AMMI Analysis of Genotype × Environment Interaction on Sugar Beet (Beta vulgaris L.) Yield, Sugar Content and Production in Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Genetic Materials and Environments
2.2. Experimental Design and Agronomic Practices
2.3. Statistical Analysis
3. Results and Discussion
3.1. Combined Variance Analysis for Yield, Sugar Content, Sugar Production, and Its Related Traits
3.2. The AMMI Variance Analysis for Yield, Sugar Content, and Sugar Production
3.3. The AMMI Stability Value (ASV) for Yield, Sugar Content, and Sugar Production
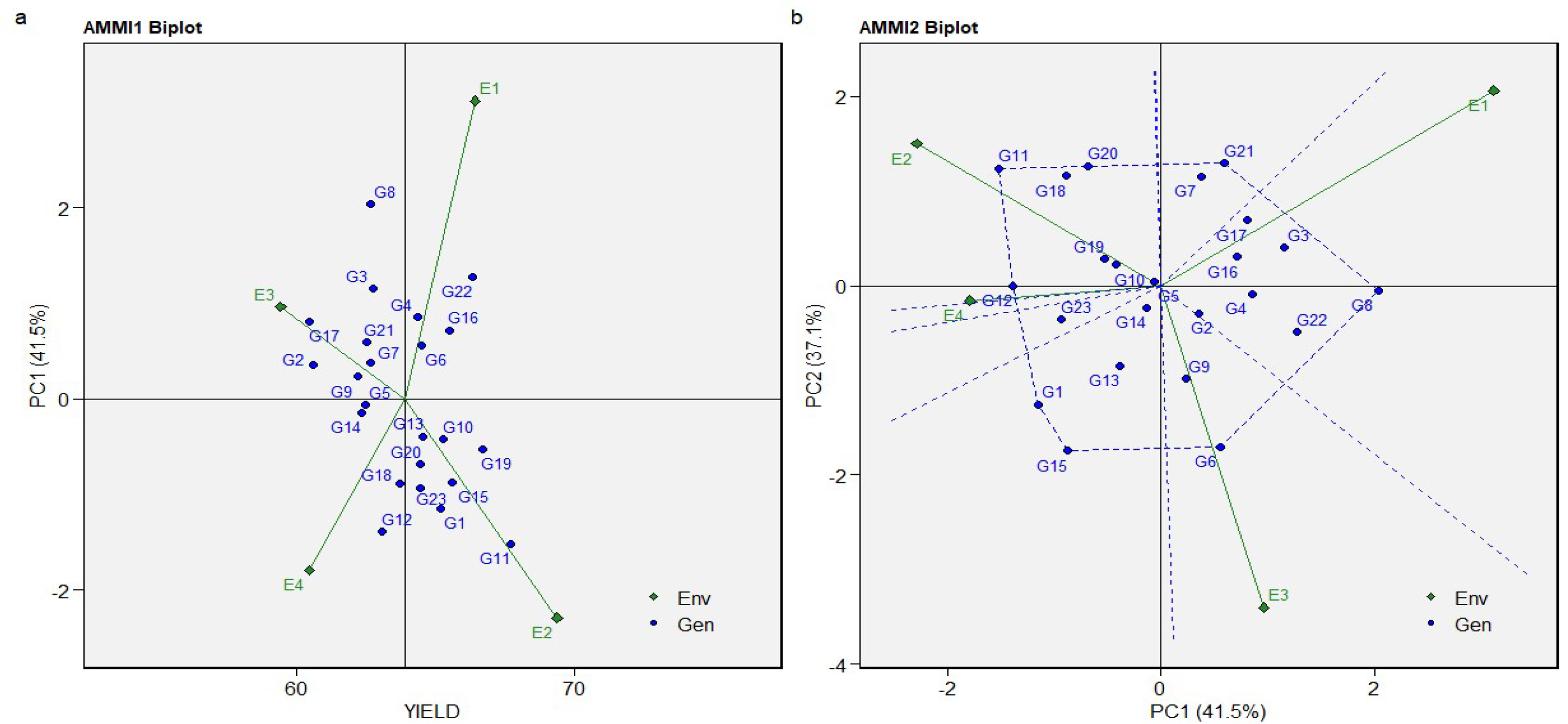
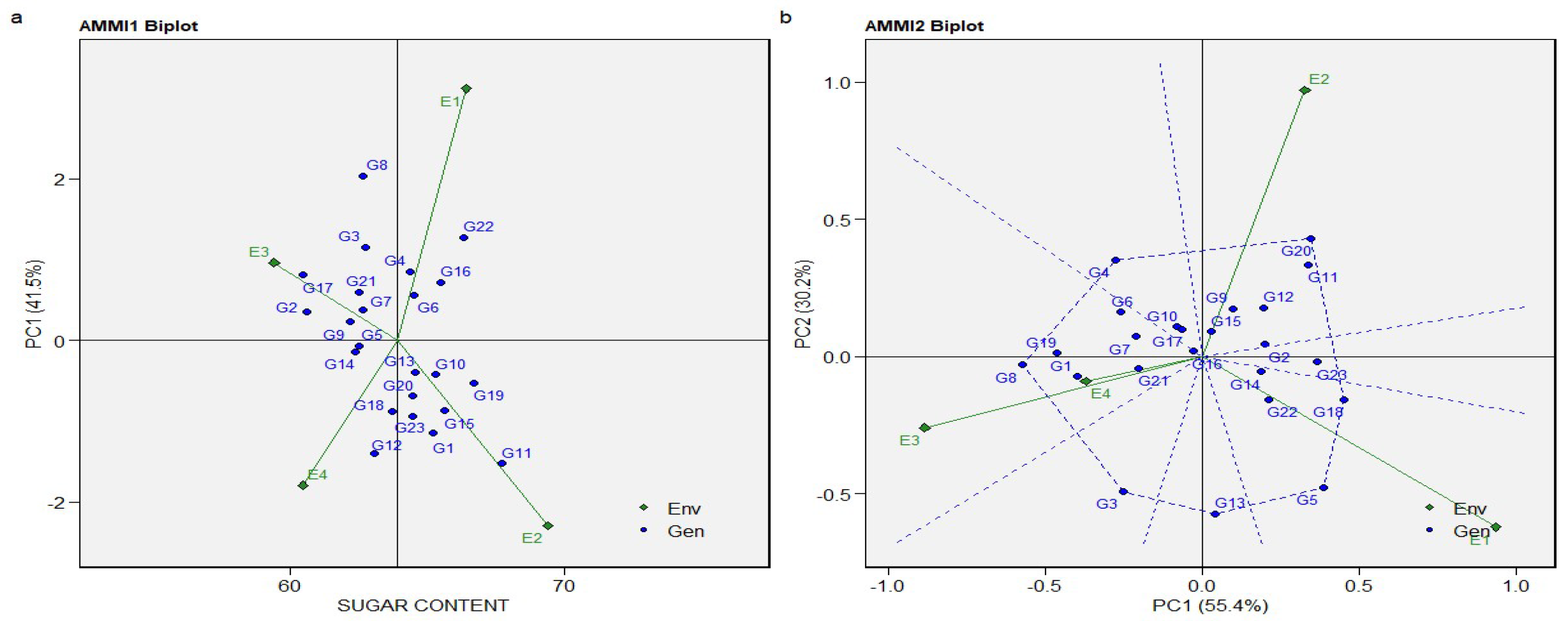
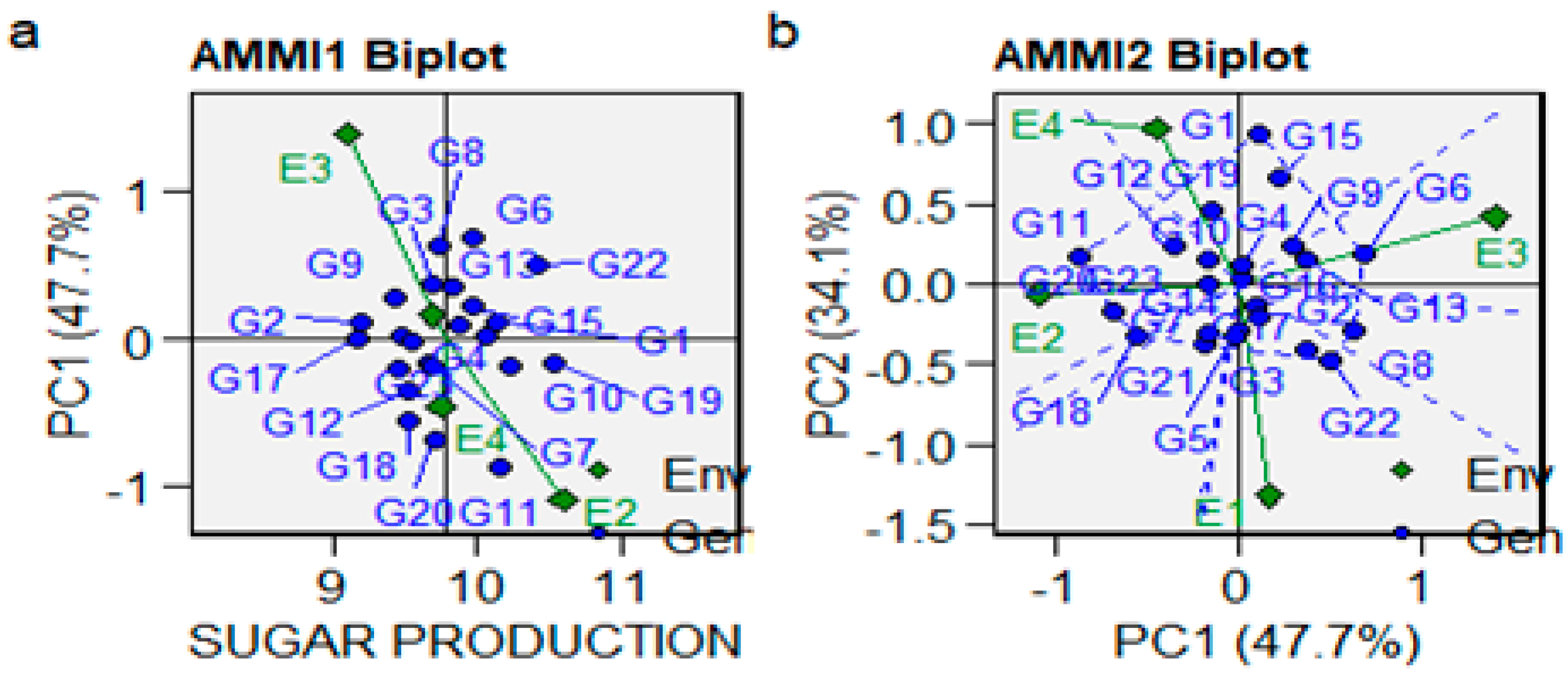
