The Effect of Temperature and Moisture Content on Population Growth of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Experimental Design
2.3. Statistical Analysis
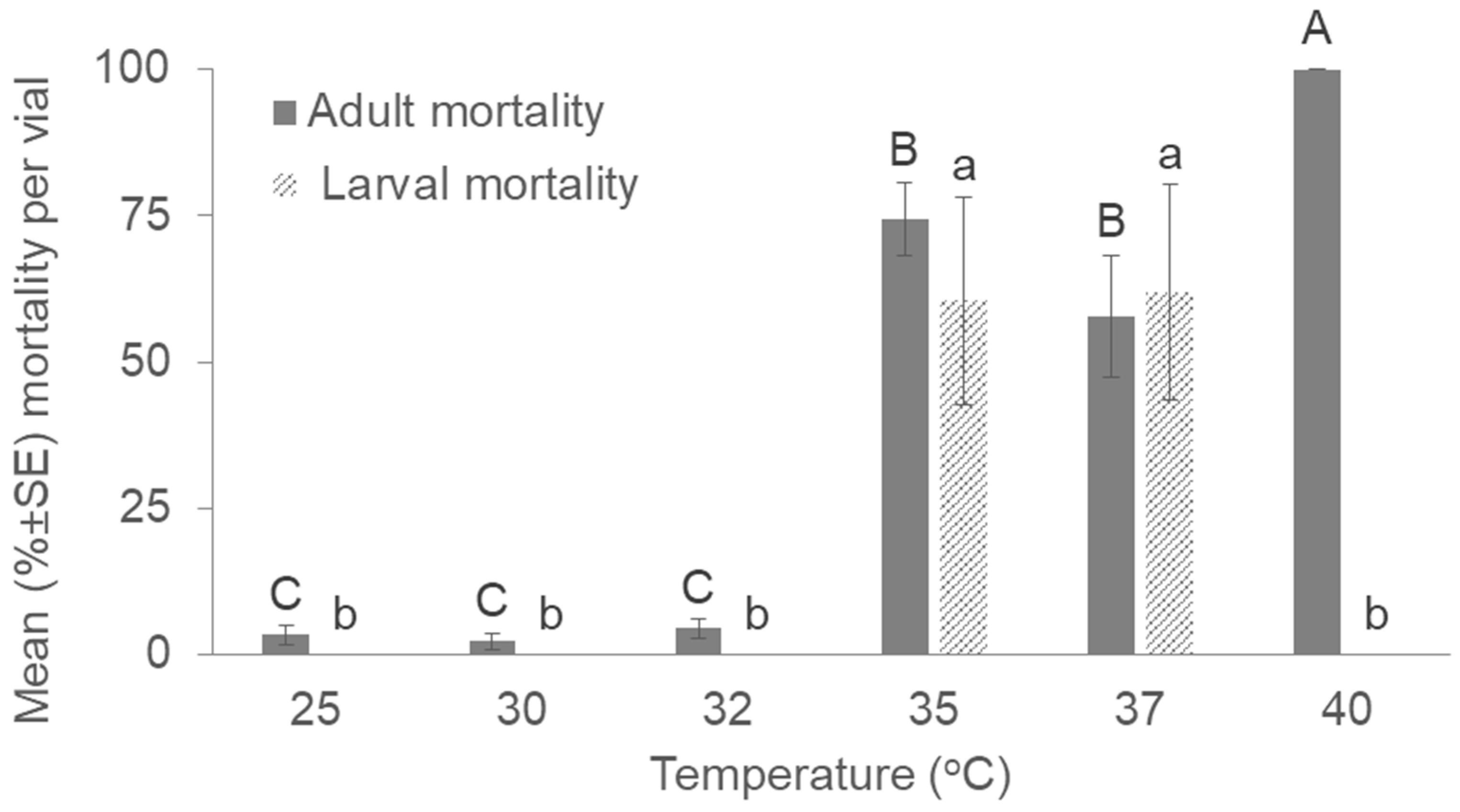
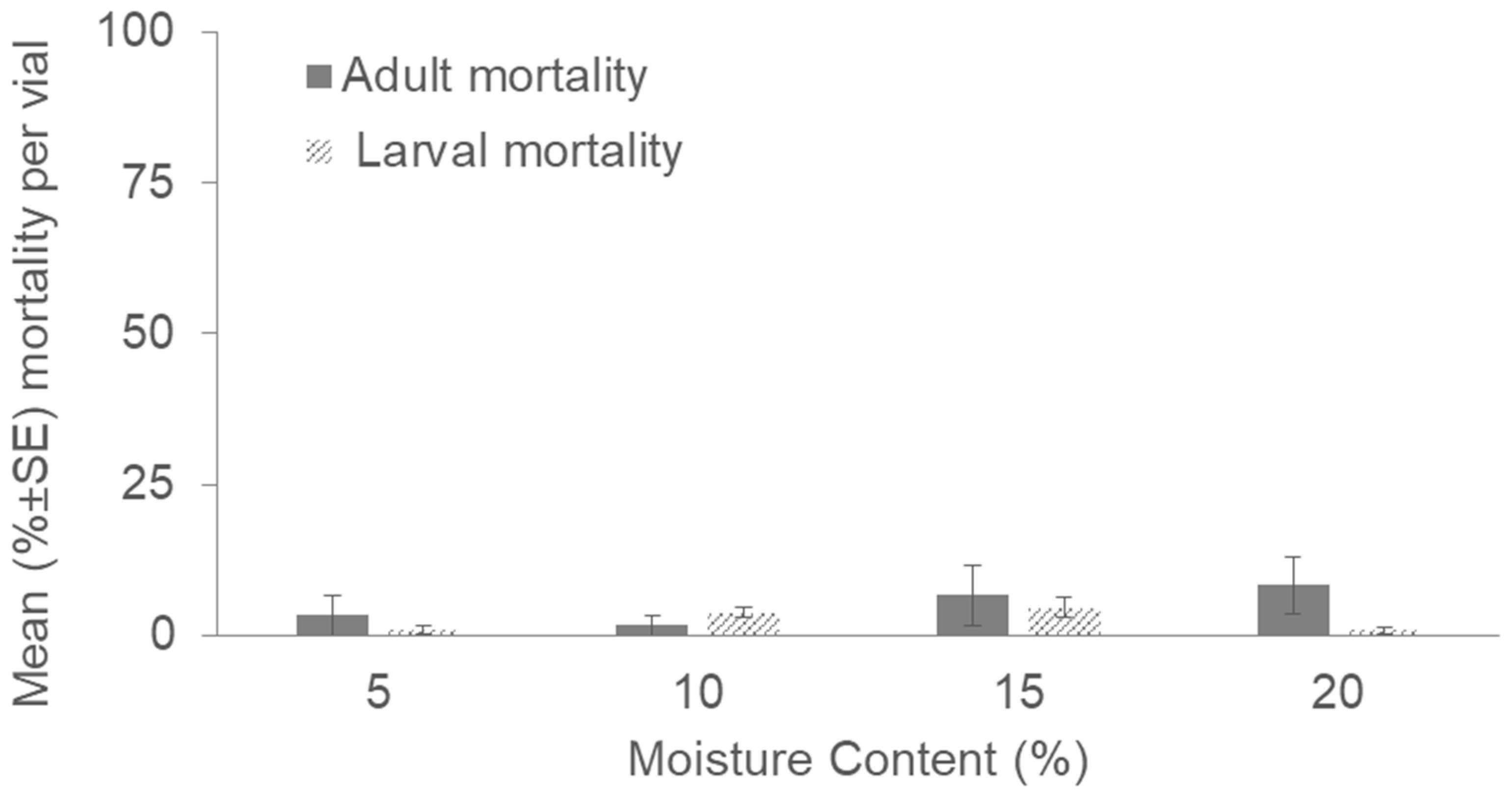
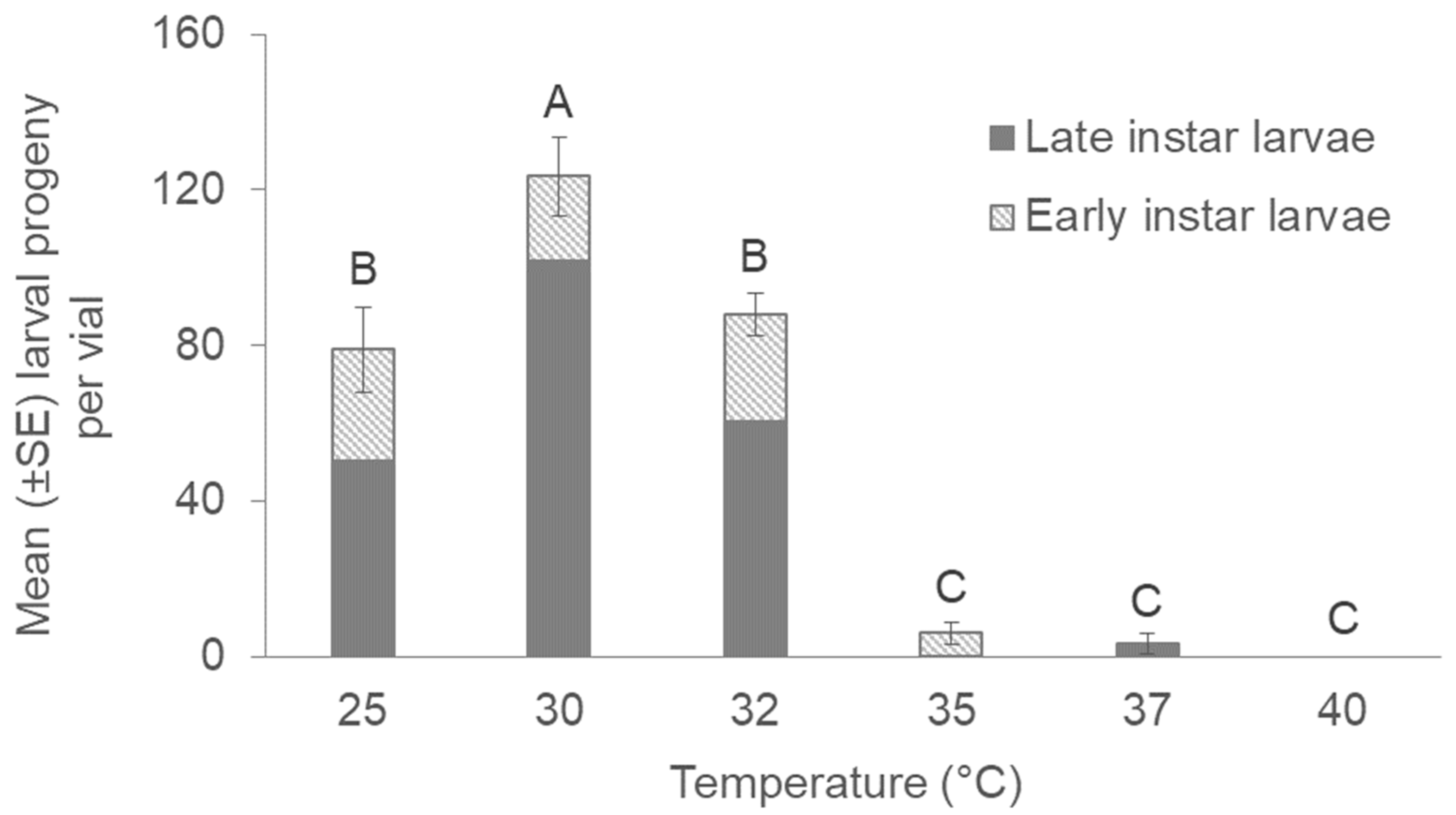
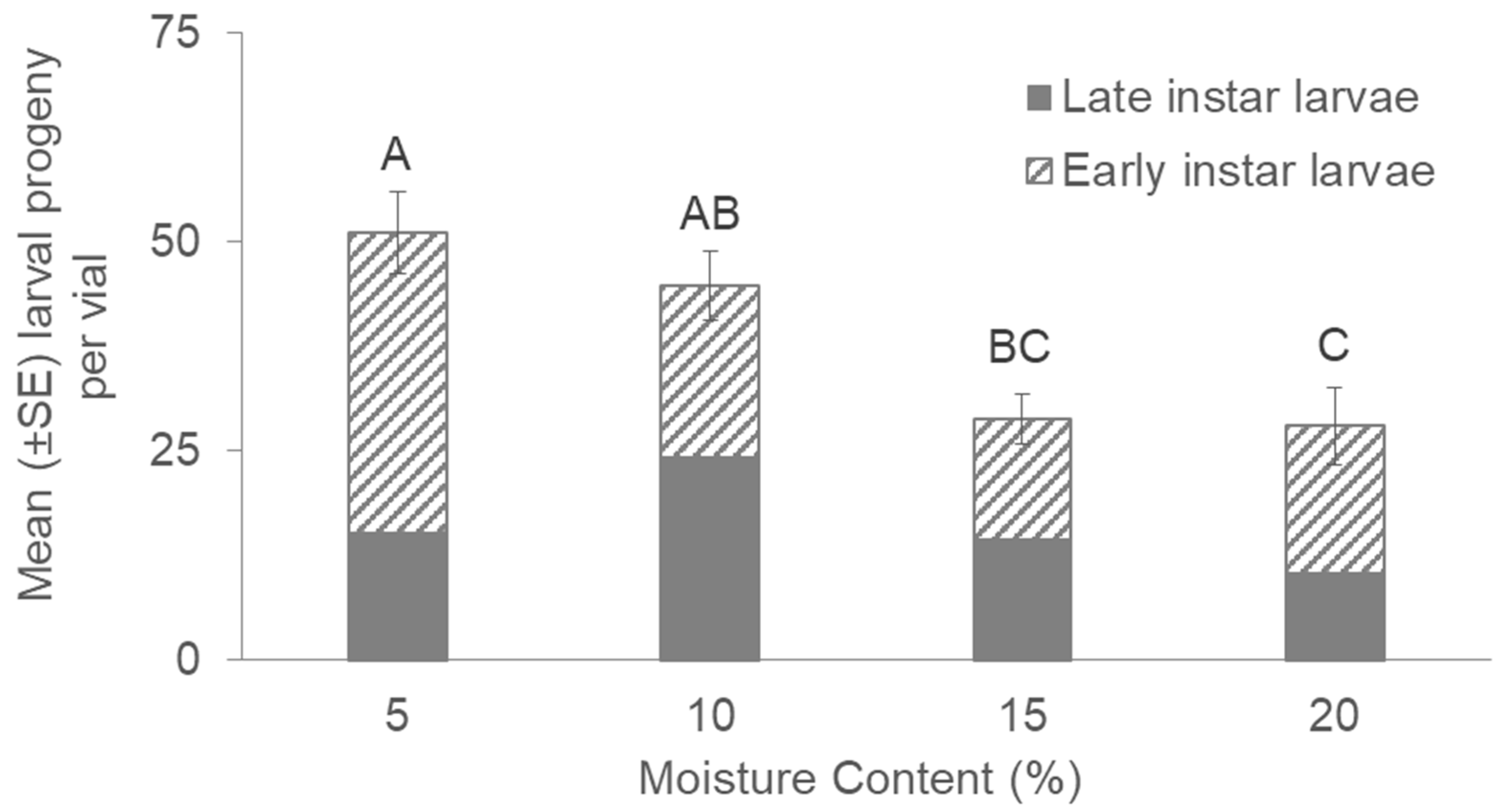
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’connor, J. Alphitobius diaperinus (Panzer)(Col. Tenebrionidae) damaging polystyrene insulation in Irish piggery. Entomol. Mon. Mag. 1987, 123, 1472–1475. [Google Scholar]
- Axtell, R. Biology and economic importance of the darkling beetle in poultry houses. In Proceedings of the North Carolina State University Poultry Supervisors’ Short Course, Raleigh, NC, USA, 19 April 1994; pp. 8–17. [Google Scholar]
- Ducatelle, R.; Van Immerseel, F. Management and sanitation procedures to control Salmonella in laying hen flocks. In Improving the Safety and Quality of Eggs and Egg Products; Elsevier: Amsterdam, The Netherlands, 2011; pp. 146–162. [Google Scholar]
- Crippen, T.L.; Sheffield, C.L.; Esquivel, S.V.; Droleskey, R.E.; Esquivel, J.F. The acquisition and internalization of Salmonella by the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae). Vector-Borne Zoonotic Dis. 2009, 9, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Cox, N.; Richardson, L.; Buhr, R.; Cason, J.; Fairchild, B.; Hinkle, N. Transmission of Salmonella to broilers by contaminated larval and adult lesser mealworms, Alphitobius diaperinus (Coleoptera: Tenebrionidae). Poult. Sci. 2009, 88, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, W.L. Beetles (Coleoptera). In Medical and Veterinary Entomology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–143. [Google Scholar]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Athanassiou, C.G. The lesser mealworm Alphitobius diaperinus: A noxious pest or a promising nutrient source? Rev. Aquac. 2019, 11, 1418–1437. [Google Scholar] [CrossRef]
