Soil Carbon Management Index under Different Land Use Systems and Soil Types of Sanjiang Plain in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling and Laboratory Procedures
2.3. Soil Aggregate and C Fractionation
2.4. CMI
2.5. Statistical Analyses
3. Results
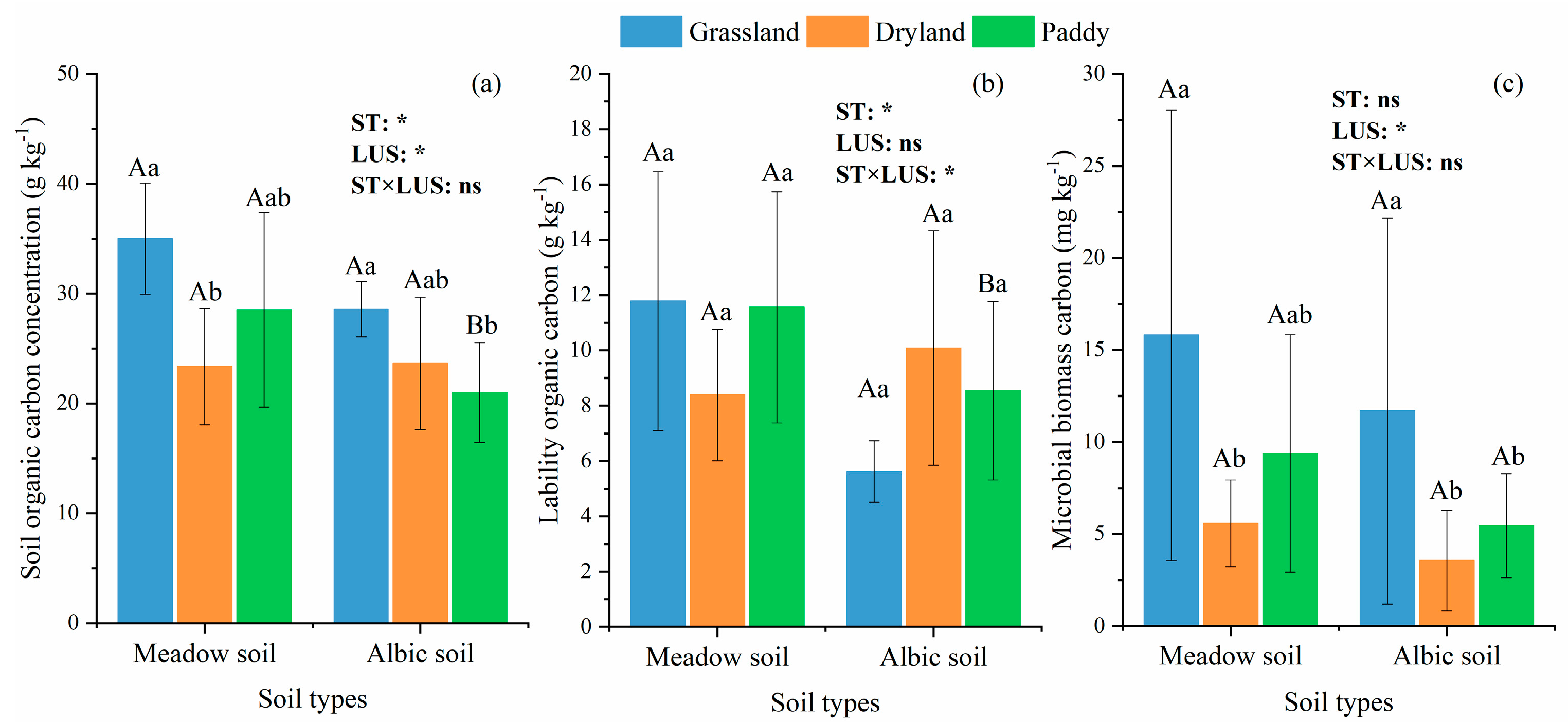
3.1. Soil Properties under Different ST and LUS
3.2. Soil Aggregates and SOC under Different ST and LUS
3.3. Soil C Content under Different ST and Land Uses
3.4. CMI under Different ST and LUS
4. Discussions
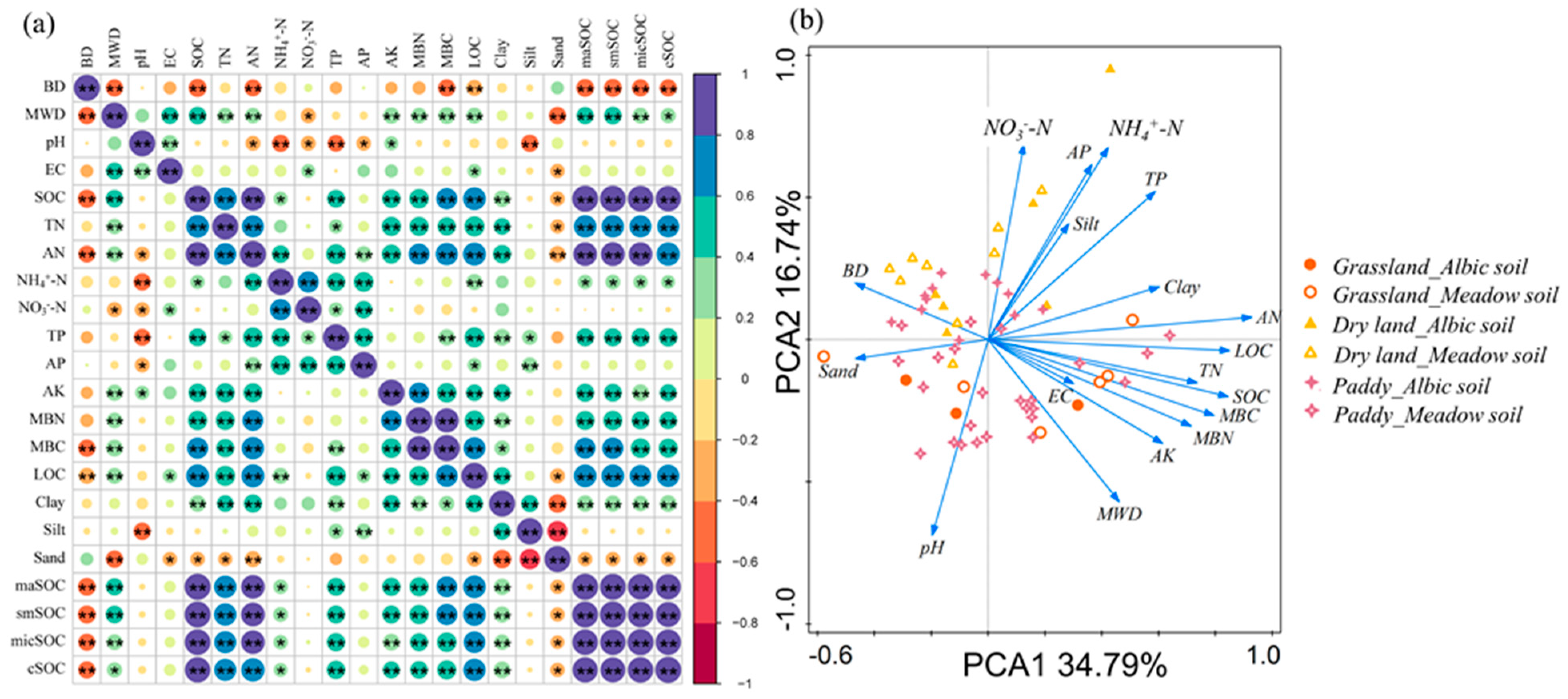
4.1. SOC Associated with Aggregate Size Fractions under Different ST and LUS
4.2. C Management under Different LUS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Change 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Lal, R.; Smith, P.; Jungkunst, H.F.; Mitsch, W.J.; Lehmann, J.; Nair, P.K.R.; McBratney, A.B.; Sa, J.C.D.; Schneider, J.; Zinn, Y.L.; et al. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018, 73, A145–A152. [Google Scholar] [CrossRef]
- Paustian, K.; Collier, S.; Baldock, J.; Burgess, R.; Creque, J.; DeLonge, M.; Dungait, J.; Ellert, B.; Frank, S.; Goddard, T.; et al. Quantifying carbon for agricultural soil management: From the current status toward a global soil information system. Carbon Manag. 2019, 10, 567–587. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Parras-Alcantara, L.; Lozano-Garcia, B.; Obregon-Romero, R. Soil organic carbon distribution in Mediterranean areas under a climate change scenario via multiple linear regression analysis. Sci. Total Environ. 2017, 592, 134–143. [Google Scholar] [CrossRef]
- Hu, P.L.; Liu, S.J.; Ye, Y.Y.; Zhang, W.; He, X.Y.; Su, Y.R.; Wang, K.L. Soil carbon and nitrogen accumulation following agricultural abandonment in a subtropical karst region. Appl. Soil Ecol. 2018, 132, 169–178. [Google Scholar] [CrossRef]
- Li, C.H.; Veum, K.S.; Goyne, K.W.; Nunes, M.R.; Acosta-Martinez, V. A chronosequence of soil health under tallgrass prairie reconstruction. Appl. Soil Ecol. 2021, 164, 103939. [Google Scholar] [CrossRef]
- Hobley, E.; Baldock, J.; Hua, Q.; Wilson, B. Land-use contrasts reveal instability of subsoil organic carbon. Glob. Change Biol. 2017, 23, 955–965. [Google Scholar] [CrossRef]
- Sainepo, B.M.; Gachene, C.K.; Karuma, A. Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Bal. Manage. 2018, 13, 4. [Google Scholar] [CrossRef]
- Pham, T.G.; Nguyen, H.T.; Kappas, M. Assessment of soil quality indicators under different agricultural land uses and topographic aspects in Central Vietnam. Int. Soil Water Conse. 2018, 6, 280–288. [Google Scholar] [CrossRef]