3.4. The AMMI Mono- and Biplot Model for Yield, Sugar Content, and Sugar Production
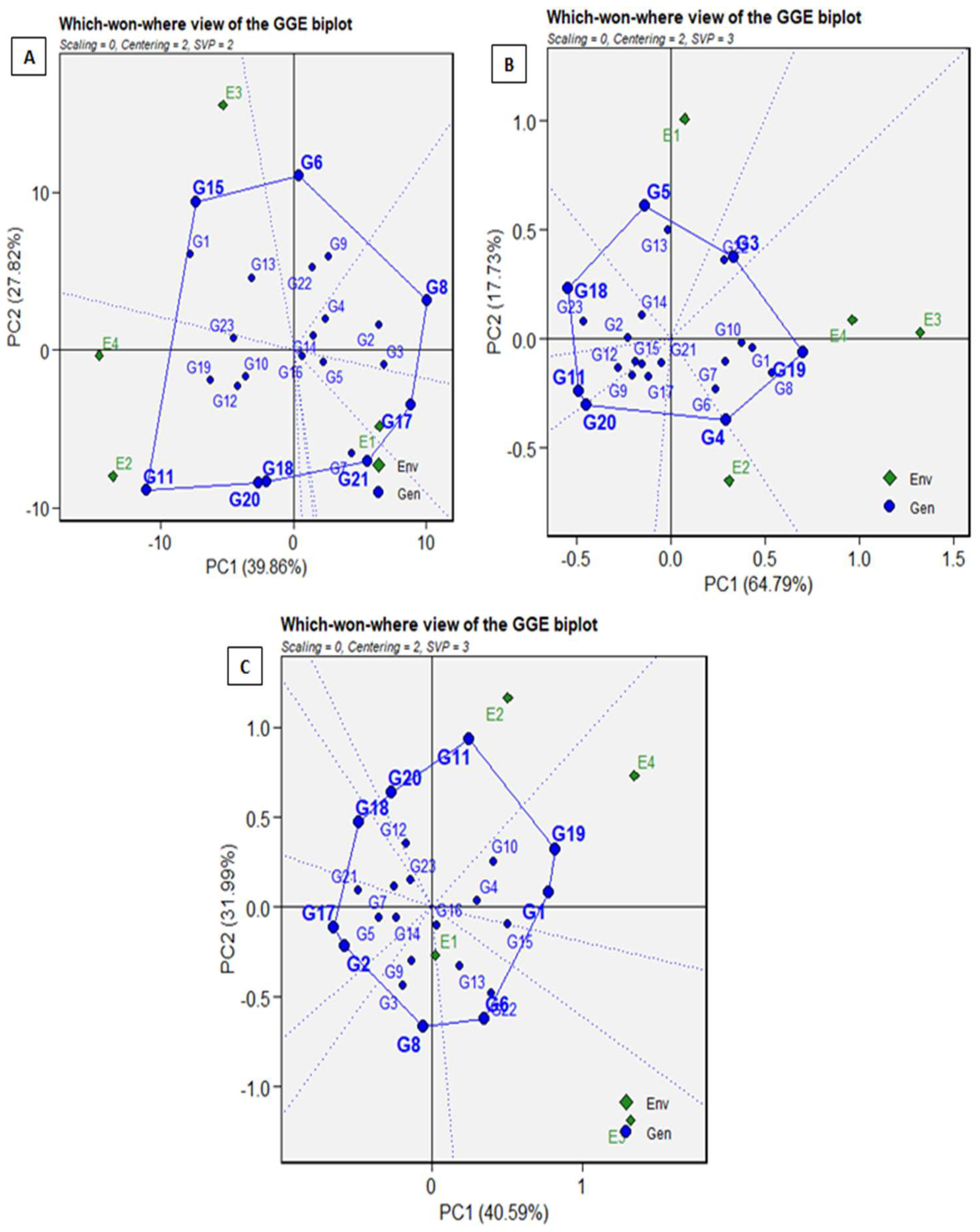
3.5. The GGE Biplot (‘Which-Won-Where’ Pattern) for Yield, Sugar Content, and Sugar Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Agriculture an Rural Development. 2022. Available online: www.madr.ro (accessed on 17 July 2023).
