Effects of Substituting B with FR and UVA at Different Growth Stages on the Growth and Quality of Lettuce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Conditions
2.2. Light Treatments
2.3. Biometric Measurements
2.4. Phytochemical Determination
2.4.1. Chlorophyll (Chl) and Carotenoid Contents
2.4.2. Soluble Sugar Content
2.4.3. Soluble Protein Content
2.4.4. Nitrate Content
2.4.5. Vitamin C Content
2.4.6. Polyphenol Content
2.4.7. Flavonoid Content
2.4.8. DPPH Radical-Scavenging Rate
2.4.9. Ferric-Reducing Antioxidant Power
2.5. Statistical Analysis
3. Results
3.1. Growth and Biomass
3.2. Photosynthetic Pigment Contents
3.3. The Contents of Soluble Sugar, Soluble Protein, Vitamin C, and Nitrate
3.4. Antioxidant Content and Capacity
3.5. Correlation Analysis and Comprehensive Analysis
4. Discussion
4.1. The Growth of Lettuce Was Affected by Light Combinations in the Growth Stage
4.2. The Nutrition of Lettuce Was Affected by Light Combinations in the Growth Stage
4.3. The Antioxidant Contents and Activity of Lettuce Were Affected by Light Combinations in Growth Stage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Majid, M.; Khan, J.N.; Ahmad Shah, Q.M.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of Hydroponic Systems for the Cultivation of Lettuce (Lactuca Sativa L., Var. Longifolia) and Comparison with Protected Soil-Based Cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- van der Maesen, L.J.G.; Nijhuis, H.J.G. Human Sciences and Climate Change: Quo Vadis? Int. J. Soc. Qual. 2022, 12, v–xxii. [Google Scholar] [CrossRef]
- Treftz, C.; Omaye, S.T. Hydroponics: Potential for Augmenting Sustainable Food Production in Non-Arable Regions. NFS 2016, 46, 672–684. [Google Scholar] [CrossRef]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Raza, M.A.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-Red Light: A Regulator of Plant Morphology and Photosynthetic Capacity. Crop. J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant Factories Are Heating Up: Hunting for the Best Combination of Light Intensity, Air Temperature and Root-Zone Temperature in Lettuce Production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef]