- Despins, J.L.; Axtell, R.C. Feeding behavior and growth of turkey poults fed larvae of the darkling beetle, Alphitobius diaperinus. Poult. Sci. 1994, 73, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Despins, J.L.; Axtell, R.C. Feeding behavior and growth of broiler chicks fed larvae of the darkling beetle, Alphitobius diaperinus. Poult. Sci. 1995, 74, 331–336. [Google Scholar] [CrossRef]
- Herrero, M.; Wirsenius, S.; Henderson, B.; Rigolot, C.; Thornton, P.; Havlík, P.; De Boer, I.; Gerber, P.J. Livestock and the environment: What have we learned in the past decade? Annu. Rev. Environ. Resour. 2015, 40, 177–202. [Google Scholar] [CrossRef]
- Van Huis, A. Edible insects contributing to food security? Agric. Food Secur. 2015, 4, 20. [Google Scholar] [CrossRef]
- Montanari, F.; Pinto de Moura, A.; Miguel Cunha, L. The EU Regulatory Framework for Insects as Food and Feed and Its Current Constraints. In Production and Commercialization of Insects as Food and Feed: Identification of the Main Constraints in the European Union; Springer: Cham, Switzerland, 2021; pp. 41–78. [Google Scholar]
- Bukkens, S.G. The nutritional value of edible insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- Kummu, M.; De Moel, H.; Porkka, M.; Siebert, S.; Varis, O.; Ward, P.J. Lost food, wasted resources: Global food supply chain losses and their impacts on freshwater, cropland, and fertiliser use. Sci. Total Environ. 2012, 438, 477–489. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Bliamplias, D.; Gourgouta, M.; Michail, V.; Athanassiou, C.G. Rearing Tenebrio molitor and Alphitobius diaperinus larvae on seed cleaning process byproducts. Insects 2021, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed. 