- Kühnel, S.; Schols, H.A.; Gruppen, H. Aiming for the Complete Utilization of Sugar-Beet Pulp: Examination of the Effects of Mild Acid and Hydrothermal Pretreatment Followed by Enzymatic Digestion. Biotechnol. Biofuels 2011, 4, 14. [Google Scholar] [CrossRef]
- Berłowska, J.; Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Patelski, P.; Dziugan, P.; Kręgiel, D. Simultaneous Saccharification and Fermentation of Sugar Beet Pulp for Efficient Bioethanol Production. BioMed Res. Int. 2016, 2016, 3154929. [Google Scholar] [CrossRef]
- Ahmed, S.; Shahzad, F.; Rahman, H.-U.; Rajab, M. Effects of Sugar Beet Pulp Based Total Mixed Ration on Growth Performance and Blood Profile Status in Male Nili Ravi Buffalo Calves. Turk. J. Veter-Anim. Sci. 2020, 44, 928–933. [Google Scholar] [CrossRef]
- Broccanello, C.; McGrath, J.M.; Panella, L.; Richardson, K.; Funk, A.; Chiodi, C.; Biscarini, F.; Barone, V.; Baglieri, A.; Squartini, A.; et al. A SNP mutation affects rhizomania-virus content of sugar beets grown on resistance-breaking soils. Euphytica 2018, 214, 14. [Google Scholar] [CrossRef]
- Jaggard, K.W.; Clark, C.J.A.; Draycott, A.P. The weight and processing quality of components of the storage roots of sugar beet (Beta vulgaris L). J. Sci. Food Agric. 1999, 79, 1389–1398. [Google Scholar] [CrossRef]
- Milford, G.F.J.; Houghton, B.J. An analysis of the variation in crown size in sugar-beet (Beta vulgaris) grown in England. Ann. Appl. Biol. 1999, 134, 225–232. [Google Scholar] [CrossRef]
- Verma, A.; Deepti, S. Abiotic stress and crop improvement: Current scenario. Adv. Plants Agric. Res. 2016, 4, 345–346. [Google Scholar]
- Hoberg, F.; Ladewig, E.; Kenter, C. Genotype environment interactions in sugar beet in Germany. In Proceedings of the IIRB-Congress, Brussels, Belgium, 16–17 February 2016. [Google Scholar]
- Gholami, R.; Zahedi, S.M. Identifying superior drought-tolerant olive genotypes and their biochemical and some physiological responses to various irrigation levels. J. Plant Nutr. 2019, 42, 2057–2069. [Google Scholar] [CrossRef]
- Okorie, V.O.; Mphambukeli, T.N.; Amusan, S.O. Exploring the political economy of water and food security nexus in BRICS. Afr. Insight 2019, 48, 21–38. [Google Scholar]
- Phuke, R.M.; Anuradha, K.; Radhika, K.; Jabeen, F.; Anuradha, G.; Ramesh, T.; Hariprasanna, K.; Mehtre, S.P.; Deshpande, S.P.; Anil, G.; et al. Genetic Variability, Genotype × Environment Interaction, Correlation, and GGE Biplot Analysis for Grain Iron and Zinc Concentration and Other Agronomic Traits in RIL Population of Sorghum (Sorghum bicolor L. Moench). Front. Plant Sci. 2017, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Diyah, H.S.; Hadi, A.F. AMMI Model for Yield Estimation in Multi-Environment Trials: A Comparison to BLUP. Agric. Agric. Sci. Procedia 2016, 9, 163–169. [Google Scholar] [CrossRef]
