; Denat, M.; Minebois, R.; Heras, J.M.; Guillamón, J.M.; Ferreira, V.; Querol, A. Modulation of Aroma and Chemical Composition of Albariño Semi-Synthetic Wines by Non-Wine Saccharomyces Yeasts and Bottle Aging. Food Microbiol. 2022, 104, 103981. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Q. Malolactic Fermentation in Wine—beyond Deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.L.; Costa, C.E.; Cunha, J.T.; Soares, P.O.; Domingues, L. Metabolic Engineering of Saccharomyces Cerevisiae for the Production of Top Value Chemicals from Biorefinery Carbohydrates. Biotechnol. Adv. 2021, 47, 107697. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces Cerevisiae and Non-Saccharomyces Strains on Alcoholic Fermentation Behavior and Aroma Profile of Yellow-Fleshed Peach Wine. LWT 2022, 155, 112993. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A New Approach to the Use of Apple Pomace in Cider Making for the Recovery of Phenolic Compounds. LWT 2020, 126, 109316. [Google Scholar] [CrossRef]
- Way, M.L.; Jones, J.E.; Longo, R.; Dambergs, R.G.; Swarts, N.D. A Preliminary Study of Yeast Strain Influence on Chemical and Sensory Characteristics of Apple Cider. Fermentation 2022, 8, 455. [Google Scholar] [CrossRef]
- He, W.; Liu, S.; Heponiemi, P.; Heinonen, M.; Marsol-Vall, A.; Ma, X.; Yang, B.; Laaksonen, O. Effect of Saccharomyces Cerevisiae and Schizosaccharomyces Pombe Strains on Chemical Composition and Sensory Quality of Ciders Made from Finnish Apple Cultivars. Food Chem. 2021, 345, 128833. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of Polyphenols and Organic Acids during the Fermentation of Apple Cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, F.; Ji, B.; Nout, R.M.J.; Fang, Q.; Yang, Z. Determination of Organic Acids Evolution during Apple Cider Fermentation Using an Improved HPLC Analysis Method. Eur. Food Res. Technol. 2008, 227, 1183–1190. [Google Scholar] [CrossRef]
- Herrero, M.; Cuesta, I.; García, L.A.; Díaz, M. Changes in Organic Acids During Malolactic Fermentation at Different Temperatures in Yeast-Fermented Apple Juice. J. Inst. Brew. 1999, 105, 191–196. [Google Scholar] [CrossRef]
- Han, Y.; Du, J. A Comparative Study of the Effect of Bacteria and Yeasts Communities on Inoculated and Spontaneously Fermented Apple Cider. Food Microbiol. 2023, 111, 104195. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and Bacterial Modulation of Wine Aroma and Flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Changes in the Profile of Volatile Compounds and Amino Acids during Cider Fermentation Using Dessert Variety of Apples. Eur. Food Res. Technol. 2014, 239, 67–77. [Google Scholar] [CrossRef]
- Joshi, V.K.; Kumar, V. Influence of Different Sugar Sources, Nitrogen Sources and Inocula on the Quality Characteristics of Apple Tea Wine. J. Inst. Brew. 2017, 123, 268–276. [Google Scholar] [CrossRef]