- Kusuma, P.; Bugbee, B. Improving the Predictive Value of Phytochrome Photoequilibrium: Consideration of Spectral Distortion Within a Leaf. Front. Plant Sci. 2021, 12, 596943. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.W.; Bugbee, B. Photosynthesis in Sun and Shade: The Surprising Importance of Far-red Photons. New Phytol. 2022, 236, 538–546. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and Physiological Properties of Indoor Cultivated Lettuce in Response to Additional Far-Red Light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding Far-Red to Red-Blue Light-Emitting Diode Light Promotes Yield of Lettuce at Different Planting Densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Yoon, H.I.; Kim, J.; Ahn, T.I.; Son, J.E. Ray-Tracing Analysis on the Far-Red Induced Light-Capturing Ability of Kale. Sci. Hortic. 2023, 311, 111806. [Google Scholar] [CrossRef]
- He, R.; Li, Y.; Ou, S.; Gao, M.; Zhang, Y.; Song, S.; Liu, H. Regulation of Growth and Main Health-Promoting Compounds of Chinese Kale Baby-Leaf by UV-A and FR Light. Front. Plant Sci. 2021, 12, 799376. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Runkle, E.S. Far-Red Radiation Interacts with Relative and Absolute Blue and Red Photon Flux Densities to Regulate Growth, Morphology, and Pigmentation of Lettuce and Basil Seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef]
- Choi, D.-S.; Nguyen, T.K.L.; Oh, M.-M. Growth and Biochemical Responses of Kale to Supplementary Irradiation with Different Peak Wavelengths of UV-A Light-Emitting Diodes. Hortic. Environ. Biotechnol. 2022, 63, 65–76. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Jiang, H.; He, R.; Shi, R.; Song, S.; Liu, H. UVA-Radiation Exposure of Different Durations Promoted the Growth, Phytochemicals and Glucosinolate Biosynthesis of Chinese Kale. IJMS 2022, 23, 7619. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; He, R.; Tan, J.; Liu, K.; Chen, Y.; Liu, H. Effect of Supplemental UV-A Intensity on Growth and Quality of Kale under Red and Blue Light. IJMS 2022, 23, 6819. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Ji, Y.; Larsen, D.H.; Huang, Y.; Heuvelink, E.; Marcelis, L.F.M. Gradually Increasing Light Intensity during the Growth Period Increases Dry Weight Production Compared to Constant or Gradually Decreasing Light Intensity in Lettuce. Sci. Hortic. 2023, 311, 111807. [Google Scholar] [CrossRef]
- Min, Q.; Marcelis, L.F.M.; Nicole, C.C.S.; Woltering, E.J. High Light Intensity Applied Shortly Before Harvest Improves Lettuce Nutritional Quality and Extends the Shelf Life. Front. Plant Sci. 2021, 12, 615355. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Haimi, P.; Miliauskienė, J. Photoresponse to Different Lighting Strategies during Red Leaf Lettuce Growth. J. Photochem. Photobiol. B Biol. 2020, 202, 111726. [Google Scholar] [CrossRef]
- Viršilė, A.; Miliauskienė, J.; Haimi, P.J.; Laužikė, K.; Samuolienė, G. The Comparison of Constant and Dynamic Red and Blue Light Irradiation Effects on Red and Green Leaf Lettuce. Agronomy 2020, 10, 1802. [Google Scholar] [CrossRef]
- Gratani, L. A Non-Destructive Method to Determine Chlorophyll Content of Leaves. Photosynthetica 1992, 26, 469–473. [Google Scholar]
- Kohyama, K.; Nishinari, K. Effect of Soluble Sugars on Gelatinization and Retrogradation of Sweet Potato Starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and Determination of Ascorbate and Dehydroascorbate from Plant Tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Xie, Y.; Zheng, Y.; Dai, X.; Wang, Q.; Cao, J.; Xiao, J. Seasonal Dynamics of Total Flavonoid Contents and Antioxidant Activity of Dryopteris Erythrosora. Food Chem. 2015, 186, 113–118. [Google Scholar] [CrossRef]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol Inhibition of Lipid Peroxidation. Free Radic. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L. Principal Component Analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Miliauskienė, J.; Samuolienė, G.; Novičkovas, A.; Duchovskis, P. Nitrate, Nitrite, Protein, Amino Acid Contents, and Photosynthetic and Growth Characteristics of Tatsoi Cultivated under Various Photon Flux Densities and Spectral Light Compositions. Sci. Hortic. 2019, 258, 108781. [Google Scholar] [CrossRef]
