Reduction in N2O Emissions and Improvement in Nitrifier and Denitrifier Communities through Bamboo-Biochar-Based Fertilization in Pomelo Orchard Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Biochar-Based Fertilizer
2.3. Experimental Design
2.4. Sampling and Measurements
2.5. Gas Flux Measurements
2.6. DNA Extraction
2.7. Quantitative PCR Assay
2.8. Sequencing
2.9. Statistical Analyses
3. Results
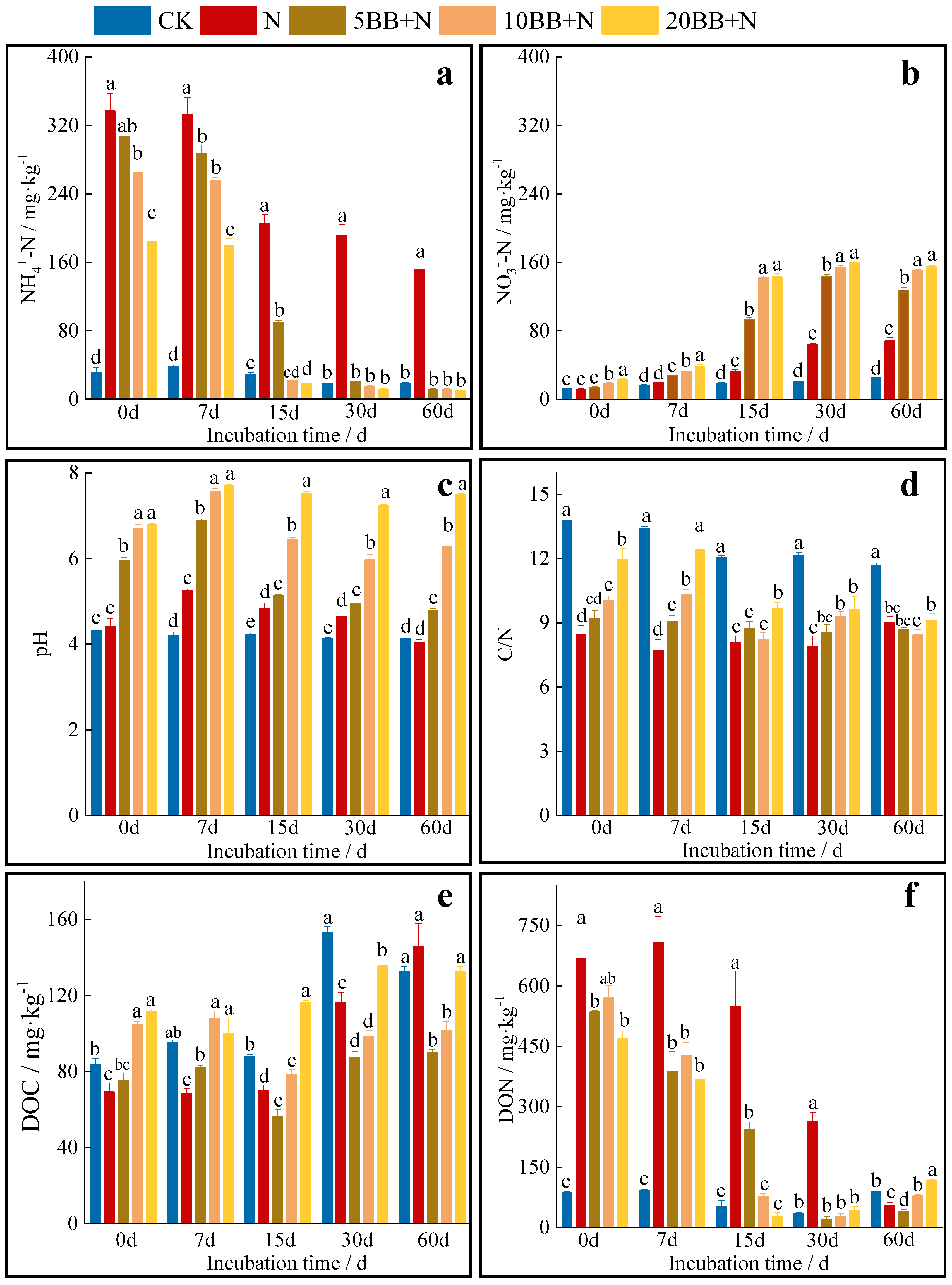
3.1. Soil Chemical Properties
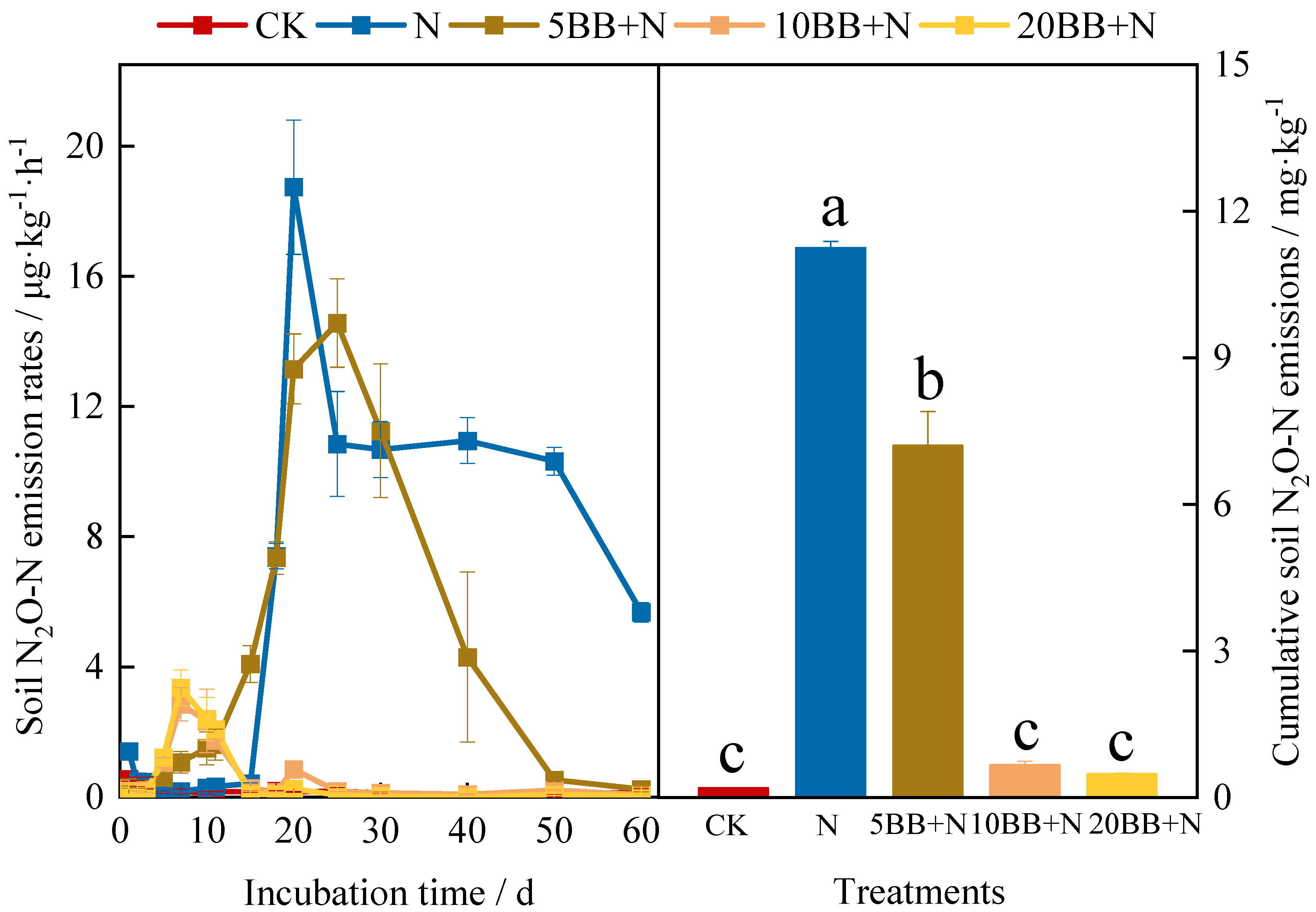
3.2. N2O Emissions
3.3. Soil Gene Copy Numbers of Nitrification and Denitrification
3.4. α Diversity of Nitrification and Denitrification Genes
3.5. Compositions of Nitrification and Denitrification Genes
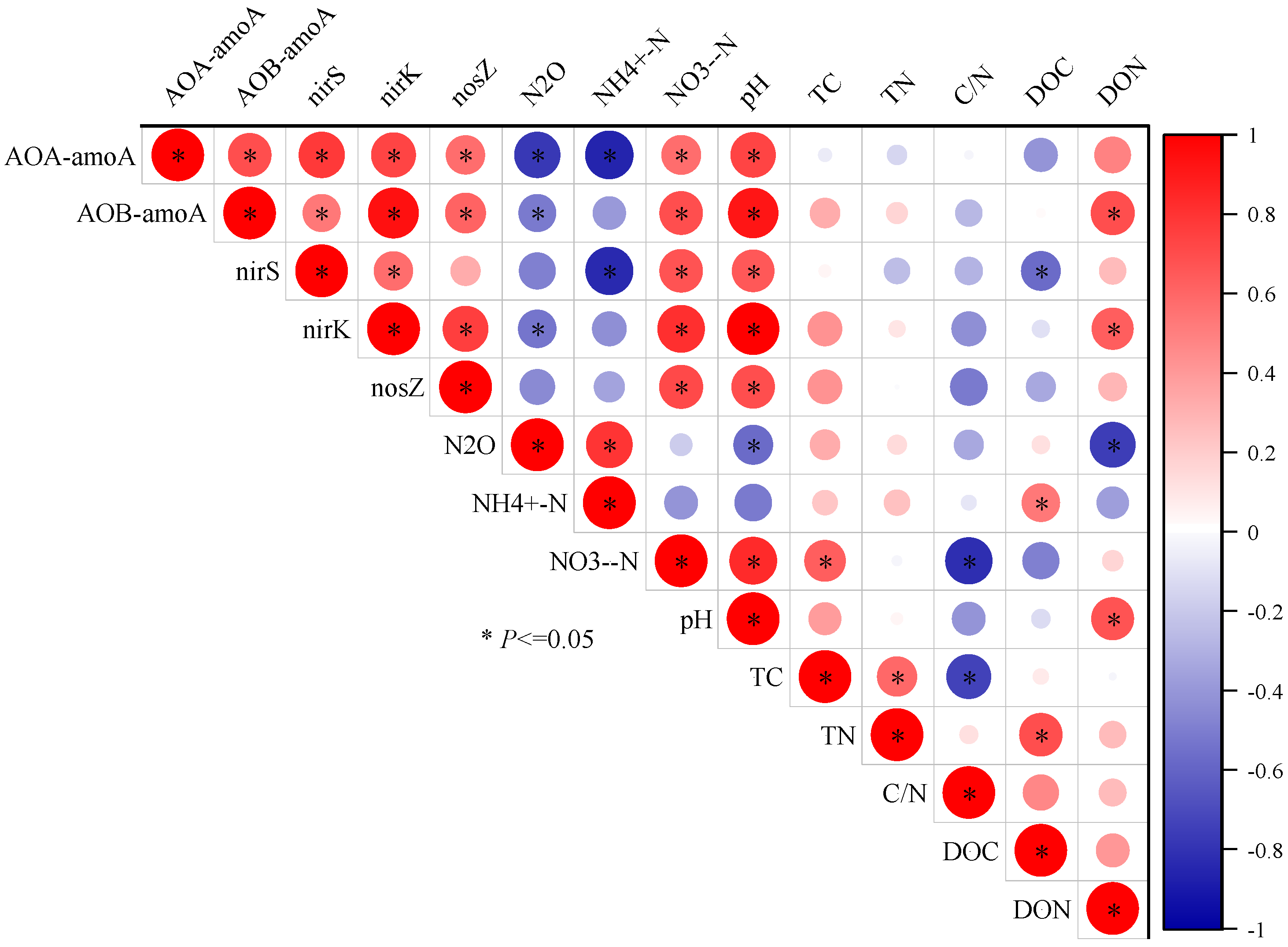
3.6. Correlation of Nitrification and Denitrification Gene Copy Numbers with Soil Chemical Properties and N2O Emissions
3.7. Correlations between Environmental Factors and the Communities of Nitrification and Denitrification Genes
4. Discussion
4.1. Response of Soil N2O Emissions to Bamboo-Biochar-Based Fertilizer and Urea
4.2. Response of Soil Nitrification to Biochar-Based Fertilizer and Urea
4.3. Response of Soil Denitrification to Biochar-Based Fertilizer and Urea
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, Y.; Su, D.; Wang, M.; Ye, D.; Tong, L.; Wu, L.; Weng, X.; Liu, D.; Xu, X.; Zhang, S. Long-term excessive phosphorus fertilization alters soil phosphorus fractions in the acidic soil of pomelo orchards. Soil. Tillage Res. 2022, 215, 105214. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Q.; Qian, X.; Chen, H.; Wang, F.; Yi, Z. Effects of the combined application of biochar-based fertilizer and urea on N2O emissions, nitrifier, and denitrifier communities in the acidic soil of pomelo orchards. J. Soils Sediments 2022, 22, 3119–3136. [Google Scholar] [CrossRef]
- Li, Q.; Ma, X.; Cheng, Q.; Pan, D.; Yu, D.; Luo, Y.; Yuan, J.; Kong, F. Effects of nitrogen fertilizer on the accumulation and allocation of dry matter and nitrogen for maize cultivars with different low nitrogen tolerance. J. Zhejiang Univ. (Agric. Life Sci.) 2015, 41, 527–536. [Google Scholar] [CrossRef]