- Schimel, D.S.; House, J.I.; Hibbard, K.A.; Bousquet, P.; Ciais, P.; Peylin, P.; Braswell, B.H.; Apps, M.J.; Baker, D.; Bondeau, A.; et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 2001, 414, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Wu, X.; Yao, Z.; Bruggemann, N.; Shen, Z.Y.; Wolf, B.; Dannenmann, M.; Zheng, X.; Butterbach-Bahl, K. Effects of soil moisture and temperature on CO2 and CH4 soil atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Kidinda, L.K.; Doetterl, S.; Kalbitz, K.; Bukombe, B.; Babin, D.; Mujinya, B.B.; Vogel, C. Relationships between geochemical properties and microbial nutrient acquisition in tropical forest and cropland soils. Appl. Soil Ecol. 2023, 181, 104653. [Google Scholar] [CrossRef]
- Romkens, P.F.A.M.; van der Plicht, J.; Hassink, J. Soil organic matter dynamics after the conversion of arable land to pasture. Biol. Fert. Soils 1999, 28, 277–284. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Yifru Abera and Belachew, T. Effect of land use on soil organic carbon and nitrogen in soils of Bale, Southeastern Ethiopia. Trop. Subtrop. Agroecosystems 2011, 14, 229–235. [Google Scholar]
- Haile, G.; Lemenhi, M.; Itanna, F.; Senbeta, F. Impacts of land uses changes on soil fertility, carbon and nitrogen stock under smallholder farmers in central highlands of Ethiopia: Implication for sustainable agricultural landscape management around Butajira area. N. Y. Sci. J. 2014, 7, 27–44. [Google Scholar]
- Ketema, H.; Yimer, F. Soil property variation under agroforestry based conservation tillage and maize based conventional tillage in Southern Ethiopia. Soil Till. Res. 2014, 141, 25–31. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; Van Wesemael, B.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone—Carbon response functions as a model approach. Glob. Change Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Tighe, M.; Cowie, A.; Wilson, B.R.; Schwenke, G.; Mcleod, M.; Badgery, W.; Baldock, J. The relationships between land uses, soil management practices, and soil carbon fractions in South Eastern Australia. Agr. Ecosyst. Environ. 2014, 197, 41–52. [Google Scholar] [CrossRef]
- Mathieu, J.A.; Hatte, C.; Balesdent, J.; Parent, E. Deep soil carbon dynamics are driven more by soil type than by climate: A worldwide meta-analysis of radiocarbon profiles. Glob. Change Biol. 2015, 21, 4278–4292. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.L.; Fang, J.Y.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009-U82. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, M.; Thum, T.; Jarvinen, H.; Fronzek, S.; Berg, B.; Harmon, M.; Trofymow, J.A.; Sevanto, S.; Liski, J. Leaf litter decomposition-Estimates of global variability based on Yasso07 model. Ecol. Model. 2009, 220, 3362–3371. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.B.; Xue, S.; Sun, C.L. Soil organic carbon and total nitrogen storage as affected by land use in a small watershed of the Loess Plateau, China. Eur. J. Soil Biol. 2013, 54, 16–24. [Google Scholar] [CrossRef]