- Emerson, R.; Chalmers, R.; Cederstrand, C. Some Factors Influencing the Long-Wave Limit of Photosynthesis. Proc. Natl. Acad. Sci. USA 1957, 43, 133–143. [Google Scholar] [CrossRef]
- Emerson, R.; Rabinowitch, E. Red Drop and Role of Auxiliary Pigments in Photosynthesis. Plant Physiol. 1960, 35, 477. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-red Light Enhances Photochemical Efficiency in a Wavelength-dependent Manner. Physiol. Plant 2019, 167, 21–33. [Google Scholar] [CrossRef]
- Kim, D.; Son, J.E. Adding Far-Red to Red, Blue Supplemental Light-Emitting Diode Interlighting Improved Sweet Pepper Yield but Attenuated Carotenoid Content. Front. Plant Sci. 2022, 13, 938199. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Liu, Y.; Fan, S.; Ma, Q. Progress of Research on the Regulatory Pathway of the Plant Shade-Avoidance Syndrome. Front. Plant Sci. 2020, 11, 439. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Substituting Far-Red for Traditionally Defined Photosynthetic Photons Results in Equal Canopy Quantum Yield for CO2 Fixation and Increased Photon Capture During Long-Term Studies: Implications for Re-Defining PAR. Front. Plant Sci. 2020, 11, 581156. [Google Scholar] [CrossRef]
- Ohtake, N.; Ju, Y.; Ishikura, M.; Suzuki, H.; Adachi, S.; Yamori, W. Alternating Red/Blue Light Increases Leaf Thickness and Mesophyll Cell Density in the Early Growth Stage, Improving Photosynthesis and Plant Growth in Lettuce. Environ. Control. Biol. 2021, 59, 59–67. [Google Scholar] [CrossRef]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of Light Quality on CO2 Assimilation, Chlorophyll-Fluorescence Quenching, Expression of Calvin Cycle Genes and Carbohydrate Accumulation in Cucumis sativus. J. Photochem. Photobiol. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shang, W.; Ma, D.; Wang, Z.; He, S.; Shi, L.; Shen, Y.; He, D.; Wang, E.; Wang, X. Effect on the Growth and Photosynthetic Characteristics of Anthurium Andreanum (‘Pink Champion’, ‘Alabama’) under Hydroponic Culture by Different LED Light Spectra. Horticulturae 2022, 8, 389. [Google Scholar] [CrossRef]