- Tanveer, S.K.; Zhang, J.; Lu, X.; Wen, X.; Liao, Y.C. Effect of Corn Residue Mulch and N Fertilizer Application on Nitrous Oxide (N2O) Emission and Wheat Crop Productivity under Rain-fed Condition of Loess Plateau China. Int. J. Agric. Biol. 2014, 16, 505–512. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Bouwman, A.F. The Role of Soils and Land Use in the Greenhouse Effect. Neth. J. Agric. Sci. 1989, 37, 13–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.; Zhang, J.; Dong, W.; Wang, Y.; Song, L. Research advances on source/sink intensities and greenhouse effects of CO2, CH4 and N2O in agricultural soils. Chin. J. Eco-Agric. 2011, 19, 966–975. [Google Scholar] [CrossRef]
- Crutzen, P.J. Atmospheric chemical processes of the oxides of nitrogen, including nitrous oxide [Including nitrogen fertilizer]. In Denitrification Nitrification & Atmospheric Nitrous Oxid; Zubal Books: Cleveland, OH, USA, 1981; pp. 151–170. [Google Scholar]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil. Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Braker, G.; Fesefeldt, A.; Witzel, K.-P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263. [Google Scholar] [CrossRef]
- He, F.; Jiang, R.; Chen, Q.; Zhang, F.; Su, F. Nitrous oxide emissions from an intensively managed greenhouse vegetable cropping system in Northern China. Environ. Pollut. 2009, 157, 1666–1672. [Google Scholar] [CrossRef]
- Sánchez-Martín, L.; Vallejo, A.; Dick, J.; Skiba, U.M. The influence of soluble carbon and fertilizer nitrogen on nitric oxide and nitrous oxide emissions from two contrasting agricultural soils. Soil. Biol. Biochem. 2008, 40, 142–151. [Google Scholar] [CrossRef]
- Long, X.E.; Shen, J.P.; Wang, J.T.; Zhang, L.M.; Di, H.; He, J.Z. Contrasting response of two grassland soils to N addition and moisture levels: N2O emission and functional gene abundance. J. Soils Sediments 2016, 17, 384–392. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, X.; Hu, R.; Wu, M.; Wu, J.; Wei, W. Impact of Long-Term Fertilization on the Composition of Denitrifier Communities Based on Nitrite Reductase Analyses in a Paddy Soil. Microb. Ecol. 2010, 60, 850–861. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lu, S.E.; Xiang, Q.J.; Yu, L.M.; Zhao, K.; Zhang, X.P.; Tu, S.H.; Gu, Y.F. Responses of N2O reductase gene (nosZ)-denitrifier communities to long-term fertilization follow a depth pattern in calcareous purplish paddy soil. J. Integr. Agric. 2017, 016, 2597–2611. [Google Scholar] [CrossRef]
- Qiu, Q.; Wu, L.; Ouyang, Z.; Li, B.; Xu, Y. Effects of different forms of plant-derived organic matter on nitrous oxide emissions. Env. Sci. Process Impacts 2016, 18, 854–862. [Google Scholar] [CrossRef]
- Li, X.H. Present Status, Problems and Developmental Countermeasures of the Bamboo Industry in the North of Fujian Province. J. Fujian For. Sci. Technol. 2004, 02, 109–112. [Google Scholar] [CrossRef]
- Kuehl, Y. Resources, Yield, and Volume of Bamboos; Springer: Berlin/Heidelberg, Germany, 2015; Volume 10, pp. 91–111. [Google Scholar] [CrossRef]
- Won, K.S.; Hwa, C.Y.; Ju, Y.J.; Hyuk, P.J.; Hwan, P.J.; Cheol, S.D.; Sik, C.J. Effect of biochar application on nitrogen use efficiency for sustainable and productive agriculture under different field crops. J. Plant Nutr. 2021, 44, 2849–2862. [Google Scholar] [CrossRef]
- He, X.; Yin, H.; Fang, C.; Xiong, J.; Han, L.; Yang, Z.; Huang, G. Metagenomic and q-PCR analysis reveals the effect of powder bamboo biochar on nitrous oxide and ammonia emissions during aerobic composting. Bioresour. Technol. 2021, 323, 124567. [Google Scholar] [CrossRef]
- Guo, H.; Gu, J.; Wang, X.; Yu, J.; Nasir, M.; Zhang, K.; Sun, W. Microbial driven reduction of N2O and NH3 emissions during composting: Effects of bamboo charcoal and bamboo vinegar. J. Hazard. Mater. 2020, 390, 121292. [Google Scholar] [CrossRef]
- Case, S.D.C.; Uno, H.; Nakajima, Y.; Jensen, L.S.; Akiyama, H. Bamboo biochar does not affect paddy soil N2O emissions or source following slurry or mineral fertilizer amendment—A 15N tracer study. J. Plant Nutr. Soil. Sci. 2018, 181, 90–98. [Google Scholar] [CrossRef]
- Li, C.J.; Yang, W.H.; Zhou, B.Q.; Zhang, Y.; Lin, Y.; Xing, S.H. Effects of biochar based fertilizer on soil nutrients, tea output and quality in an acidified tea field. Chin. J. Soil. Sci. 2021, 52, 387–397. [Google Scholar] [CrossRef]