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable use of Hermetia illucens insect biomass for feed and food: Attributional and consequential life cycle assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Für Naturforschung C 2017, 72, 337–349. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an alternative protein source for monogastric animal: A review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed. Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.-J.; Biancarosa, I.; Gasco, L. Insects as Raw Materials in Compound Feed for Aquaculture. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 263–276. [Google Scholar]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed. Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Ribeiro, N.; Abelho, M.; Costa, R. A review of the scientific literature for optimal conditions for mass rearing Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2018, 53, 434–454. [Google Scholar]
- Cossins, A.R.; Schwarzbaum, P.J.; Wieser, W. Effects of Temperature on Cellular ion Regulation and Membrane Transport systems. In Biochemistry and Molecular Biology of Fishes; Elsevier: Amsterdam, The Netherlands, 1995; Volume 5, pp. 101–126. [Google Scholar]
- Salin, C.; Vernon, P.; Vannier, G. The supercooling and high temperature stupor points of the adult lesser mealworm Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 1998, 34, 385–394. [Google Scholar] [CrossRef]
- Opit, G.; Arthur, F.; Bonjour, E.; Jones, C.; Phillips, T.W. Efficacy of heat treatment for disinfestation of concrete grain silos. J. Econ. Entomol. 2011, 104, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Gazoni, F.; Flores, F.; Bampi, R.; Silveira, F.; Boufleur, R.; Lovato, M. Evaluation of the resistance of mealworms (Alphitobius Diaperinus)(Panzer)(Coleoptera: Tenebrionidae) at different temperatures. Arq. Do Inst. Biológico 2012, 79, 69–74. [Google Scholar] [CrossRef]