- Ma, D.; Stützel, H. Prediction of winter wheat cultivar performance in Germany: At national, regional and location scale. Eur. J. Agron. 2014, 52, 210–217. [Google Scholar] [CrossRef]
- Yan, W. Analysis and handling of G × E in a practical breeding program. Crop. Sci. 2016, 56, 2106–2118. [Google Scholar] [CrossRef]
- Rodrigues, P.C.; Malosetti, M.; Gauch, H.G.; van Eeuwijk, F.A. A weighted AMMI algorithm to study genotype-by-environment interaction and QTL-by-environment interaction. Crop. Sci. 2014, 54, 1555–1570. [Google Scholar] [CrossRef]
- Li, W.; Yan, Z.-H.; Wei, Y.-M.; Lan, X.-J.; Zheng, Y.-L. Evaluation of Genotype × Environment Interactions in Chinese Spring Wheat by the AMMI Model, Correlation and Path Analysis. J. Agron. Crop. Sci. 2006, 192, 221–227. [Google Scholar] [CrossRef]
- Agahi, K.; Ahmadi, J.; Oghan, H.A.; Fotokian, M.H.; Orang, S.F. Analysis of genotype × environment interaction for seed yield in spring oilseed rape using the AMMI model. Crop. Breed. Appl. Biotechnol. 2020, 20, e26502012. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE Biplot vs. AMMI Analysis of Genotype-by-Environment Data. Crop Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef]
- Hoffmann, C.M.; Huijbregts, T.; van Swaaij, N.; Jansen, R. Impact of different environments in Europe on yield and quality of sugar beet genotypes. Eur. J. Agron. 2009, 30, 17–26. [Google Scholar] [CrossRef]
- Vargas, M.; Crossa, J.; Van Eeuwijk, F.; Sayre, K.D.; Reynolds, M.P. Interpreting treatment × environment interaction in agronomy trials. Agron. J. 2001, 93, 949–960. [Google Scholar] [CrossRef]
- Hassani, M.; Heidari, B.; Dadkhodaie, A.; Stevanato, P. Genotype by environment interaction components underlying variations in root, sugar and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica 2018, 214, 79. [Google Scholar] [CrossRef]
- Moradi, F.M.; Safari, H.; Jalililan, A. Study of genotype × environment interaction for sugar beet monogerm cultivars using AMMI method. J. Sugar Beet 2012, 28, 55–66. [Google Scholar] [CrossRef]
- Flores, F.; Moreno, M.; Cubero, J. A comparison of univariate and multivariate methods to analyze environments. Field Crop. Res. 1998, 56, 271–286. [Google Scholar] [CrossRef]
- Jafari, T.; Farshadfar, E. Stability analysis of bread wheat genotypes (Triticum aestivum L.) by GGE biplot. Cereal Res. 2018, 8, 199–208. [Google Scholar] [CrossRef]
- Yang, R.-C.; Crossa, J.; Cornelius, P.L.; Burgueño, J. Biplot analysis of genotype × environment interaction: Proceed with caution. Crop. Sci. 2009, 49, 1564–1576. [Google Scholar] [CrossRef]
- Yaghotipoor, A.; Farshadfar, E. Non-parametric estimation and component analysis of phenotypic stability in chickpea (Cicer arietinum L.). Pak. J. Biol. Sci. 2007, 10, 2646–2652. [Google Scholar]