- Gschaedler, A.; Iñiguez-Muñoz, L.E.; Flores-Flores, N.Y.; Kirchmayr, M.; Arellano-Plaza, M. Use of Non-Saccharomyces Yeasts in Cider Fermentation: Importance of the Nutrients Addition to Obtain an Efficient Fermentation. Int. J. Food Microbiol. 2021, 347, 109169. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces Cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Villière, A.; Arvisenet, G.; Lethuaut, L.; Prost, C.; Sérot, T. Selection of a Representative Extraction Method for the Analysis of Odourant Volatile Composition of French Cider by GC–MS–O and GC×GC–TOF-MS. Food Chem. 2012, 131, 1561–1568. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Han, Y. Quantification of Volatile Compounds in Chinese Ciders by Stir Bar Sorptive Extraction (SBSE) and Gas Chromatography-Mass Spectrometry (GC-MS). J. Inst. Brew. 2011, 117, 61–66. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, W.; Qian, M.C. Characterization of Aroma Compounds in Apple Cider Using Solvent-Assisted Flavor Evaporation and Headspace Solid-Phase Microextraction. J. Agric. Food Chem. 2007, 55, 3051–3057. [Google Scholar] [CrossRef]
- Nešpor, J.; Karabín, M.; Štulíková, K.; Dostálek, P. An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production. Molecules 2019, 24, 2117. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of Yeasts for Apple Juice Fermentation and Production of Cider Volatile Compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Cordente, A.G.; Schmidt, S.; Beltran, G.; Torija, M.J.; Curtin, C.D. Harnessing Yeast Metabolism of Aromatic Amino Acids for Fermented Beverage Bioflavouring and Bioproduction. Appl. Microbiol. Biotechnol. 2019, 103, 4325–4336. [Google Scholar] [CrossRef]
- Ma, S.; Neilson, A.P.; Lahne, J.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free Amino Acid Composition of Apple Juices with Potential for Cider Making as Determined by UPLC-PDA. J. Inst. Brew. 2018, 124, 467–476. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Effects of Sequential Mixed Cultures of Wickerhamomyces Anomalus and Saccharomyces Cerevisiae on Apple Cider Fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Machado dos Santos, T.P.; Ferreira Zielinski, A.A.; Eleutério dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on Chemical Profile in Apple Juice and Cider Made from Unripe, Ripe and Senescent Dessert Varieties. LWT Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- dos Santos, C.M.E.; de Arruda Moura Pietrowski, G.; Braga, C.M.; Rossi, M.J.; Ninow, J.; Machado dos Santos, T.P.; Wosiacki, G.; Jorge, R.M.M.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, C1170–C1177. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ou, J.; Huang, Y.; Li, Q.; Xu, G.; Liu, Z.; Yang, S. Determination of 21 Free Amino Acids in Fruit Juices by HPLC Using a Modification of the 6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate (AQC) Method. Food Anal. Methods 2015, 8, 428–437. [Google Scholar] [CrossRef]