- Lu, R.K. Methods for Soil Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Jantalia, C.P.; Santos, H.P.D.; Urquiaga, S.; Boddey, R.M.; Alves, B.J.R. Fluxes of nitrous oxide from soil under different crop rotations and tillage systems in the South of Brazil. Nutr. Cycl. Agroecosyst. 2008, 82, 161–173. [Google Scholar] [CrossRef]
- Allen, D.E.; Kingston, G.; Rennenberg, H.; Dalal, R.C.; Schmidt, S. Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agric. Ecosyst. Environ. 2010, 136, 209–217. [Google Scholar] [CrossRef]
- Zhang, L.M.; Offre, P.R.; He, J.Z.; Verhamme, D.T.; Nicol, G.W.; Prosser, J.I. Autotrophic ammonia oxidation by soil thaumarchaea. Proc. Natl. Acad. Sci. USA 2010, 107, 17240–17245. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.A.; Santoro, A.E.; Oakley, B.B.; Beman, J.M.; Roberts, K.J. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [PubMed]
- Witzel, K.P.; Rotthauwe, J.H. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wang, Y.; Deng, J. Multiple-year nitrous oxide emissions from a greenhouse vegetable field in China: Effects of nitrogen management. Sci. Total Environ. 2018, 616-617, 1139–1148. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Liu, B.; Mz, C.; Bla, C. Integrated systematic approach increase greenhouse tomato yield and reduce environmental losses. J. Environ. Manag. 2020, 266, 110569. [Google Scholar] [CrossRef]
- Jung, J.; Yeom, J.; Kim, J.; Han, J.; Lim, H.S.; Park, H.; Hyun, S.; Park, W. Change in gene abundance in the nitrogen biogeochemical cycle with temperature and nitrogen addition in Antarctic soils. Res. Microbiol. 2011, 162, 1018–1026. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Müller, C.; Cai, Z. The Mechanisms of High N2O Emissions from Greenhouse Vegetable Field Soils. Clean-Soil. Air Water 2017, 45, 1600210. [Google Scholar] [CrossRef]