- Sabaghnia, N.; Sabaghpour, S.H.; Dehghani, H. The use of an AMMI model and its parameters to analyse yield stability in multi-environment trials. J. Agric. Sci. 2008, 146, 571–581. [Google Scholar] [CrossRef]
- Naroui Rad, M.R.; Abdul Kadir, M.; Rafii, Y.M.; Hawa, Z.E.J.; Naghavi, M.R.; Ahmadi, A. Genotype 9 environment interaction by AMMI and GGE biplot analysis in three consecutive generations of wheat (Triticum aestivum) under normal and drought stress conditions. Aust. J. Crop Sci. 2013, 7, 956–961. [Google Scholar]
- Khan, M.H.; Rafii, M.Y.; Ramlee, S.I.; Jusoh, M.; Al Mamun, A. Genetic analysis and selection of Bambara groundnut (Vigna subterranea L. Verdc.) landraces for high yield revealed by qualitative and quantitative traits. Sci. Rep. 2021, 11, 22791. [Google Scholar] [CrossRef]
- Mostavafi, K.; Orazizadeh, M.; Rajabi, A.; Ilkaei, M.N. Stability and adaptability analysis in sugar beet varieties for sugar content using GGE-biplot and AMMI methods. Bulg. J. Agric. Sci. 2018, 24, 40–45. [Google Scholar]
- Taleghani, D.; Rajabi, A.; Saremirad, A.; Fasahat, P. Stability analysis and selection of sugar beet (Beta vulgaris L.) genotypes using AMMI, BLUP, GGE biplot and MTSI. Sci. Rep. 2023, 13, 10019. [Google Scholar] [CrossRef] [PubMed]
- Akinwale, R.O.; Fakorede, M.A.B.; Badu-Apraku, B.; Oluwaranti, A. Assessing the usefulness of GGE biplot as a statistical tool for plant breeders and agronomists. Cereal. Res. Commun. 2014, 42, 534–546. [Google Scholar] [CrossRef]
- Xu, Y. Envirotyping for deciphering environmental impacts on crop plants. Theor. Appl. Genet. 2016, 129, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Gauch, H.G., Jr.; Zobel, R.W. Optimal Replication in Selection Experiments. Crop. Sci. 1996, 36, 838–843. [Google Scholar] [CrossRef]
- Studnicki, M.; Lenartowicz, T.; Noras, K.; Wójcik-Gront, E.; Wyszyński, Z. Assessment of Stability and Adaptation Patterns of White Sugar Yield from Sugar Beet Cultivars in Temperate Climate Environments. Agronomy 2019, 9, 405. [Google Scholar] [CrossRef]
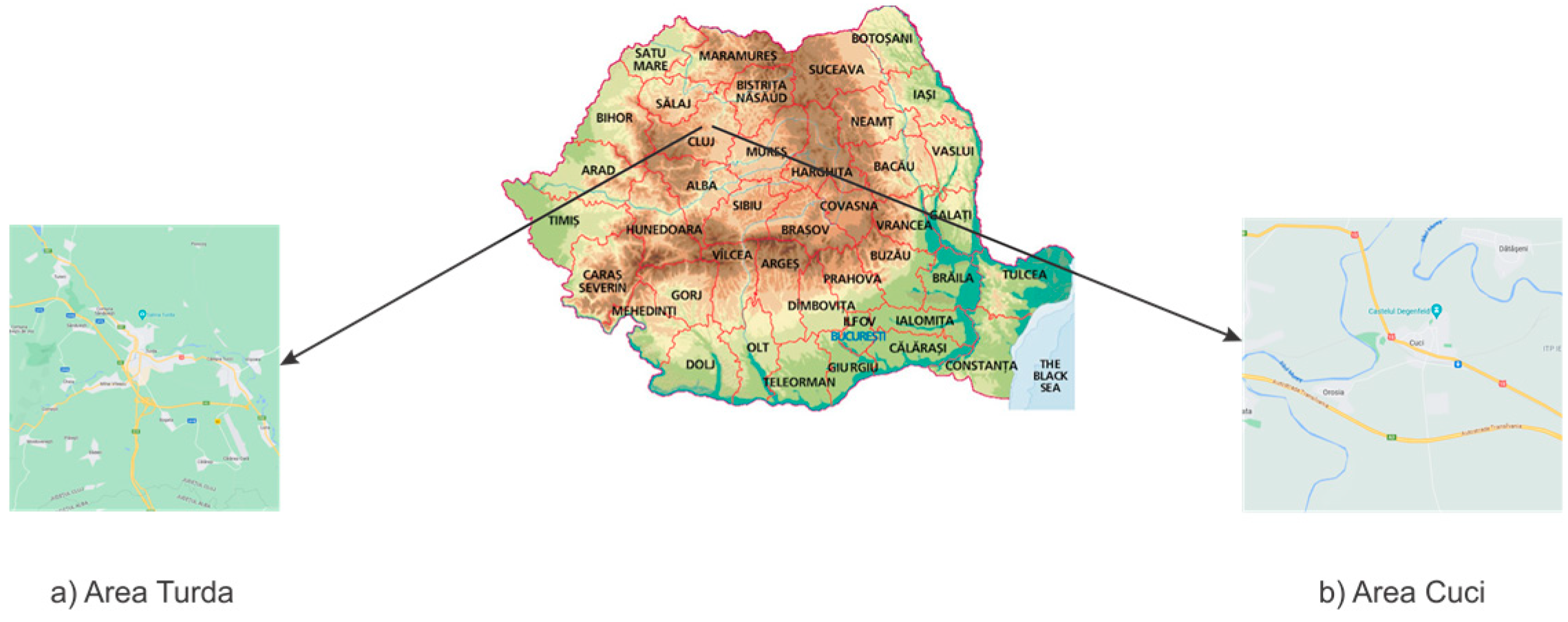
| Environment | Location | Year | Altitude (m) | Latitude (°N) | Longitude (°E) | Low Temp (°C) | High Temp (°C) | Mean Temp (°C) | Rainfall (mm) | Soil Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| E1 | Bogata (Turda) | 2020 | 343 | 46.5143337 | 23.8054982 | 0.0 | 32 | 18.98 | 336 | chernozem argiloiluvial |
| E3 | Cuci | 2020 | 257 | 46.4468099 | 24.1705663 | 1.0 | 31 | 17.22 | 316 | chernozem argiloiluvial |
| E2 | Bogata (Turda) | 2021 | 343 | 46.5115843 | 23.7937994 | 1.50 | 30 | 17.50 | 342 | chernozem argiloiluvial |
| E4 | Cuci | 2021 | 254 | 46.4555663 | 23.7937994 | 2.37 | 27 | 13.40 | 321 | chernozem argiloiluvial |
| Source of Variation | Df | SS | MS | F Value | p Value | % of Total Variance Explained |
|---|---|---|---|---|---|---|
| The sugar beet yield | ||||||
| ENV | 3 | 6267.51 | 2089.17 | 114.6182 | 0.00000 | 37.44 |