- Zheng, J.F.; Chen, J.H.; Pan, G.X.; Wang, G.M.; Liu, X.Y.; Zhang, X.H.; Li, L.Q.; Bian, R.J.; Cheng, K.; Zheng, J.W. A long-term hybrid poplar plantation on cropland reduces soil organic carbon mineralization and shifts microbial community abundance and composition. Appl. Soil Ecol. 2017, 111, 94–104. [Google Scholar] [CrossRef]
- Yan, M.; Deng, W.; Chen, P. Climate change in the Sanjiang Plain disturbed by large-scale reclamation. J. Geogr. Sci. 2002, 12, 405–412. [Google Scholar]
- Zhang, B.; Chang, L.; Nia, Z.; Callaham, M.A.; Sun, X.; Wu, D.H. Effects of land use changes on winter-active Collembola in Sanjiang Plain of China. Appl. Soil Ecol. 2014, 83, 51–58. [Google Scholar] [CrossRef]
- Kueppers, L.M.; Snyder, M.A.; Sloan, L.C. Irrigation cooling effect: Regional climate forcing by land-use change. Geophys. Res. Lett. 2007, 34, L03703. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, S.; Su, F. Variations in ecosystem services in response to paddy expansion in the Sanjiang Plain, Northeast China. Int. J. Agr. Sustain. 2019, 17, 158–171. [Google Scholar] [CrossRef]
- Ouyang, W.; Xu, Y.; Hao, F.; Wang, X.; Siyang, C.; Lin, C. Effect of long-term agricultural cultivation and land use conversion on soil nutrient contents in the Sanjiang Plain. Catena 2013, 104, 243–250. [Google Scholar] [CrossRef]
- Zhao, Q.; Brocks, S.; Lenz-Wiedemann, V.I.S.; Miao, Y.; Zhang, F.; Bareth, G. Detecting spatial variability of paddy rice yield by combining the DNDC model with high resolution satellite images. Agr. Syst. 2017, 152, 47–57. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Stratification of Carbon Fractions and Carbon Management Index in Deep Soil Affected by the Grain-to-Green Program in China. PLoS ONE 2014, 9, e99657. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Silva, M.L.N.; Beiniach, A.; Bispo, D.F.A.; Contins, J.G.P.; Curi, N. Relationship between soil organic matter fractions and cover plants in Olive post planting. Rev. Bras. Frutic. 2018, 40. [Google Scholar] [CrossRef]
- Bispo, D.F.A.; Guimarães, D.V.; Marques, J.J.G.d.S.e.M.; Beniaich, A.; Acuña-Guzman, S.F.; Silva, M.L.N.; Curi, N. Soil Organic Carbon as Response to Reforestation Age and Land Use Changes: A Qualitative Approach to Ecosystem Services. Sustainability 2023, 15, 6863. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartage, K.H. Bulk density. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle size analysis. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. A. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agro-Chemical Analysis; China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil—V: A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate Structure and Carbon, Nitrogen, and Phosphorus in Native and Cultivated Soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.; Nelson, S. Gas Displacement and Aggregate Stability of Soils. Soil Sci. Soc. Am. J. 1985, 49, 25–28. [Google Scholar] [CrossRef]