- Guo, Y.; Zhong, Y.; Mo, L.; Zhang, W.; Chen, Y.; Wang, Y.; Chen, H.; Wang, Z.; Song, X.; Meng, X. Different Combinations of Red and Blue LED Light Affect the Growth, Physiology Metabolism and Photosynthesis of in Vitro-Cultured Dendrobium Nobile ‘Zixia’. Hortic. Environ. Biotechnol. 2023, 64, 393–407. [Google Scholar] [CrossRef]
- Shang, W.; Song, Y.; Zhang, C.; Shi, L.; Shen, Y.; Li, X.; Wang, Z.; He, S. Effects of Light Quality on Growth, Photosynthetic Characteristics, and Endogenous Hormones in in Vitro-Cultured Lilium Plantlets. Hortic. Environ. Biotechnol. 2022, 64, 65–81. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Wang, L.; Guo, W. Red and Blue Wavelengths Affect the Morphology, Energy Use Efficiency and Nutritional Content of Lettuce (Lactuca Sativa L.). Sci. Rep. 2021, 11, 8374. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of Nitrogen Application Rate on the Photosynthetic Pigment, Leaf Fluorescence Characteristics, and Yield of Indica Hybrid Rice and Their Interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Nuñez Ocaña, D.; Choe, D.; Larsen, D.H.; Marcelis, L.F.M.; Heuvelink, E. Far-red Radiation Stimulates Dry Mass Partitioning to Fruits by Increasing Fruit Sink Strength in Tomato. New Phytol. 2020, 228, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, X. The Effect of Light in Vitamin C Metabolism Regulation and Accumulation in Mung Bean (Vigna Radiata) Germination. Plant Foods Hum. Nutr. 2020, 75, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xue, X.; Guo, W.; Wang, L.; Qiao, X. Growth and Nutritional Properties of Lettuce Affected by Mixed Irradiation of White and Supplemental Light Provided by Light-Emitting Diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of Ascorbate Accumulation and Metabolism in Lettuce by the Red:Blue Ratio of Continuous Light Using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Zushi, K.; Suehara, C.; Shirai, M. Effect of Light Intensity and Wavelengths on Ascorbic Acid Content and the Antioxidant System in Tomato Fruit Grown in Vitro. Sci. Hortic. 