- Yang, T.; Li, F.; Zhou, X.; Xu, C.C.; Fang, F. Impact of nitrogen fertilizer, greenhouse, and crop species on yield-scaled nitrous oxide emission from vegetable crops: A meta-analysis. Ecol. Indic. 2019, 105, 717–726. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Ju, X. Linkage between N2O emission and functional gene abundance in an intensively managed calcareous fluvo-aquic soil. Sci. Rep. 2017, 7, 43283. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Lehmann, J.; Rilling, M.C.; Thies, J.; Masiello, C.A.; Cam, C.; Wch, D.; Dc, E. Biochar effects on soil biota: A review. Soil. Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.J.; Zhang, Y.H.; Hu, T.L.; Lin, Z.B.; Liu, G. Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands: Options and mitigation strength in a global perspective. Glob. Chang. Biol. 2019, 25, 2077–2093. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Zhang, A.; Rahaman, M.A.; Yang, Z. Inhibited effect of biochar application on N2O emissions is amount and time-dependent by regulating denitrification in a wheat-maize rotation system in North China-ScienceDirect. Sci. Total Environ. 2020, 721, 137636. [Google Scholar] [CrossRef]
- Xu, X.; Huang, H.; Zhang, Y.; Xu, Z.; Cao, X. Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef]
- Shi, R.Y.; Jiu-Yu, L.I.; Ni, N.I.; Ren-Kou, X.U. Understanding the biochar’s role in ameliorating soil acidity. J. Integr. Agric. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Yoo, G.; Kim, Y.J.; Lee, Y.O.; Ding, W.X. Investigation of greenhouse gas emissions from the soil amended with rice straw biochar. KSCE J. Civ. Eng. 2016, 20, 2197–2207. [Google Scholar] [CrossRef]
- Urovec, O.; Wall, D.P.; Brennan, F.P.; Krol, D.J.; Richards, K.G. Increasing soil pH reduces fertiliser derived N2O emissions in intensively managed temperate grassland. Agric. Ecosyst. Environ. 2021, 311, 107319. [Google Scholar] [CrossRef]
- Chen, H.; Yin, C.; Fan, X.; Ye, M.; Peng, H.; Li, T.; Zhao, Y.; Wakelin, S.A.; Chu, G.; Liang, Y. Reduction of N2O emission by biochar and/or 3,4-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations. Sci. Total Environ. 2019, 694, 133658. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Bent, E.; Abalos, D.; Wagner-Riddle, C.; Dunfield, K.E. soil microbial communities as potential regulators of in situ N2O fluxes in annual and perennial cropping systems. Soil. Biol. Biochem. 2016, 103, 262–273. [Google Scholar] [CrossRef]