- Wolf, J.; Potrich, M.; Lozano, E.R.; Gouvea, A.; Pegorini, C.S. Combined physical and chemical methods to control lesser mealworm beetles under laboratory conditions. Poult. Sci. 2015, 94, 1145–1149. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Adler, P.H.; Myers, H.M. Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environ. Entomol. 2009, 38, 930–934. [Google Scholar] [CrossRef]
- Holmes, L.; Vanlaerhoven, S.; Tomberlin, J. Substrate effects on pupation and adult emergence of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2013, 42, 370–374. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.; Niassy, S.; van Loon, J.J.; Dicke, M. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e0206097. [Google Scholar]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.)(Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Mutchmor, J. Effects of temperature, relative humidity and period of exposure on the survival capacity of Tenebrio molitor (Coleoptera: Tenebrionidae). J. Kans. Entomol. Soc. 1980, 53, 260–270. [Google Scholar]
- Rueda, L.; Axtell, R. Temperature-dependent development and survival of the lesser mealworm, Alphitobius diaperinus. Med. Vet. Entomol. 1996, 10, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.H.; Miner, F.D. Influence of temperature on development of the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Kans. Entomol. Soc. 1969, 42, 294–303. [Google Scholar]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.-H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kotsou, K.; Rumbos, C.I.; Baliota, G.V.; Gourgouta, M.; Athanassiou, C.G. Influence of temperature, relative humidity and protein content on the growth and development of larvae of the lesser mealworm, Alphitobius diaperinus (Panzer). Sustainability 2021, 13, 11087. [Google Scholar] [CrossRef]