- Way, M.L.; Jones, J.E.; Nichols, D.S.; Dambergs, R.G.; Swarts, N.D. A Comparison of Laboratory Analysis Methods for Total Phenolic Content of Cider. Beverages 2020, 6, 55. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A.; Tyrakowska, B. Quality of Commercial Apple Juices Evaluated on the Basis of the Polyphenol Content and the TEAC Antioxidant Activity. J. Food Sci. 2003, 68, 1844–1849. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, C.; Guo, Y.; Wang, X.; Meng, Y. Polyphenols in Fermented Apple Juice: Beneficial Effects on Human Health. J. Funct. Foods 2021, 76, 104294. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of Apple Cultivar, Ripening Stage, Fermentation Type and Yeast Strain on Phenolic Composition of Apple Ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zuriarrain-Ocio, A.; Zuriarrain, J.; Vidal, M.; Dueñas, M.T.; Berregi, I. Antioxidant Activity and Phenolic Profiles of Ciders from the Basque Country. Food Biosci. 2021, 41, 100887. [Google Scholar] [CrossRef]
- Riekstina-Dolge, R.; Kruma, Z.; Dimins, F.; Straumite, E.; Karklina, D. Phenolic Composition and Sensory Properties of Ciders Produced from Latvian Apples. Proc. Latv. Univ. Agric. 2014, 31, 39–45. [Google Scholar] [CrossRef]
- Heinmaa, L.; Moor, U.; Põldma, P.; Raudsepp, P.; Kidmose, U.; Lo Scalzo, R. Content of Health-Beneficial Compounds and Sensory Properties of Organic Apple Juice as Affected by Processing Technology. LWT Food Sci. Technol. 2017, 85, 372–379. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Zhang, X.; Guo, H.; Yuan, Y.; Yue, T. Multi-Omics Discovery of Aroma-Active Compound Formation by Pichia Kluyveri during Cider Production. LWT 2022, 159, 113233. [Google Scholar] [CrossRef]
- Benvenutti, L.; Bortolini, D.G.; Nogueira, A.; Zielinski, A.A.F.; Alberti, A. Effect of Addition of Phenolic Compounds Recovered from Apple Pomace on Cider Quality. LWT 2019, 100, 348–354. [Google Scholar] [CrossRef]



| Compounds/Chemical Parameters | AJ | C1 | C2 | C3 | C4 | p-Value | Sig. |
|---|---|---|---|---|---|---|---|
| Ethanol (% v/v) | nd | 6.10 ± 0.03 b | 6.20 ± 0.02 a | 6.20 ± 0.05 a | 6.10 ± 0.01 b | p < 0.01 | ** |
| Total acidity (g L−1 malic acid) | 6.61 ± 0.02 a | 5.42 ± 0.03 b | 3.14 ± 0.03 d | 2.94 ± 0.03 e | 4.28 ± 0.02 c | p < 0.001 | *** |
| pH | 3.46 ± 0.04 c | 3.55 ± 0.03 c | 3.79 ± 0.03 ab | 3.85 ± 0.04 a | 3.71 ± 0.03 b | p < 0.001 | *** |
| Sugars | |||||||
| Glucose | 42.54 ± 0.56 | nd | nd | nd | nd | ||
| Fructose | 61.11 ± 0.63 a | 5.46 ± 0.27 b | 1.85 ± 0.07 d | 2.01 ± 0.09 d | 3.79 ± 0,11 c | p < 0.001 | *** |
| Sorbitol | 0.98 ± 0.05 d | 1.01 ± 0.05 d | 1.31 ± 0.06 c | 1.44 ± 0.05 b | 1.67 ± 0.06 a | p < 0.001 | *** |
| Erythritol | nd | 0.10 ± 0.02 c | 0.15 ± 0.03 b | 0.17 ± 0.02 ab | 0.22 ± 0.02 a | p < 0.01 | ** |
| Organic acids | |||||||