2020, 274, 109673. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen Assimilation in Plants: Current Status and Future Prospects. J. Genet. Genomics. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Dietzen, D.J. 13—Amino Acids, Peptides, and Proteins. In Principles and Applications of Molecular Diagnostics; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–380. ISBN 978-0-12-816061-9. [Google Scholar]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of Different LED Sources on the Growth and Nitrogen Metabolism of Lettuce. Plant Cell Tiss Organ Cult. 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional Quality, Mineral and Antioxidant Content in Lettuce Affected by Interaction of Light Intensity and Nutrient Solution Concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Cheng, R.; Wang, Y.; Yang, Q.; Lu, C. Effect of Green Light on Nitrate Reduction and Edible Quality of Hydroponically Grown Lettuce (Lactuca Sativa L.) under Short-Term Continuous Light from Red and Blue Light-Emitting Diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Yu, J.; Liu, H. Accumulation and Primary Metabolism of Nitrate in Lettuce (Lactuca Sativa L. Var. Youmaicai) Grown Under Three Different Light Sources. Commun. Soil Sci. Plant Anal. 2016, 47, 1994–2002. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of Light Quality on Main Health-Promoting Compounds and Antioxidant Capacity of Chinese Kale Sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.E.; Zhen, S.; Kim, J. Mild-Intensity UV-A Radiation Applied Over a Long Duration Can Improve the Growth and Phenolic Contents of Sweet Basil. Front. Plant Sci. 2022, 13, 858433. [Google Scholar] [CrossRef]
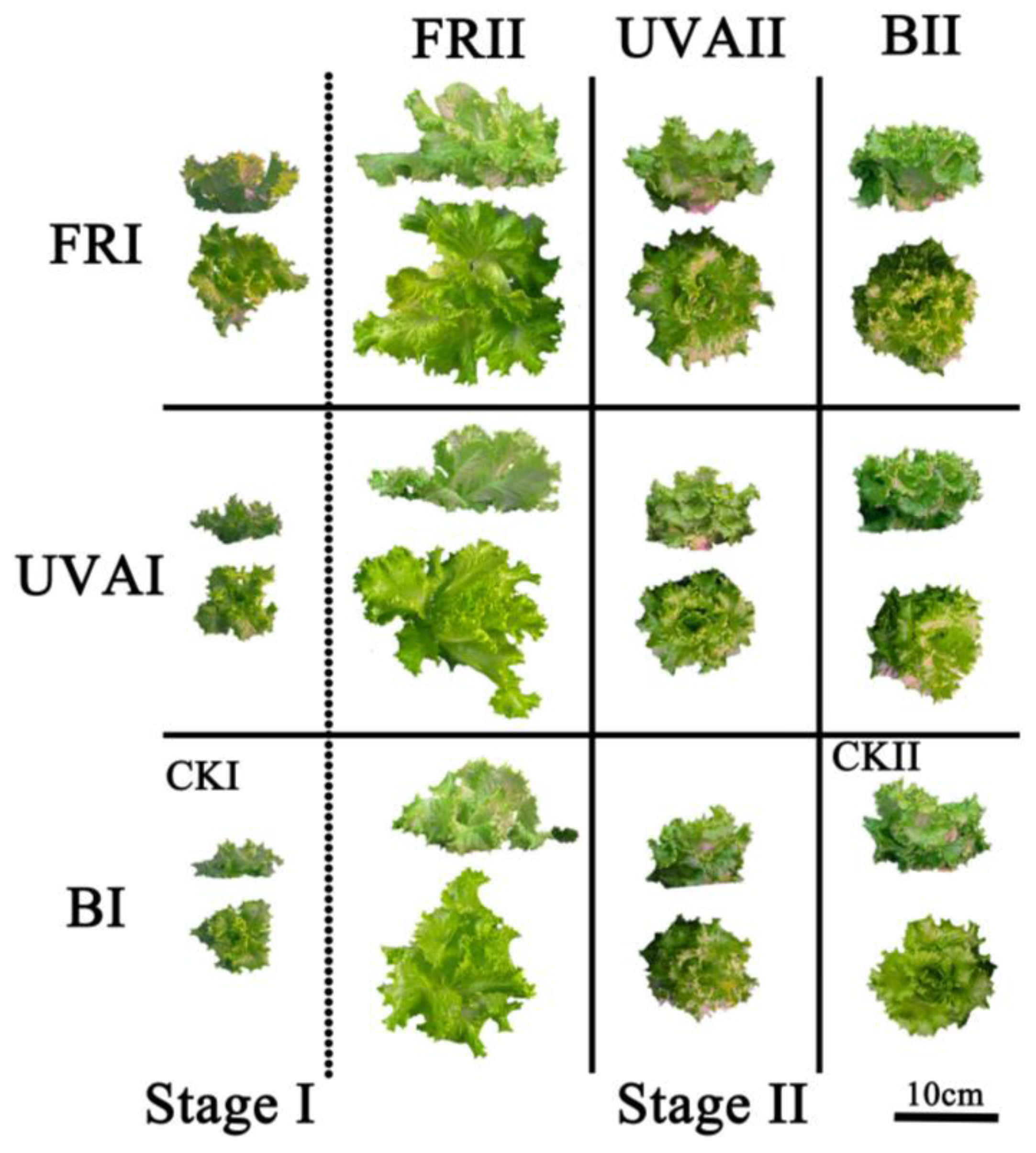
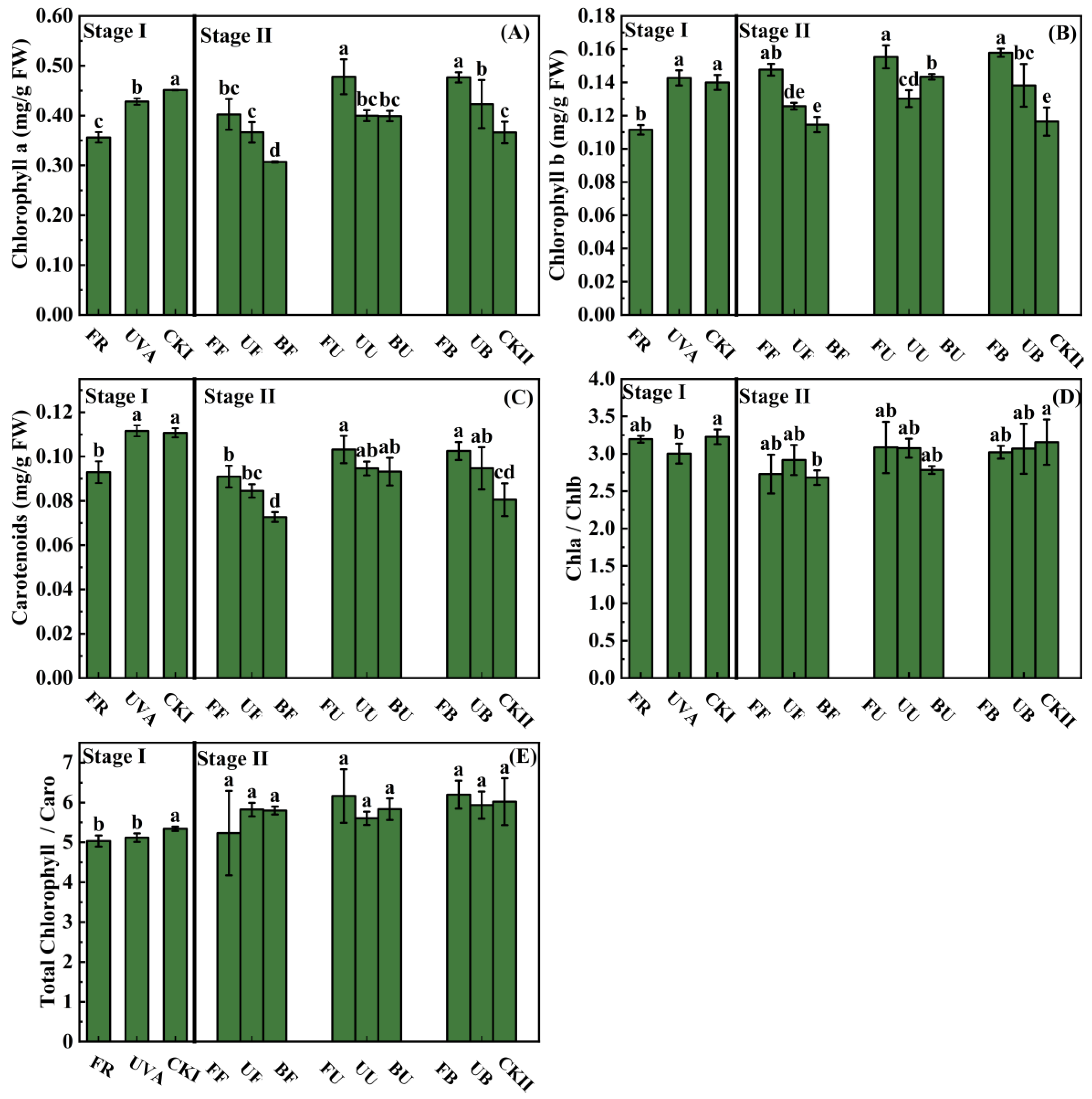
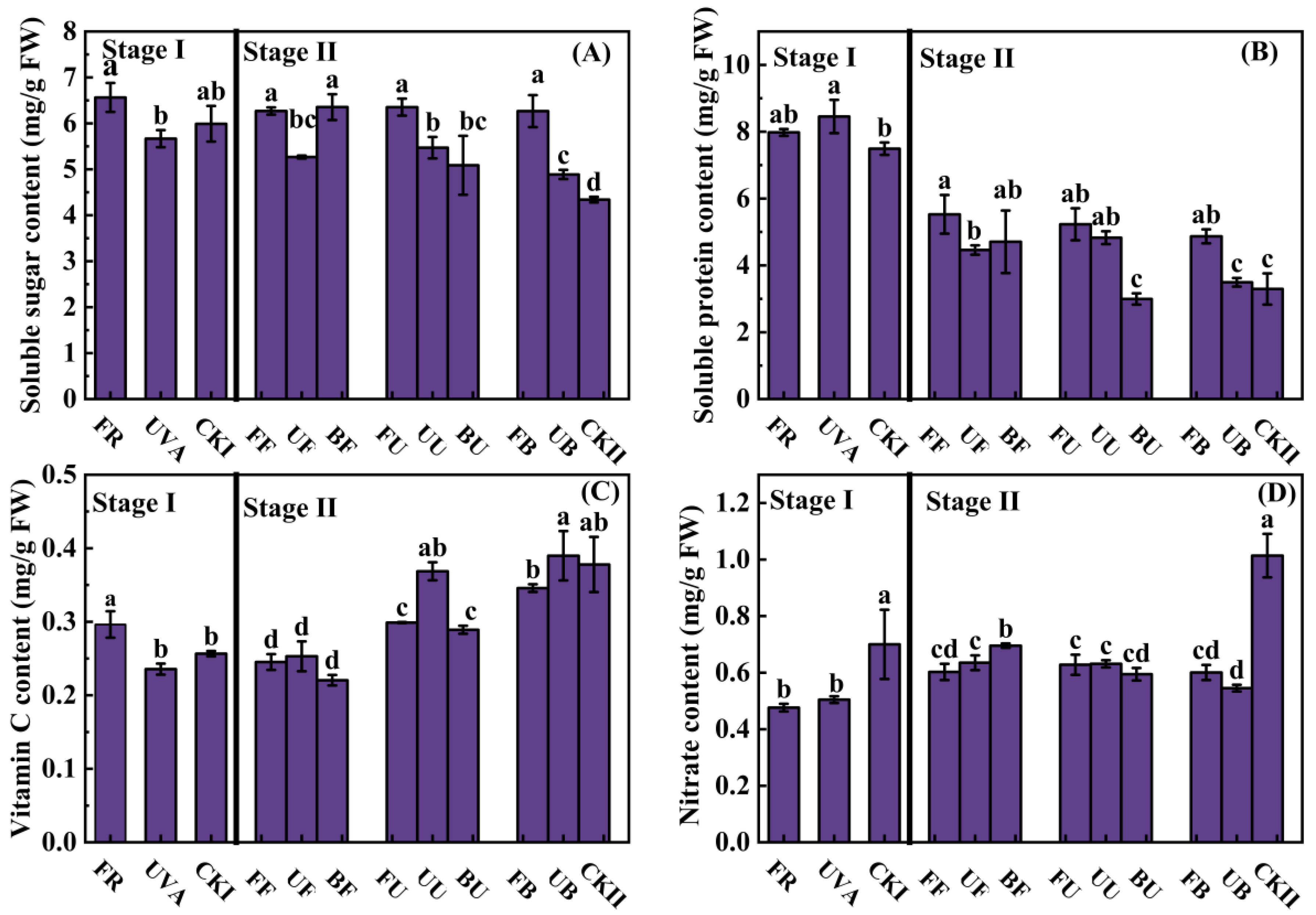