- Kemper, W.D.; Koch, E.J. Aggregate Stability of Soils from Western USA and Canada; USDA Technical Bulletin; US Government Printing Office: Washington, DC, USA, 1966; p. 1355.
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agr. Res. 1995, 46, 1459. [Google Scholar] [CrossRef]
- Li, J.B.; Zhao, Y.; Zhang, A.F.; Song, B.; Hill, R.L. Effect of grazing exclusion on nitrous oxide emissions during freeze-thaw cycles in a typical steppe of Inner Mongolia. Agric. Ecosyst. Environ. 2021, 307, 107217. [Google Scholar] [CrossRef]
- Eynard, A.; Schumacher, T.E.; Lindstrom, M.J.; Malo, D.D. Effect of agricultural management systems on soil organic C in aggregates of Ustoll and Usterts. Soil Till. Res. 2005, 81, 253–263. [Google Scholar] [CrossRef]
- Xue, S.; Liu, G.B.; Pan, Y.P.; Dai, Q.H.; Zhang, C.; Yu, N. Evolution of soil labile organic matter and carbon management index in the Artificial robinia of Loess hilly area. Sci. Agric. Sin. 2009, 42, 1458–1464. [Google Scholar]
- Abiven, S.; Menasseri, S.; Chenu, C. The effects of organic inputs over time on soil aggregate stability—A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Feng, H.J.; Wang, S.Y.; Gao, Z.D.; Pan, H.; Zhuge, Y.P.; Ren, X.Q.; Hu, S.W.; Li, C.L. Aggregate stability and organic carbon stock under different land uses integrally regulated by binding agents and chemical properties in saline-sodic soils. Land Degrad. Dev. 2021, 32, 4151–4161. [Google Scholar] [CrossRef]
- Xiao, L.; Yao, K.H.; Li, P.; Liu, Y.; Chang, E.H.; Zhang, Y.; Zhu, T.T. Increased soil aggregate stability is strongly correlated with root and soil properties along a gradient of secondary succession on the Loess Plateau. Ecol. Eng. 2020, 143, 105671. [Google Scholar] [CrossRef]
- Kukal, S.S.; Kaur, M.; Bawa, S.S.; Gupta, N. Water-drop stability of PVA-treated natural soil aggregates from different land uses. Catena 2007, 70, 475–479. [Google Scholar] [CrossRef]
- Gajic, B.; Dugalic, G.; Djurovic, N. Comparison of soil organic matter content, aggregatecomposition and water stability of gleyic fluvisol from adjacentforest and cultivated areas. Agron. Res. 2006, 4, 499–508. [Google Scholar]
- Lal, R. Tillage Effects on Soil Degradation, Soil Resilience, Soil Quality, and Sustainability—Introduction. Soil Till. Res. 1993, 27, 1–8. [Google Scholar] [CrossRef]