| Malic | 2.65 ± 0.11 a | 0.14 ± 0.03 b | 0.16 ± 0.02 b | 0.18 ± 0.02 b | 0.18 ± 0.02 b | p < 0.05 | * |
| Lactic | nd | 1.41 ± 0.08 d | 2.83 ± 0.09 b | 3.32 ± 0.10 a | 2.23 ± 0.05 c | p < 0.001 | *** |
| Pyruvic | 0.38 ± 0.04 a | 0.33 ± 0.04 ab | 0.30 ± 0.03 ab | 0.24 ± 0.04 b | 0.28 ± 0.03 b | p < 0.05 | * |
| Citric | 0.24 ± 0.02 a | 0.11 ± 0.01 c | 0.14 ± 0.02 bc | 0.24 ± 0.03 a | 0.19 ± 0.02 b | p < 0.05 | * |
| Succinic | 0.40 ± 0.03 cd | 0.57 ± 0.02 b | 0.35 ± 0.02 d | 0.43 ± 0.02 c | 0.65 ± 0.04 a | p < 0.01 | ** |
| Acetic | nd | 0.07 ± 0.01 b | 0.13 ± 0.03 a | 0.15 ± 0.03 a | 0.07 ± 0.01 b | p < 0.05 | * |
| Volatile Compounds | AJ | C1 | C2 | C3 | C4 | p-Value | Sig. |
|---|---|---|---|---|---|---|---|
| 2-Methylbut-1-ene | 40.97 ± 2.81 a | 27.79 ± 0.23 b | 13.75 ± 0.21 c | 15.67 ± 0.22 c | 24.71 ± 2.53 b | p < 0.01 | ** |
| heptane | 15.92 ± 1.56 a | 15.91 ± 2.36 a | 11.10 ± 0.33 b | 12.82 ± 0.32 ab | 16.34 ± 1.47 a | p < 0.01 | ** |
| 2-Methylpentan-3-ol | 3.56 ± 0.07 a | 2.01 ± 0.33 b | 1.53 ± 0.04 c | 2.07 ± 0.04 b | 2.30 ± 0.10 b | p < 0.01 | ** |
| butyl acetate | 3.36 ± 0.29 a | 0.81 ± 0.08 b | 0.29 ± 0.01 c | 0.47 ± 0.03 c | nd | p < 0.001 | *** |
| 2-Methylpropan-1-ol | nd | 2.30 ± 0.35 b | 2.60 ± 0.04 ab | 2.74 ± 0.06 a | 2.62 ± 0.06 ab | p < 0.05 | * |
| 3-Methylbutyl acetate | nd | 2.72 ± 0.27 a | 0.82 ± 0.03 b | nd | nd | p < 0.001 | *** |
| butan-1-ol | 6.03 ± 0.69 a | 1.33 ± 0.22 b | 1.66 ± 0.02 b | 1.67 ± 0.03 b | 1.58 ± 0.08 b | p < 0.01 | ** |
| 3-Methylbutan-1-ol | nd | 31.44 ± 2.32 c | 39.08 ± 0.35 ab | 41.36 ± 0.20 a | 37.10 ± 0.51 b | p < 0.001 | *** |
| 2-Methylbutan-1-ol | 0.94 ± 0.23 | nd | nd | nd | nd | ||
| hexyl acetate | 1.48 ± 0.27 a | 0.22 ± 0.04 b | 0.21 ± 0.02 b | nd | nd | p < 0.01 | ** |
| 3-Hydroxybutan-2-one | nd | nd | 0.31 ± 0.02 | nd | nd | ||
| ethyl 2-hydroxypropanoate | nd | nd | 0.52 ± 0.02 b | 3.54 ± 0.05 a | nd | p < 0.001 | *** |
| Hexan-1-ol | 3.46 ± 0.44 a | 0.41 ± 0.03 c | 0.61 ± 0.02 bc | 0.98 ± 0.03 b | 0.86 ± 0.04 bc | p < 0.001 | *** |
| Butane-2.3-diol | nd | 0.23 ± 0.04 c | 1.49 ± 0.03 a | 1.05 ± 0.02 b | nd | p < 0.001 | *** |
| 2-Methylpropanoic acid | nd | 0.25 ± 0.05 a | 0.16 ± 0.02 b | 0.21 ± 0.04 ab | nd | p < 0.01 | ** |
| Butanoic acid | nd | 0.23 ± 0.04 a | 0.25 ± 0.03 a | nd | nd | p > 0.05 | ns |
| Hexanoic acid | nd | 0.38 ± 0.06 b | 0.67 ± 0.04 a | 0.70 ± 0.04 a | nd | p < 0.01 | ** |
| 2-Methylbutanoic acid | nd | nd | 0.21 ± 0.03 a | 0.22 ± 0.01 a | nd | p > 0.05 | ns |
| 3-Methylsulfanylpropan-1-ol | nd | 0.39 ± 0.03 c | 0.63 ± 0.04 a | 0.55 ± 0.02 b | nd | p < 0.001 | *** |
| Ethyl acetate | nd | nd | 0.25 ± 0.01 a | 0.22 ± 0.01 a | nd | p > 0.05 | ns |
| Methyl 4-hydroxybutanoate | nd | nd | 0.41 ± 0.02 b | 0.37 ± 0.02 b | 0.85 ± 0.04 a | p < 0.01 | ** |
| 2-Phenylethyl acetate | nd | 2.00 ± 0.03 a | 0.88 ± 0.02 b | nd | nd | p < 0.001 | *** |
| 2-Phenylethanol | nd | 5.12 ± 0.22 c | 8.09 ± 0.15 b | 9.54 ± 0.07 a | 8.23 ± 1.12 ab | p < 0.001 | *** |