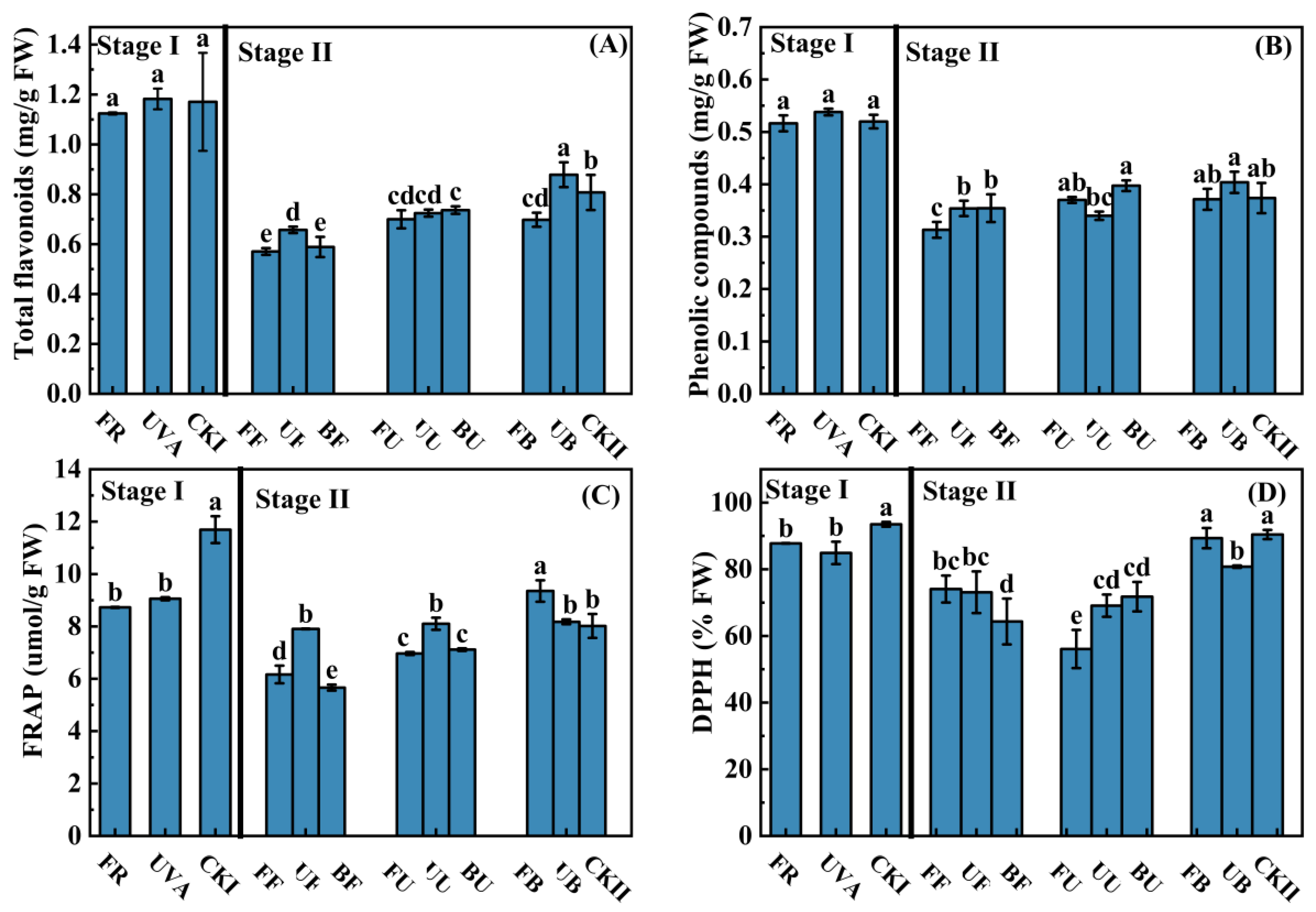

| Treatment | R (660 ± 10 nm) | B (460 ± 10 nm) | FR (730 ± 10 nm) | UV-A (380 ± 10 nm) | PPFD | YPFD | Unit |
|---|---|---|---|---|---|---|---|
| FR | 150 | 50 | 50 | 200 | 250 | μmol·m−2·s−1 | |
| UVA | 150 | 50 | 50 | 200 | 250 | ||
| B (CKI) | 150 | 100 | 250 | 250 |
| Stage I (20 Days before Harvesting) | Stage II (10 Days before Harvesting) |
|---|---|
| FR | FR (FF) |
| UVA (FU) | |
| B (FB) | |
| UVA | FR (UF) |
| UVA (UU) | |
| B (UB) | |
| B | FR (BF) |
| UVA (BU) | |
| B (CKII) |
| Leaf | Leaf | Leaf | Specific | Weight (g/Plant) | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Width | Length | Area | Leaf | ||||
| cm | cm | cm2 | Weight | Root | Shoot | Root | Shoot | |
| kg/cm2 | FW | FW | DW | DW | ||||
| Stage I | ||||||||
| FR | 6.46 ± 1.01 a | 9.13 ± 1.18 a | 270.24 ± 50.61 a | 2.13 ± 0.17 b | 2.18 ± 0.13 a | 11.25 ± 0.85 a | 0.11 ± 0.01 a | 0.57 ± 0.04 a |
| UVA | 5.13 ± 0.55 b | 6.84 ± 0.54 b | 208.42 ± 29.20 b | 2.57 ± 0.34 a | 2.41 ± 0.15 a | 9.30 ± 0.46 b | 0.12 ± 0.01 a | 0.53 ± 0.02 a |
| CKI | 5.20 ± 0.56 b | 7.00 ± 0.83 b | 177.29 ± 28.44 b | 2.83 ± 0.34 a | 2.16 ± 0.13 a | 8.03 ± 0.67 b | 0.12 ± 0.01 a | 0.5 ± 0.02 a |
| Stage II | ||||||||
| FF | 11.00 ± 0.63 ab | 15.44 ± 0.99 b | 1203.83 ± 102.08 ab | 1.95 ± 0.19 c–e | 4.45 ± 0.27 a | 65.76 ± 3.01 a | 0.24 ± 0.04 ab | 2.35 ± 0.34 a |
| UF | 10.54 ± 0.28 b | 16.37 ± 0.83 a | 1138.42 ± 90.55 b | 1.90 ± 0.12 df | 3.78 ± 0.17 b | 64.91 ± 1.97 a | 0.18 ± 0.04 cd | 2.17 ± 0.24 ab |
| BF | 11.33 ± 0.55 a | 16.25 ± 0.29 a | 1242.92 ± 69.84 a | 1.75 ± 0.25 f | 3.66 ± 0.22 b | 61.01 ± 1.74 ab | 0.19 ± 0.03 cd | 2.17 ± 0.36 ab |
| FU | 9.97 ± 0.69 c | 12.07 ± 0.3 de | 919.28 ± 127.81 c | 2.19 ± 0.11 b | 4.44 ± 0.2 a | 57.70 ± 2.60 b | 0.22 ± 0.03 bc | 2.03 ± 0.21 bd |
| UU | 11.05 ± 0.65 ab | 12.98 ± 0.78 c | 884.23 ± 75.81 c | 2.06 ± 0.1 bd | 3.65 ± 0.08 b | 50.65 ± 1.18 cd | 0.21 ± 0.04 cd | 1.82 ± 0.2 cd |
| BU | 9.35 ± 0.35 de | 13.03 ± 0.51 c | 828.78 ± 71.81 c | 2.12 ± 0.19 bc | 3.82 ± 0.31 b | 48.48 ± 2.33 de | 0.18 ± 0.03 d | 1.76 ± 0.28 de |