- Samson, P.; Keating, J. Effect of relative humidity on the biological activity of insecticides in impregnated paper assays. J. Stored Prod. Res. 1987, 23, 177–181. [Google Scholar] [CrossRef]
- Subramanyam, B.; Roesli, R. Inert Dusts. In Alternatives to Pesticides in Stored-Product IPM; Springer: Boston, MA, USA, 2000; pp. 321–380. [Google Scholar]
- Fields, P.; Korunic, Z. The effect of grain moisture content and temperature on the efficacy of diatomaceous earths from different geographical locations against stored-product beetles. J. Stored Prod. Res. 2000, 36, 1–13. [Google Scholar] [CrossRef]
- Athanassiou, C.; Kavallieratos, N.; Meletsis, C. Insecticidal effect of three diatomaceous earth formulations, applied alone or in combination, against three stored-product beetle species on wheat and maize. J. Stored Prod. Res. 2007, 43, 330–334. [Google Scholar] [CrossRef]
- Baliota, G.V.; Lampiri, E.; Athanassiou, C.G. Differential effects of abiotic factors on the insecticidal efficacy of diatomaceous earth against three major stored product beetle species. Agronomy 2022, 12, 156. [Google Scholar] [CrossRef]
- Francisco, O.; Do Prado, A. Characterization of the larval stages of Alphitobius diaperinus (Panzer)(Coleoptera: Tenebrionidae) using head capsule width. Rev. Bras. De Biol. 2001, 61, 125–131. [Google Scholar] [CrossRef]
- Brindley, T.A. The Growth and Development of Ephestia Kuehniella Zeller (Lepidoptera) and Tri-Bolium Confusum Duval (Coleoptera) under Controlled Conditions of Temperature and Relative Humidity. Ann. Entomol. Soc. Am. 1930, 23, 741–757. [Google Scholar] [CrossRef]