| Octanoic acid | nd | nd | 0.89 ± 0.04 b | 1.08 ± 0.05 ab | 1.31 ± 0.25 a | p < 0.05 | * |
| Octane-1.3-diol | 9.62 ± 0.66 a | 1.77 ± 0.28 c | 2.71 ± 0.09 b | 3.35 ± 0.07 b | 2.63 ± 0.09 bc | p < 0.01 | ** |
| Pentan-2-ol | 1.33 ± 0.18 | nd | nd | nd | nd | ||
| Heneicosane | 1.75 ± 0.11 | nd | nd | nd | nd | ||
| Ethyl 3-methylbutanoate | nd | 0.21 ± 0.01 | nd | nd | nd | ||
| 2-Butoxyethyl acetate | nd | 0.15 ± 0.01 | nd | nd | nd | ||
| Ethyl octanoate | nd | nd | nd | 0.18 ± 0.02 | nd | ||
| 3-Methylsulfanylpropan-1-ol | nd | nd | nd | nd | 0.53 ± 0.02 | ||
| Butyl acetate | nd | nd | nd | nd | 0.30 ± 0.04 |
| Amino Acids | AJ | C1 | C2 | C3 | C4 | p-Value | Sig. |
|---|---|---|---|---|---|---|---|
| Alanine | 16.6 ± 0.52 a | 6.2 ± 0.16 c | 4.55 ± 0.12 d | 5.9 ± 0.13 c | 13.07 ± 0.17 b | p < 0.001 | *** |
| Isoleucine | 3.21 ± 0.08 | nd | nd | nd | nd | ||
| Serine | 8.35 ± 0.17 | nd | nd | nd | nd | ||
| Proline | 2.13 ± 0.09 | nd | nd | nd | nd | ||
| Asparagine | 287.76 ± 4.82 a | 3.61 ± 0.16 b | nd | nd | 2.78 ± 0.08 b | p < 0.01 | ** |
| Aspartate | 58.96 ± 1.16 | nd | nd | nd | nd | ||
| Glutamic acid | 44.68 ± 1.44 | nd | nd | nd | nd | ||
| Phenylalanine | nd | 1.55 ± 0.11 b | 2.01 ± 0.17 a | 1.99 ± 0.10 a | 1.93 ± 0.06 a | p < 0.05 | * |
| α-Aminoadipic acid | 24.42 ± 0.86 | nd | nd | nd | nd | ||
| Glutamine | nd | nd | nd | nd | 5.00 ± 0.20 | ||
| Lysine | 3.85 ± 0.09 a | 3.89 ± 0.17 a | 3.16 ± 0.17 b | 3.25 ± 0.20 b | 2.24 ± 0.15 c | p < 0.01 | ** |
| Histidine | nd | nd | 3.88 ± 0.09 a | 3.92 ± 0.09 a | 3.95 ± 0.14 a | p > 0.05 | ns |
| Tyrosine | nd | nd | nd | nd | 1.26 ± 0.07 | ||
| Tryptophan | nd | 2.28 ± 0.08 b | 2.66 ± 0.14 a | 2.81 ± 0.17 a | 2.74 ± 0.14 a | p < 0.05 | * |
| Phenolic Compounds | AJ | C1 | C2 | C3 | C4 | p-Value | Sig. |
|---|---|---|---|---|---|---|---|
| p-Anisaldehida | 21.01 ± 1.35 a | 2.41 ± 0.04 b | 0.13 ± 0.01 cd | 1.68 ± 0.04 bc | 0.04 ± 0.01 d | p < 0.001 | *** |
| Gallic acid-glucoside | 6.55 ± 0.16 a | 1.71 ± 0.06 c | 1.41 ± 0.06 d | 0.96 ± 0.05 e | 4.51 ± 0.10 b | p < 0.001 | *** |
| 2,3-Dihydroxybenzoic acid | 6.08 ± 0.19 c | 3.73 ± 0.12 d | 8.75 ± 0.13 b | 8.32 ± 0.18 b | 9.45 ± 0.26 a | p < 0.001 | *** |
| Gallic acid | 2.81 ± 0.08 a | 0.04 ± 0.01 d | 0.33 ± 0.03 c | 0.07 ± 0.01 d | 0.84 ± 0.05 b | p < 0.001 | *** |
| Gentisic acid | 2.92 ± 0.10 c | 2.42 ± 0.10 d | 1.62 ± 0.09 e | 3.47 ± 0.09 b | 3.94 ± 0.10 a | p < 0.001 | *** |
| Protocatechuic acid | 1.65 ± 0.07 b | 0.07 ± 0.01 d | 0.09 ± 0.02 d | 0.67 ± 0.04 c | 2.27 ± 0.05 a | p < 0.001 | *** |
| Neochlorogenic acid | 9.81 ± 0.13 a | 3.40 ± 0.04 e | 4.86 ± 0.15 d | 5.27 ± 0.13 c | 6.81 ± 0.16 b | p < 0.001 | *** |
| Procyanidin dimer B1 | 26.86 ± 0.75 a | 13.08 ± 0.43 d | 16.37 ± 0.83 c | 14.87 ± 0.88 cd | 22.49 ± 1.05 b | p < 0.001 | *** |
| Catechin | 24.86 ± 0.98 b | 19.58 ± 0.56 c | 24.35 ± 0.86 b | 26.06 ± 1.55 b | 30.97 ± 1.25 a | p < 0.001 | *** |