| FB | 9.81 ± 0.54 cd | 11.75 ± 0.55 e | 836.97 ± 78.97 c | 2.53 ± 0.15 a | 5.02 ± 0.22 a | 55.86 ± 1.46 bc | 0.27 ± 0.04 a | 2.12 ± 0.23 ac |
| UB | 9.20 ± 0.27 e | 11.81 ± 0.47 e | 865.36 ± 91.14 c | 2.16 ± 0.25 b | 3.71 ± 0.09 b | 49.40 ± 1.41 de | 0.19 ± 0.03 cd | 1.88 ± 0.31 bd |
| CKII | 9.14 ± 0.39 e | 12.59 ± 0.46 cd | 831.84 ± 71.58 c | 1.83 ± 0.09 ef | 3.41 ± 0.14 b | 43.99 ± 1.24 e | 0.19 ± 0.01 cd | 1.53 ± 0.18 e |
| Treatment | Score of Each Principal Component | Variance Contribution % | Total Score | Rank | ||||
|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | PC1 | PC2 | PC3 | |||
| Stage I | ||||||||
| FR | 4.02 | −0.73 | −0.05 | 46.15% | 24.35% | 10.21% | 1.67 | 1 |
| UVA | −1.16 | 2.79 | 0.04 | 0.15 | 2 | |||
| CKI | −2.86 | −2.06 | 0.01 | −1.82 | 3 | |||
| Stage II | ||||||||
| FF | 4.49 | 1.49 | −0.02 | 39.57% | 27.56% | 7.31% | 2.19 | 1 |
| UF | 1.98 | −1.27 | 0.47 | 0.47 | 5 | |||
| BF | 4.06 | −2.91 | 0.05 | 0.81 | 2 | |||
| FU | 0.12 | 2.54 | −0.49 | 0.71 | 4 | |||
| UU | −0.47 | −0.16 | 0.35 | −0.2 | 6 | |||
| BU | −1.94 | −0.95 | −2.18 | −1.19 | 7 | |||
| FB | −1.29 | 4.3 | 1.32 | 0.77 | 3 | |||
| UB | −3.35 | 0.22 | −1.06 | −1.34 | 8 | |||
| CKII | −3.62 | −3.26 | 1.56 | −2.22 | 9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; He, R.; Ju, J.; Zhang, S.; He, X.; Li, Y.; Liu, X.; Liu, H. Effects of Substituting B with FR and UVA at Different Growth Stages on the Growth and Quality of Lettuce. Agronomy 2023, 13, 2547. https://doi.org/10.3390/agronomy13102547
Hu Y, He R, Ju J, Zhang S, He X, Li Y, Liu X, Liu H. Effects of Substituting B with FR and UVA at Different Growth Stages on the Growth and Quality of Lettuce. Agronomy. 2023; 13(10):2547. https://doi.org/10.3390/agronomy13102547
Chicago/Turabian StyleHu, Youzhi, Rui He, Jun Ju, Shuchang Zhang, Xinyang He, Yamin Li, Xiaojuan Liu, and Houcheng Liu. 2023. "Effects of Substituting B with FR and UVA at Different Growth Stages on the Growth and Quality of Lettuce" Agronomy 13, no. 10: 2547. https://doi.org/10.3390/agronomy13102547