- Holeplass, H.; Singh, B.R.; Lal, R. Carbon sequestration in soil aggregates under different crop rotation and nitrogen fertilization in an Inceptisol in southeastern Norway. Nutr. Cycl. Agroecosys. 2004, 70, 167–177. [Google Scholar] [CrossRef]
- Ashagrie, Y.; Zech, W.; Guggenberger, G. Transformation of a Podocarpus falcatus dominated natural forest into a monoculture Eucalyptus globulus plantation at Munesa, Ethiopia: Soil organic C, N and S dynamics in primary particle and aggregate-size fractions. Agr. Ecosyst. Environ. 2005, 106, 89–98. [Google Scholar] [CrossRef]
- Giniyatullin, K.G.; Shinkarev, A.A.; Shinkarev, A.A.; Krinari, G.A.; Lygina, T.Z.; Gubaidullina, A.M.; Suchkova, G.G. Binding of organic matter into an oxidation-resistant form during the interaction of clay minerals with plant residues. Eurasian Soil Sci. 2010, 43, 1159–1173. [Google Scholar] [CrossRef]
- Fang, X.M.; Chen, F.S.; Wan, S.Z.; Yang, Q.P.; Shi, J.M. Topsoil and Deep Soil Organic Carbon Concentration and Stability Vary with Aggregate Size and Vegetation Type in Subtropical China. PLoS ONE 2015, 10, e0139380. [Google Scholar] [CrossRef]
- Puget, P.; Chenu, C.; Balesdent, J. Total and Young Organic-Matter Distributions in Aggregates of Silty Cultivated Soils. Eur. J. Soil Sci. 1995, 46, 449–459. [Google Scholar] [CrossRef]
- Jastrow, J.D. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Sodhi, G.P.S.; Beri, V.; Benbi, D.K. Soil aggregation and distribution of carbon and nitrogen in different fractions under long-term application of compost in rice-wheat system. Soil Till. Res. 2009, 103, 412–418. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Sitaula, B.K.; Singh, B.R.; Bajracharya, R.M. Soil organic carbon stocks in soil aggregates under different land use systems in Nepal. Nutr. Cycl. Agroecosys. 2004, 70, 201–213. [Google Scholar] [CrossRef]
- Beare, M.H.; Hendrix, P.F.; Coleman, D.C. Water-Stable Aggregates and Organic-Matter Fractions in Conventional-Tillage and No-Tillage Soils. Soil Sci. Soc. Am. J. 1994, 58, 777–786. [Google Scholar] [CrossRef]
- Luo, Z.K.; Wang, E.L.; Baldock, J.; Xing, H.T. Potential soil organic carbon stock and its uncertainty under various cropping systems in Australian cropland. Soil Res. 2014, 52, 463–475. [Google Scholar] [CrossRef]
- Bolinder, M.A.; Katterer, T.; Poeplau, C.; Borjesson, G.; Parent, L.E. Net primary productivity and below-ground crop residue inputs for root crops: Potato (Solanum tuberosum L.) and sugar beet (Beta vulgaris L.). Can. J. Soil Sci. 2015, 95, 87–93. [Google Scholar] [CrossRef]
- Chen, L.D.; Gong, J.; Fu, B.J.; Huang, Z.L.; Huang, Y.L.; Gui, L.D. Effect of land use conversion on soil organic carbon sequestration in the loess hilly area, loess plateau of China. Ecol. Res. 2007, 22, 641–648. [Google Scholar] [CrossRef]