- Zhong, W.H.; Bian, B.Y.; Gao, Y.; Zhao, A.Z. Nitrogen fertilization induced changes in ammonia oxidation are attributablemostly to bacteria rather than archaea in greenhouse-based high N input vegetable soil. Soil. Biol. Biochem. 2016, 93, 150–159. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, P.; Wu, Z.; Xiong, Z. Aged biochar stimulated ammonia-oxidizing archaea and bacteria-derived N2O and NO production in an acidic vegetable soil. Sci. Total Environ. 2019, 687, 433–440. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. Isme J. Multidiscip. J. Microb. Ecol. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Prommer, J.; Wanek, W.; Hofhansl, F.; Trojan, D.; Offre, P.; Urich, T.; Schleper, C.; Sassmann, S.; Kitzler, B.; Soja, G. Biochar Decelerates Soil Organic Nitrogen Cycling but Stimulates Soil Nitrification in a Temperate Arable Field Trial. PLoS ONE 2014, 9, e86388. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Ma, B.; Chang, S.X.; Gong, J. Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol. Fertil. Soils 2014, 50, 321–332. [Google Scholar] [CrossRef]
- He, L.; Shan, J.; Zhao, X.; Wang, S.; Yan, X. Variable responses of nitrification and denitrification in a paddy soil to long-term biochar amendment and short-term biochar addition. Chemosphere 2019, 234, 558–567. [Google Scholar] [CrossRef]
- Xu, H.J.; Wang, X.H.; Hu, L.; Yao, H.Y.; Su, J.Q.; Zhu, Y.G. Biochar Impacts Soil Microbial Community Composition and Nitrogen Cycling in an Acidic Soil Planted with Rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, C.; Wang, Y.; Yu, X.; Zhang, Z.; Zhang, D. Effect of long-term fertilization on humic redox mediators in multiple microbial redox reactions. Environ. Pollut. 2018, 234, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xia, H.; Lv, B.; Chong, M.; Song, W.; Jiang, C. Short-Term Effect of Biochar Amendments on Total Bacteria and Ammonia Oxidizers Communities in Different Type Soils. Sci. Agric. Sin. 2019, 52, 1260–1271. [Google Scholar] [CrossRef]