- Berger, D.; Walters, R.; Gotthard, K. What limits insect fecundity? Body size-and temperature-dependent egg maturation and oviposition in a butterfly. Funct. Ecol. 2008, 22, 523–529. [Google Scholar] [CrossRef]
- Gilbert, N.; Raworth, D. Insects and temperature—A general theory. Can. Entomol. 1996, 128, 1–13. [Google Scholar] [CrossRef]
- Dosland, O.; Subramanyam, B.; Sheppard, K.; Mahroof, R. Temperature Modification for Insect Control. In Insect Management for Food Storage and Processing; Elsevier: Amsterdam, Netherlands, 2006; pp. 89–103. [Google Scholar]
- Chidawanyika, F.; Terblanche, J.S. Rapid thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2011, 57, 108–117. [Google Scholar] [CrossRef]
- Machekano, H.; Mutamiswa, R.; Singano, C.; Joseph, V.; Chidawanyika, F.; Nyamukondiwa, C. Thermal resilience of Prostephanus truncatus (Horn): Can we derive optimum temperature-time combinations for commodity treatment? J. Stored Prod. Res. 2020, 86, 101568. [Google Scholar] [CrossRef]
- Eberle, S.; Schaden, L.-M.; Tintner, J.; Stauffer, C.; Schebeck, M. Effect of temperature and photoperiod on development, survival, and growth rate of mealworms, Tenebrio molitor. Insects 2022, 13, 321. [Google Scholar] [CrossRef]
- Ichinose, T.; Shibazaki, S.; Ohta, M. Studies on the biology and mode of infestation of the tenebrionid beetle, Alphitobius diaperinus (Panzer) harmful to broiler-chicken houses. Jpn. J. Appl. Entomol. Zool 1980, 24, 167–174. [Google Scholar] [CrossRef]
- Howe, R. The effects of temperature and humidity on the rate of development and the mortality of Tribolium confusum Duval (Coleoptera, Tenebrionidae). Ann. Appl. Biol. 1960, 48, 363–376. [Google Scholar] [CrossRef]
- Skourti, A.; Kavallieratos, N.G.; Papanikolaou, N.E. Laboratory evaluation of development and survival of Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae) under constant temperatures. J. Stored Prod. Res. 2019, 83, 305–310. [Google Scholar] [CrossRef]