- Saha, D.; Kukal, S.S.; Sharma, S. Landuse impacts on SOC fractions and aggregate stability in typic ustochrepts of Northwest India. Plant Soil 2011, 339, 457–470. [Google Scholar] [CrossRef]
- Dhumgond, P.; Prakash, S.S.; Punith Raj, T.S.; Reddy, S.B.; Shivakumar, K.M. Dynamics of soil organic carbon and their fractions under different cropping systems in hill zone acid soils of Karnataka. Madras Agric. J. 2012, 99, 762–768. [Google Scholar] [CrossRef]
- Lal, R. Development in East-Africa. Geoderma 1995, 67, 159–164. [Google Scholar] [CrossRef]
- Nath, A.J.; Lal, R.; Sileshi, G.W.; Das, A.K. Managing India’s small landholder farms for food security and achieving the “4 per Thousand” target. Sci. Total Environ. 2018, 634, 1024–1033. [Google Scholar] [CrossRef]
- Schwendenmann, L.; Pendall, E. Effects of forest conversion into grassland on soil aggregate structure and carbon storage in Panama: Evidence from soil carbon fractionation and stable isotopes. Plant Soil 2006, 288, 217–232. [Google Scholar] [CrossRef]
- Zhang, J.B.; Song, C.C.; Yang, W.Y. Land use effects on the distribution of labile organic carbon fractions through soil profiles. Soil Sci. Soc. Am. J. 2006, 70, 1037. [Google Scholar]
- Meena, V.S.; Ghosh, B.N.; Singh, R.J.; Bhattacharyya, R.; Sharma, N.K.; Alam, N.M.; Meena, S.K.; Mishra, P.K. Land use types and topographic position affect soil aggregation and carbon management in the mountain agro-ecosystems of the Indian Himalayas. Land Degrad. Dev. 2021, 32, 3992–4003. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration and aggregation by cover cropping. J. Soil Water Conserv. 2015, 70, 329–339. [Google Scholar] [CrossRef]
- Moharana, P.C.; Sharma, B.M.; Biswas, D.R.; Dwivedi, B.S.; Singh, R.V. Long-term effect of nutrient management on soil fertility and soil organic carbon pools under a 6-year-old pearl millet-wheat cropping system in an Inceptisol of subtropical India. Field Crop. Res. 2012, 136, 32–41. [Google Scholar] [CrossRef]
- Ghosh, S.; Wilson, B.; Ghoshal, S.; Senapati, N.; Mandal, B. Organic amendments influence soil quality and carbon sequestration in the Indo-Gangetic plains of India. Agr. Ecosyst. Environ. 2012, 156, 134–141. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leiros, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Vieira, F.C.B.; Bayer, C.; Zanatta, J.A.; Dieckow, J.; Mielniczuk, J.; He, Z.L. Carbon management index based on physical fractionation of soil organic matter in an Acrisol under long-term no-till cropping systems. Soil Till. Res. 2007, 96, 195–204. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Meena, V.S.; Alam, N.M.; Dogra, P.; Bhattacharyya, R.; Sharma, N.K.; Mishra, P.K. Impact of conservation practices on soil aggregation and the carbon management index after seven years of maize-wheat cropping system in the Indian Himalayas. Agr. Ecosyst. Environ. 2016, 216, 247–257. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 2015, 237, 149–158. [Google Scholar] [CrossRef]