- Konneke, M.; Schubert, D.M.; Brown, P.C.; Hugler, M.; Standfest, S.; Schwander, T.; Schada von Borzyskowski, L.; Erb, T.J.; Stahl, D.A.; Berg, I.A. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc. Natl. Acad. Sci. USA 2014, 111, 8239–8244. [Google Scholar] [CrossRef] [PubMed]
- Kessel, M.V.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op, D.; Kartal, B.; Jetten, M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Jiang, W.T.; Tian, L.B.; Zhu, G.D.; Tang, R.G.; Lin, Y.X.; Pan, L.Q.; Cai, Y.J. Effects of different forms of nitrogen addition on N2O emissions from the soil of Moso bamboo (Phyllostachys edulis) forest. J. Plant Nutr. Fertil. 2022, 28, 857–868. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Zhang, Q. Effects of combined biochar and organic fertilizer on nitrous oxide fluxes and the related nitrifier and denitrifier communities in a saline-alkali soil. Sci. Total Environ. 2019, 686, 199–211. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Y.; Liu, J.; Li, J.; Gao, M. Variation in N2O emission and N2O related microbial functional genes in straw- and biochar-amended and non-amended soils. Appl. Soil. Ecol. 2019, 137, 57–68. [Google Scholar] [CrossRef]
- Prakash, O.; Green, S.J.; Jasrotia, P.; Overholt, W.A.; Canion, A.; Watson, D.B.; Brooks, S.C.; Kostka, J.E. Rhodanobacter denitrificans sp. nov., isolated from nitrate-rich zones of a contaminated aquifer. Int. J. Syst. Evol. Microbiol. 2012, 62, 2457–2462. [Google Scholar] [CrossRef]
- Taylor, A.E.; Vajrala, N.; Giguere, A.T.; Gitelman, A.I.; Bottomley, P.J. Use of Aliphatic n-Alkynes To Discriminate Soil Nitrification Activities of Ammonia-Oxidizing Thaumarchaea and Bacteria. Appl. Environ. Microbiol. 2013, 79, 6544–6551. [Google Scholar] [CrossRef]



| Target Gene | Primer | Sequence (5′–3′) | Annealing Temperature (°C) | Product Fragment Size (bp) | References |
|---|---|---|---|---|---|
| AOA-amoA | CHEND-arch-amoA-23F | ATGGTCTGGCTWAGACG | 55 | 629 | [29] |
| CHEND-arch-amoA-616R | GCCATCCATCTGTATGTCCA | ||||
| AOB-amoA | AmoA-1F | GGGGTTTCTACTGGTGGT | 58 | 491 | [30] |
| AmoA-2R | CCCCTCKGSAAAGCCTTCTTC | ||||