| Chlorogenic acid | 312.69 ± 4.63 a | 153.05 ± 3.53 e | 174.07 ± 3.36 d | 210.62 ± 3.10 c | 248.28 ± 5.57 b | p < 0.001 | *** |
| Procyanidin dimer B2 | 50.60 ± 1.99 a | 22.89 ± 0.73 d | 29.51 ± 0.82 c | 27.03 ± 2.30 cd | 43.39 ± 1.67 b | p < 0.001 | *** |
| Epicatechin | 43.55 ± 1.99 a | 25.13 ± 1.33 c | 37.80 ± 1.62 b | 38.63 ± 0.91 b | 46.38 ± 1.35 a | p < 0.001 | *** |
| p-Coumaroylquinic acid | 30.72 ± 1.11 a | 11.49 ± 0.65 d | 16.63 ± 0.92 c | 22.98 ± 1.54 b | 29.63 ± 0.96 a | p < 0.001 | *** |
| Quercetin-rutinoside | 5.97 ± 0.26 a | 2.26 ± 0.09 d | 3.56 ± 0.36 c | 3.84 ± 0.12 c | 5.09 ± 0.22 b | p < 0.001 | *** |
| Quercetin-glucoside | 5.44 ± 0.12 a | 2.33 ± 0.04 d | 3.11 ± 0.08 c | 3.15 ± 0.11 c | 5.18 ± 0.09 b | p < 0.001 | *** |
| Quercetin-arabinoside | 5.66 ± 0.16 a | 1.81 ± 0.10 d | 2.41 ± 0.10 c | 2.64 ± 0.09 c | 3.63 ± 0.09 b | p < 0.001 | *** |
| Phloretin-xylosyl-glucoside | 27.49 ± 0.85 a | 11.82 ± 0.09 d | 12.69 ± 0.33 cd | 13.37 ± 0.37 c | 20.19 ± 0.63 b | p < 0.001 | *** |
| Quercetin-(malonyl-glucoside) | 10.30 ± 0.23 a | 5.38 ± 0.15 d | 6.36 ± 0.43 c | 7.26 ± 0.25 b | 7.97 ± 0.39 b | p < 0.001 | *** |
| Phloridzin | 3.81 ± 0.13 b | 2.11 ± 0.07 d | 4.28 ± 0.11 b | 3.24 ± 0.17 c | 6.60 ± 0.32 a | p < 0.001 | *** |
| Procyanidin trimer C1 | 24.44 ± 1.24 a | 25.34 ± 1.29 a | 24.93 ± 1.27 a | 25.52 ± 1.14 a | 25.32 ± 0.85 a | p > 0.05 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Călugăr, P.C.; Coldea, T.E.; Pop, C.R.; Stan, L.; Gal, E.; Ranga, F.; Hegheș, S.C.; Mudura, E. Mixed Fermentations of Yeasts and Lactic Acid Bacteria as Sustainable Processes to Enhance the Chemical Composition of Cider Made of Topaz and Red Topaz Apple Varieties. Agronomy 2023, 13, 2485. https://doi.org/10.3390/agronomy13102485
Călugăr PC, Coldea TE, Pop CR, Stan L, Gal E, Ranga F, Hegheș SC, Mudura E. Mixed Fermentations of Yeasts and Lactic Acid Bacteria as Sustainable Processes to Enhance the Chemical Composition of Cider Made of Topaz and Red Topaz Apple Varieties. Agronomy. 2023; 13(10):2485. https://doi.org/10.3390/agronomy13102485
Chicago/Turabian StyleCălugăr, Paul Cristian, Teodora Emilia Coldea, Carmen Rodica Pop, Laura Stan, Emese Gal, Floricuța Ranga, Simona Codruța Hegheș, and Elena Mudura. 2023. "Mixed Fermentations of Yeasts and Lactic Acid Bacteria as Sustainable Processes to Enhance the Chemical Composition of Cider Made of Topaz and Red Topaz Apple Varieties" Agronomy 13, no. 10: 2485. https://doi.org/10.3390/agronomy13102485
APA StyleCălugăr, P. C., Coldea, T. E., Pop, C. R., Stan, L., Gal, E., Ranga, F., Hegheș, S. C., & Mudura, E. (2023). Mixed Fermentations of Yeasts and Lactic Acid Bacteria as Sustainable Processes to Enhance the Chemical Composition of Cider Made of Topaz and Red Topaz Apple Varieties. Agronomy, 13(10), 2485. https://doi.org/10.3390/agronomy13102485