| Soil Types | Meadow Soil | Albic Soil | ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Land-Use Systems | Grassland | Dryland | Paddy | Grassland | Dryland | Paddy | ST | LUS | ST × LUS |
| BD (g cm−3) | 1.2 ± 0.3 Aa | 1.3 ± 0.2 Aa | 1.2 ± 0.2 Ba | 1.2 ± 0.3 Ab | 1.3 ± 0.0 Aa | 1.3 ± 0.1 Aab | ns | ns | ns |
| Clay (%) | 37.5 ± 7.2 Aa | 37.2 ± 5.6 Aa | 34.9 ± 6.5 Aa | 34.9 ± 6.4 Aa | 37.5 ± 4.0 Aa | 36.9 ± 5.5 Aa | ns | ns | ns |
| Silt (%) | 34.9 ± 9.3 Aa | 34.5 ± 7.5 Ba | 33.6 ± 4.6 Ba | 35.1 ± 4.3 Ab | 43.1 ± 1.0. Aa | 43.1 ± 2.6 Aa | ** | ns | ns |
| Sand (%) | 27.6 ± 16.0 Aa | 28.2 ± 11.6 Aa | 31.5 ± 10.0 Aa | 30.0 ± 10.8 Aa | 19.3 ± 2.5 Ab | 19.1 ± 3.9 Bb | ns | ns | * |
| pH | 6.2 ± 0.4 Aa | 6.0 ± 0.5 Aa | 6.5 ± 0.7 Aa | 6.5 ± 0.3 Aa | 6.0 ± 0.2 Ab | 5.9 ± 0.2 Bb | ns | ns | ns |
| EC (μs/cm) | 68.1 ± 19.3 Ab | 88.1 ± 47.1 Ab | 147.9 ± 17.8 Aa | 75.2 ± 19.4 Aa | 122.2 ± 86.6 Aa | 96.4 ± 29.3 Ba | ns | * | * |
| Total N (g kg−1) | 2.8 ± 1.0Aa | 2.0 ± 0.3 Ab | 2.5 ± 0.9Aab | 2.4 ± 1.3 Aa | 2.3 ± 0.8 Aa | 2.0 ± 0.5 Ba | ns | ns | ns |
| AN (mg kg−1) | 98.7 ± 28.1 Aa | 75.4 ± 20.8 Aa | 85.8 ± 27.0 Aa | 81.2 ± 33.5 Aa | 868 ± 26.8 Aa | 69.9 ± 12.4 Ba | ns | ns | ns |
| NO3−-N (mg kg−1) | 3.3 ± 1.9Ab | 23.4 ± 18.0 Aa | 3.4 ± 1.2Ab | 6.4 ± 3.3Aa | 21.7 ± 23.3 Aa | 4.6 ± 4.3 Aa | ns | ** | ns |
| NH4+-N (mg kg−1) | 10.6 ± 7.8Ab | 17.6 ± 6.3 Aa | 10.9 ± 3.3Ab | 8.6 ± 5.0Aa | 15.0 ± 12.0 Aa | 11.7 ± 2.1 Aa | ns | ** | ns |
| TP (g kg−1) | 0.8 ± 0.2Aa | 0.8 ± 0.2 Aa | 0.7 ± 0.2Aa | 0.6 ± 0.3Ab | 1.0 ± 0.3 Aa | 0.8 ± 0.1 Aab | ns | ns | ns |
| AP (mg kg−1) | 33.3 ± 18.1 Aa | 30.4 ± 14.9 Aa | 30.2 ± 18.1 Ba | 37.4 ± 46.6 Aa | 98.2 ± 78.7 Aa | 50.6 ± 27.2 Aa | ** | * | * |
| AK (mg kg−1) | 297.8 ± 99.5 Aa | 198.4 ± 57.3 Ab | 258.8 ± 82.5 Aab | 419.4 ± 163.2 Aa | 283.7 ± 99.0 Ab | 208.7 ± 76.8 Ab | ns | ** | * |
| MBN (mg kg−1) | 395.1 ± 306.2 Aa | 98.2 ± 61.3 Ab | 210.6 ± 102.7 Ab | 292.1 ± 262.1 Aa | 139.2 ± 58.9 Ab | 144.3 ± 75.0 Ab | ns | ** | ns |
| Soil Types | Land Use Systems | >2mm (%) | 2–0.25 mm (%) | 0.25–0.053 mm (%) | <0.053 mm (%) | MWD (mm) |
|---|---|---|---|---|---|---|
| Meadow soil | Grassland | 69.9 ± 6.7 Aa | 16.4 ± 3.6 Aa | 9.4 ± 2.6 Ac | 4.3 ± 1.6 Ab | 3.7 ± 0.3 Aa |
| Dryland | 40.6 ± 13.2 Ac | 19.4 ± 3.8 Aa | 23.0 ± 6.5 Aa | 16.9 ± 8.8 Aa | 2.3 ± 0.7 Bc | |
| Paddy | 61.0 ± 5.3 Ab | 17.7 ± 3.7 Aa | 12.9 ± 2.1 Bb | 8.3 ± 2.6 Bb | 3.3 ± 0.2 Ab | |