- Banks, I.J. To Assess the Impact of Black Soldier Fly (Hermetia illucens) Larvae on Faecal Reduction in Pit Latrines. Ph.D. Thesis, London School of Hygiene & Tropical Medicine, London, UK, 2014. [Google Scholar]
- Padmanabha, M.; Kobelski, A.; Hempel, A.-J.; Streif, S. A comprehensive dynamic growth and development model of Hermetia illucens larvae. PLoS ONE 2020, 15, e0239084. [Google Scholar] [CrossRef] [PubMed]
- Throne, J.E. Effects of moisture content and initial insect density on ability of rusty grain beetles (Coleoptera: Cucujidae) to infest whole corn. J. Entomol. Sci. 1990, 25, 25–29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafeiriadis, S.; Baliota, G.V.; Athanassiou, C.G. The Effect of Temperature and Moisture Content on Population Growth of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Agronomy 2023, 13, 2535. https://doi.org/10.3390/agronomy13102535
Zafeiriadis S, Baliota GV, Athanassiou CG. The Effect of Temperature and Moisture Content on Population Growth of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Agronomy. 2023; 13(10):2535. https://doi.org/10.3390/agronomy13102535
Chicago/Turabian StyleZafeiriadis, Sofronios, Georgia V. Baliota, and Christos G. Athanassiou. 2023. "The Effect of Temperature and Moisture Content on Population Growth of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae)" Agronomy 13, no. 10: 2535. https://doi.org/10.3390/agronomy13102535
APA StyleZafeiriadis, S., Baliota, G. V., & Athanassiou, C. G. (2023). The Effect of Temperature and Moisture Content on Population Growth of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Agronomy, 13(10), 2535. https://doi.org/10.3390/agronomy13102535