| GEN | 22 | 1213.029 | 55.13767 | 3.02502 | 0.00001 | 7.25 |
| GEN × ENV | 66 | 3521.31 | 53.35318 | 2.927117 | 0.00000 | 21.04 |
| Residuals | 264 | 4811.984 | 18.22721 | 34.27 | ||
| CV (%) | 6.677775 | |||||
| The sugar content of the sugar beet | ||||||
| ENV | 3 | 109.3923 | 36.46409 | 254.3816 | 0.00000 | 52.13 |
| GEN | 22 | 21.59332 | 0.981514 | 6.847263 | 0.00000 | 7.12 |
| GEN × ENV | 66 | 26.06212 | 0.394881 | 2.754775 | 0.00000 | 12.42 |
| Residuals | 264 | 37.84283 | 0.143344 | 28.33 | ||
| CV (%) | 2.469693 | |||||
| The sugar production of the sugar beet | ||||||
| ENV | 3 | 105.105 | 35.035 | 76.86451 | 0.00000 | 26.84 |
| GEN | 22 | 45.97864 | 2.089938 | 4.585188 | 0.00000 | 11.74 |
| GEN × ENV | 66 | 95.88875 | 1.45286 | 3.18748 | 0.00000 | 24.18 |
| Residuals | 264 | 120.3317 | 0.455802 | 37.24 | ||
| CV (%) | 6.898275 | |||||
| Source of Variation | Df | SS | MS | F Value | p Value | % of Total Variance Explained |
|---|---|---|---|---|---|---|
| ENV | 3 | 6267.51 | 2089.17 | 27.08867 | 0.00001 | 30.93 |
| GEN | 22 | 1213.029 | 55.13767 | 3.02502 | 0.00001 | 5.99 |
| GEN × ENV | 66 | 3521.31 | 53.35318 | 2.927117 | 0.00000 | 17.38 |
| PC1 | 24 | 1459.829 | 60.82622 | 3.34 | 0.00000 | 41.50 |
| PC2 | 22 | 1308.164 | 59.46202 | 3.26 | 0.00000 | 37.10 |
| PC3 | 20 | 753.3161 | 37.6658 | 2.07 | 0.00540 | 21.40 |
| Residuals | 264 | 4811.984 | 18.22721 | 23.75 | ||
| CV (%) | 433 | 20260.62 | 46.79128 |
| Source of Variation | Df | SS | MS | F Value | p Value | % of Total Variance Explained |
|---|---|---|---|---|---|---|
| ENV | 3 | 109.3923 | 36.46409 | 29.27926 | 0.00001 | 46.37 |
| GEN | 22 | 21.59332 | 0.981514 | 6.847263 | 0.00000 | 9.15 |
| GEN × ENV | 66 | 26.06212 | 0.394881 | 2.754775 | 0.00000 | 11.04 |
| PC1 | 24 | 14.44013 | 0.60167 | 4.2 | 0.00000 | 55.4 |
| PC2 | 22 | 7.8755 | 0.35798 | 2.5 | 0.00030 | 30.2 |
| PC3 | 20 | 3.74649 | 0.18732 | 1.31 | 0.17190 | 14.4 |
| Residuals | 264 | 37.84283 | 0.143344 | 16.04 | ||
| CV (%) | 433 | 235.8973 | 0.544797 |
| Source of Variation | Df | SS | MS | F Value | p Value | % of Total Variance Explained |
|---|---|---|---|---|---|---|
| ENV | 3 | 105.105 | 35.035 | 17.32029 | 0.00012 | 21.56 |
| GEN | 22 | 45.97864 | 2.089938 | 4.585188 | 0.00000 | 9.43 |
| GEN × ENV | 66 | 95.88875 | 1.45286 | 3.18748 | 0.00000 | 19.67 |
| PC1 | 24 | 45.72445 | 1.90519 | 4.18 | 0.00000 | 47.70 |
| PC2 | 22 | 32.74275 | 1.48831 | 3.27 | 0.00000 | 34.10 |
| PC3 | 20 | 17.42155 | 0.87108 | 1.91 | 0.01210 | 18.20 |
| Residuals | 264 | 120.3317 | 0.455802 | 24.69 | ||
| CV (%) | 433 | 487.4661 | 1.125788 |
| GEN | Yield | ASV | Sugar Content | ASV | Sugar Production (t/ha) | ASV |
|---|---|---|---|---|---|---|
| G1 | 65.21438 | 1.793822888 | 15.56875 | 0.738432 | 10.15 | 0.953568 |
| G2 | 60.60438 | 0.490966143 | 15.19375 | 0.364398 | 9.19375 | 0.253539 |