| Albic soil | Grassland | 63.8 ± 3.2 Aa | 18.4 ± 2.3 Aa | 11.1 ± 2.7 Ab | 6.9 ± 3.3 Aa | 3.4 ± 0.2 Aa |
| Dryland | 51.7 ± 5.7 Aa | 18.5 ± 4.6 Aab | 18.4 ± 2.4 Aa | 11.4 ± 2.5 Aa | 2.8 ± 0.3 Aa | |
| Paddy | 51.2 ± 16.2 Ba | 15.5 ± 2.9 Ab | 17.0 ± 5.2 Aa | 16.3 ± 11.5 Aa | 2.8 ± 0.8 Ba | |
| ANOVA | ||||||
| ST | ns | ns | ns | ns | ns | |
| LUS | ** | ns | ** | * | ** | |
| ST × LUS | ** | ns | ** | ** | ** | |
| Soil Types | Land Use Systems | >2mm | 2–0.25 mm | 0.25–0.053 mm | <0.053 mm |
|---|---|---|---|---|---|
| Meadow soil | Grassland | 35.7 ± 4.9 Aa | 33.7 ± 4.3 Aa | 20.5 ± 2.8 Aa | 21.4 ± 5.2 Aa |
| Dryland | 24.6 ± 6.1 Ab | 23.0 ± 5.2 Ab | 18.2 ± 4.2 Ab | 15.0 ± 3.2 Ab | |
| Paddy | 28.7 ± 9.2 Ab | 28.3 ± 8.9 Ab | 19.5 ± 6.2 Ab | 14.8 ± 4.7 Ab | |
| Albic soil | Grassland | 28.5 ± 2.9 Aa | 26.8 ± 2.1 Ba | 20.5 ± 2.8 Aa | 16.2 ± 1.4 Aa |
| Dryland | 22.9 ± 5.8 Aab | 21.2 ± 5.6 Aab | 14.5 ± 4.2 Ab | 10.8 ± 3.3 Bb | |
| Paddy | 20.7 ± 4.8 Bb | 20.7 ± 4.0 Bab | 12.6 ± 3.0 Bb | 8.5 ± 2.2 Bb | |
| ANOVA | |||||
| ST | * | * | ** | ** | |
| LUS | * | * | ** | ** | |
| ST × LUS | ns | ns | ns | ns | |
| Soil Types | Land use Systems | Carbon Pool Index | Lability Index | Lability of C | Carbon Management Index |
|---|---|---|---|---|---|
| Meadow soil | Grassland | 0.75 ± 0.11 Aa | 0.81 ± 0.32 Aa | 0.52 ± 0.25 Aa | 63.4% ± 30.3% Aa |
| Dryland | 0.50 ± 0.11 Ab | 0.58 ± 0.16 Ab | 0.67 ± 0.40 Aa | 29.1% ± 10.0% Ab | |
| Paddy | 0.61 ± 0.19 Aab | 0.80 ± 0.29 Aa | 0.72 ± 0.27Aa | 53.0% ± 32.1% Aa | |
| Albic soil | Grassland | 0.83 ± 0.07 Aa | 0.53 ± 0.11 Aa | 0.24 ± 0.03 Ab | 44.7% ± 13.1% Aa |
| Dryland | 0.69 ± 0.17 Aab | 0.96 ± 0.40 Aa | 0.74 ± 0.27 Aa | 71.1% ± 45.1% Aa | |
| Paddy | 0.61 ± 0.13 Ab | 0.81 ± 0.31 Aa | 0.72 ± 0.27Aa | 52.4% ± 28.4% Aa | |
| ANOVA | |||||
| ST | ns | ns | ns | ns | |
| LUS | * | ns | ns | * | |
| ST × LUS | ns | * | * | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Han, J.; Jiao, J.; Han, J.; Zhao, X.; Hu, K.; Kang, Y.; Jaffar, M.T.; Qin, W. Soil Carbon Management Index under Different Land Use Systems and Soil Types of Sanjiang Plain in Northeast China. Agronomy 2023, 13, 2533. https://doi.org/10.3390/agronomy13102533
Zhang M, Han J, Jiao J, Han J, Zhao X, Hu K, Kang Y, Jaffar MT, Qin W. Soil Carbon Management Index under Different Land Use Systems and Soil Types of Sanjiang Plain in Northeast China. Agronomy. 2023; 13(10):2533. https://doi.org/10.3390/agronomy13102533
Chicago/Turabian StyleZhang, Man, Jiale Han, Jian Jiao, Jianqiao Han, Xiaoli Zhao, Kexin Hu, Yanhong Kang, Muhammad Tauseef Jaffar, and Wei Qin. 2023. "Soil Carbon Management Index under Different Land Use Systems and Soil Types of Sanjiang Plain in Northeast China" Agronomy 13, no. 10: 2533. https://doi.org/10.3390/agronomy13102533