| nirS | nirS-C1F | ATCGTCAACGTCAARGARACVGG | 55 | 423 | [31] |
| nirS-C1R | TTCGGGTGCGTCTTSABGAASAG | ||||
| nirK | FlaCu | ATCATGGTSCTGCCGCG | 55 | 471 | [10] |
| R3Cu | GCCTCGATCAGRTTGTGGTT | ||||
| nosZ | nosZF | CGYTGTTCMTCGACAGCCAG | 50 | 721 | [31] |
| nosZR | CATGTGCAGNGCRTGGCAGAA |
| Shannon | Simpson | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | AOA-amoA | AOB-amoA | nirS | nirK | nosZ | AOA-amoA | AOB-amoA | nirS | nirK | nosZ |
| CK | 0.80 ± 0.01 a | 0.15 ± 0.01 a | 0.13 ± 0.00 c | 0.37 ± 0.02 f | 0.11 ± 0.00 d | 0.47 ± 0.00 b | 0.93 ± 0.00 a | 0.93 ± 0.00 ab | 0.73 ± 0.04 a | 0.93 ± 0.01 a |
| N | 0.43 ± 0.04 b | 0.00 ± 0.00 a | 0.04 ± 0.00 d | 0.55 ± 0.02 d | 0.45 ± 0.01 a | 0.73 ± 0.03 a | 0.99 ± 0.00 a | 0.88 ± 0.05 b | 0.56 ± 0.02 b | 0.70 ± 0.01 b |
| 5BB+N | 0.79 ± 0.01 a | 0.04 ± 0.01 a | 0.25 ± 0.01 a | 0.45 ± 0.00 e | 0.21 ± 0.01 c | 0.48 ± 0.00 b | 0.99 ± 0.00 a | 0.91 ± 0.01 ab | 0.76 ± 0.01 a | 0.91 ± 0.01 a |
| 10BB+N | 0.82 ± 0.00 a | 0.06 ± 0.05 a | 0.14 ± 0.00 c | 0.93 ± 0.01 b | 0.24 ± 0.01 b | 0.47 ± 0.00 b | 0.98 ± 0.02 a | 0.95 ± 0.01 a | 0.44 ± 0.01 c | 0.91 ± 0.01 a |
| 20BB+N | 0.81 ± 0.00 a | 0.09 ± 0.04 a | 0.14 ± 0.01 b c | 1.42 ± 0.01 a | 0.24 ± 0.02 b | 0.48 ± 0.01 b | 0.96 ± 0.02 a | 0.95 ± 0.00 a | 0.27 ± 0.00 d | 0.91 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhao, L.; Wang, F.; Chen, H.; Qian, X. Reduction in N2O Emissions and Improvement in Nitrifier and Denitrifier Communities through Bamboo-Biochar-Based Fertilization in Pomelo Orchard Soil. Agronomy 2023, 13, 2504. https://doi.org/10.3390/agronomy13102504
Li Q, Zhao L, Wang F, Chen H, Qian X. Reduction in N2O Emissions and Improvement in Nitrifier and Denitrifier Communities through Bamboo-Biochar-Based Fertilization in Pomelo Orchard Soil. Agronomy. 2023; 13(10):2504. https://doi.org/10.3390/agronomy13102504
Chicago/Turabian StyleLi, Qinghua, Lin Zhao, Fei Wang, Hongmei Chen, and Xiaojie Qian. 2023. "Reduction in N2O Emissions and Improvement in Nitrifier and Denitrifier Communities through Bamboo-Biochar-Based Fertilization in Pomelo Orchard Soil" Agronomy 13, no. 10: 2504. https://doi.org/10.3390/agronomy13102504