| G3 | 62.75813 | 1.352812332 | 15.51875 | 0.67892 | 9.7 | 0.661424 |
| G4 | 64.3675 | 0.95798203 | 15.51875 | 0.618248 | 10.0625 | 0.127496 |
| G5 | 62.50375 | 0.085570746 | 15.35 | 0.852028 | 9.55625 | 0.319414 |
| G6 | 64.5325 | 1.816773897 | 15.45625 | 0.505736 | 9.96875 | 0.970598 |
| G7 | 62.6625 | 1.227328633 | 15.525 | 0.399781 | 9.7 | 0.396533 |
| G8 | 62.68 | 2.274888519 | 15.59375 | 1.054805 | 9.74375 | 0.923854 |
| G9 | 62.20125 | 1.017663417 | 15.175 | 0.2466 | 9.43125 | 0.461223 |
| G10 | 65.3 | 0.521050311 | 15.6875 | 0.184028 | 10.225 | 0.292545 |
| G11 | 67.72 | 2.099461427 | 15.0125 | 0.699351 | 10.16875 | 1.233068 |
| G12 | 63.10125 | 1.553317999 | 15.15 | 0.39899 | 9.5375 | 0.556771 |
| G13 | 64.575 | 0.957135137 | 15.3 | 0.577155 | 9.84375 | 0.526721 |
| G14 | 62.36375 | 0.276716107 | 15.2625 | 0.346076 | 9.475 | 0.040036 |
| G15 | 65.62063 | 1.998768325 | 15.1875 | 0.10405 | 9.975 | 0.725254 |
| G16 | 65.50313 | 0.858093709 | 15.11875 | 0.059889 | 9.875 | 0.191199 |
| G17 | 60.48875 | 1.138387692 | 15.18125 | 0.155173 | 9.16875 | 0.293401 |
| G18 | 63.72938 | 1.522914671 | 14.98125 | 0.839074 | 9.525 | 0.852712 |
| G19 | 66.69063 | 0.650662527 | 15.825 | 0.855266 | 10.5375 | 0.502168 |
| G20 | 64.4825 | 1.474864422 | 15.06875 | 0.76566 | 9.7125 | 0.970572 |
| G21 | 62.54313 | 1.453694437 | 15.16875 | 0.373913 | 9.45625 | 0.473743 |
| G22 | 66.34125 | 1.509034888 | 15.71875 | 0.41443 | 10.41875 | 0.842799 |
| G23 | 64.48625 | 1.105689217 | 15.03125 | 0.669162 | 9.675 | 0.24469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oroian, C.; Ugruțan, F.; Mureșan, I.C.; Oroian, I.; Odagiu, A.; Petrescu-Mag, I.V.; Burduhos, P. AMMI Analysis of Genotype × Environment Interaction on Sugar Beet (Beta vulgaris L.) Yield, Sugar Content and Production in Romania. Agronomy 2023, 13, 2549. https://doi.org/10.3390/agronomy13102549
Oroian C, Ugruțan F, Mureșan IC, Oroian I, Odagiu A, Petrescu-Mag IV, Burduhos P. AMMI Analysis of Genotype × Environment Interaction on Sugar Beet (Beta vulgaris L.) Yield, Sugar Content and Production in Romania. Agronomy. 2023; 13(10):2549. https://doi.org/10.3390/agronomy13102549
Chicago/Turabian StyleOroian, Camelia, Florin Ugruțan, Iulia Cristina Mureșan, Ioan Oroian, Antonia Odagiu, Ioan Valentin Petrescu-Mag, and Petru Burduhos. 2023. "AMMI Analysis of Genotype × Environment Interaction on Sugar Beet (Beta vulgaris L.) Yield, Sugar Content and Production in Romania" Agronomy 13, no. 10: 2549. https://doi.org/10.3390/agronomy13102549
APA StyleOroian, C., Ugruțan, F., Mureșan, I. C., Oroian, I., Odagiu, A., Petrescu-Mag, I. V., & Burduhos, P. (2023). AMMI Analysis of Genotype × Environment Interaction on Sugar Beet (Beta vulgaris L.) Yield, Sugar Content and Production in Romania. Agronomy, 13(10), 2549. https://doi.org/10.3390/agronomy13102549









